Introduction
Currently, the standard antiviral therapy for
patients with chronic hepatitis C is combination therapy with
pegylated interferon (PEG-IFN) and ribavirin (RBV). However, only
∼50% of patients infected with the genotype 1 subtype of hepatitis
C virus (HCV) achieve a sustained virological response (SVR) with
this regimen (1). With the use of a
triple therapy combining PEG-IFN, RBV and protease inhibitors,
including telaprevir, a SVR can be obtained at a high rate, even
for patients with HCV genotype 1b who have a high viral load
(2,3).
However, a number of adverse effects have resulted
from the use of triple drug therapy, including a rash, digestive
tract symptoms and psychiatric symptoms. Previous studies have
reported the discontinuation of treatment in a number of patients
due to these adverse effects (4,5). These
patients require treatment with standard of care (SOC) centered on
PEG-IFN with RBV for refractory chronic hepatitis C.
It was previously reported that twice daily
administration of IFN-β in the induction phase (the start of
chronic hepatitis C treatment) to patients with refractory genotype
1b chronic hepatitis C with a high viral load may improve the early
virological response of HCV and increase the SVR rate with a
transition to SOC (6). In addition,
combination therapy with IFN-β and RBV has been covered by the
National Health Insurance Program in Japan since October 2009, and
an earlier virological response is expected with the introduction
of this treatment.
Double filtration plasmapheresis (DFPP) has been
used for a number of years as a therapeutic method for autoimmune
diseases and hyperlipidemia. The therapy functions by eliminating
high molecular weight substances, mainly immunoglobulins and
lipids, from plasma based on a molecular sieving effect using
hollow fiber membranes with two different pore sizes. Since April
2008, DFPP has also been covered by the National Health Insurance
Program for the treatment of chronic hepatitis C due to its ability
to directly eliminate HCV from the plasma. Among attempts to
improve the efficacy of IFN therapy for chronic hepatitis C, an
approach that differs from the idea of combined drug use to achieve
a rapid virological response is promising (7).
Therapeutic outcomes for chronic hepatitis C
patients with genotype 1b, who have a high viral load and are
considered to have the most difficult subtype to treat, have
remained unsatisfactory. Virus eradication in the early stages of
the disease is important, which can be achieved through the use of
IFN. Specifically, a SVR is known to be high when the virus is
eliminated from the blood within two weeks from the start of
administration (8). Therefore,
whether virological reduction is associated with treatment efficacy
when these combination therapies are used, and whether a
virological response occurs at an early stage are important issues
that require further study.
Through using DFPP in addition to IFN-β/RBV
induction therapy, an early virological response may be
ascertained. In addition, predictions can be formed with regard to
which patients may achieve a SVR. The present study retrospectively
analyzed treatment outcomes following IFN-β/RBV induction and DFPP
therapy prior to SOC for chronic hepatitis C patients with the
genotype 1b subtype. These patients had a high viral load and were
cases of relapse or non-response to SOC. Furthermore, whether the
extent of virus reduction at week 2 after treatment initiation was
able to predict the development of SVR was analyzed.
Materials and methods
Patients and treatment protocol
In total, 10 patients with chronic hepatitis C of
genotype 1b were included in the study. The patients had a high
viral load and had not responded to previous IFN therapy. IFN-β
(Feron; Toray Industries, Inc., Tokyo, Japan; Daiichi Sankyo Co.,
Ltd., Tokyo, Japan) and RBV (Rebetol; MSD K.K., Tokyo, Japan)
induction therapy with DFPP was performed five times during two
weeks. During DFPP induction therapy, the patients were treated
with IFN-β at 3 million units (MU) twice daily for two weeks. The 3
MU of IFN-β (Feron; Toray Industries, Tokyo, Japan) was dissolved
in 100 ml physiological saline or 100 ml of 5% glucose for
injection, and the solution was administered twice daily by
intravenous drip infusion over 30 min in the morning and evening.
Subsequent to IFN-β treatment, 1.5 µg/kg PEG-IFN-α2b (Pegintron;
Schering-Plough, Tokyo, Japan) was administered once a week. RBV
(Rebetol; Schering-Plough) was administered at a dose of 600 mg/day
(200 mg after breakfast, 400 mg after dinner) to patients weighing
<60 kg, 800 mg/day (400 mg after breakfast, 400 mg after dinner)
to those weighing 60-8 kg, and 1,000 mg/day (400 mg after
breakfast, 600 mg after dinner) to those weighing >80 kg. The
daily dose of RBV was reduced by 200 mg once the hemoglobin levels
had decreased to <10 g/dL, and RBV was discontinued once the
hemoglobin levels had decreased to <8.5 g/dL. When side effects
made continued administration difficult, or when HCV RNA levels did
not decrease, IFN was discontinued at the discretion of the
physician.
DFPP
For DFPP, a double lumen catheter (Unitika Ltd.,
Osaka, Japan) was placed in the femoral vein from the right
inguinal region as vascular access. A Plasauto iQ21 (Asahi Kasei
Medical Co., Ltd., Tokyo, Japan) apheresis system was used for
DFPP, with a Plasmaflo OP (Asahi Kasei Medical Co., Ltd.) plasma
separator (primary membrane) and a Cascadeflo EC-50 W (Asahi Kasei
Medical Co., Ltd.) plasma component separator (secondary membrane).
The plasma processing volume used for DFPP was 50 ml/kg and the
blood flow rate was 120–130 ml/min, with one session lasting ∼2 h.
Nafamostat mesilate was used as an anticoagulant for the first DFPP
session only, after which heparin sodium was used for the second
and subsequent sessions. DFPP replacement fluid was not required.
DFPP was performed a total of five times over a period of two
weeks, but never on more than two consecutive days in order to
prevent the blood fibrinogen levels decreasing to <100 mg/dl
(9).
Evaluation of IFN therapy
The quantity of HCV RNA was measured using the
COBAS® TaqMan® HCV test (detection limit, 1.2 log IU/ml; Roche
Diagnostics, Tokyo, Japan) and the high-range AMPLICOR™ HCV monitor
method (detection limit, 5 kIU/ml; Roche Diagnosis).
A rapid virological response (RVR) was defined as
undetectable serum levels of HCV RNA at week 4, while an early
virological response was defined as undetectable serum levels of
HCV RNA at week 12. A late virological response was defined as
detectable serum levels of HCV RNA at week 12 and a SVR was defined
as an undetectable serum level of HCV RNA at week 24 after the
initiation of treatment. No virological response was defined as
being HCV RNA-positive throughout the treatment period.
Ethical considerations
Prior to the initiation of the study, written
informed consent was obtained from each patient. The The present
study was approved by the Ethical Committee of Saiseikai Niigata
Daini Hospital (Niigata, Japan), and was conducted in accordance
with the principles of the Declaration of Helsinki.
Statistical analysis
Statistical analyses were performed using
χ2 or Fisher's exact tests for categorical data, and
Student's t-test for continuous data. Subgroup analyses were
performed to determine the contributing factors to EVR and SVR, and
these factors were evaluated by logistic regression analysis.
Multivariate analysis was conducted to identify independent
prognostic factors using the Cox proportional hazards model to
calculate the adjusted hazard ratio and 95% confidence interval.
Statistical analyses were performed using StatView version 5.0
software (SAS Institute, Inc., Cary, NC, USA). All reported P
values are 2-sided, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics
In total, 10 subjects were included, of which six
were male and four were female. The mean age of the patients was
63.0±6.6 years (range, 54–73 years), and the average HCV RNA level
was 7.0±0.5 log IU/ml (range, 5.5–7.1), as shown in Table I.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Patients |
|---|
| Gender, male/female
(n) | 6/4 |
| Age (years) | 63.0±6.6
(54–73)a |
| BMI
(kg/m2) | 22.0±2.4
(20–27)a |
| Liver biopsy (n) |
|
| Stage
(F0/F1/F2/F3/F4) | 0/4/4/0/1 |
| Grade
(A0/A1/A2/A3) | 0/4/4/1 |
| ALT (IU/l) | 80.5±76.9
(23–282)a |
| AST (IU/l) | 63.5±46.4
(30–173)a |
| PLT
(×104/µl) | 13.6±4.8
(8.7–23.0)a |
| Hb (g/dl) | 13.0±1.4
(11.0–16.0)a |
| γGTP (IU/l) | 41.0±24.8
(14.0–92.0)a |
| HbA1c (%) | 5.1±1.1
(4.9–8.4)a |
| Fibrinogen
(mg/dl) | 209.3±31.2
(176–257)a |
| TC (mg/dl) | 145.5±25.6
(125–207)a |
| LDL-C (mg/dl) | 80.8±14.7
(54–100)a |
| HDL-C (mg/dl) | 43.0±12.7
(28–63)a |
| TG (mg/dl) | 116.5±67.7
(57–265)a |
| HCV RNA (log
IU/ml) | 7.0±0.5
(5.5–7.1)a |
No proteinuria or depressive symptoms were observed
in any of the patients during IFN-β/RBV administration; thus, the
treatment was continued for two weeks.
HCV RNA levels
The mean decrease in the level of HCV RNA at week 2
after the start of treatment was 3.6±1.4 log IU/ml. The achievement
rate of a decrease of ≥2 log IU/ml after two weeks was 90% (9/10
patients), while a decrease of ≥4 log IU/ml was achieved in 40% of
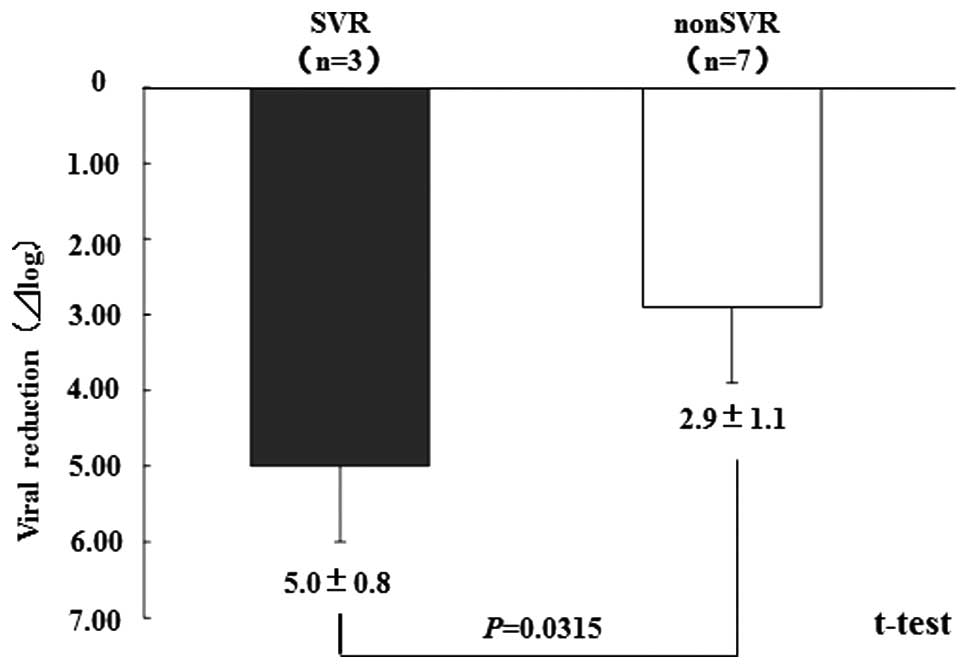
the patients (4/10 patients). The virus reduction in the SVR
patients was 5.0±0.8 log IU/ml, while that in the non-SVR patients
was 2.9±1.1 log IU/ml; the difference of which statistically
significant (Fig. 1).
None of the background factors were found to be
clinically significant in contributing to SVR (Table II).
 | Table II.Factors contributing to a SVR. |
Table II.
Factors contributing to a SVR.
| Characteristics | SVR (n=3) | non-SVR (n=7) | P-value (t-test) |
|---|
| Gender, male/female
(n) | 1/2 | 5/2 | - |
| Age (years) | 57.0±9.6
(54–72)a | 63.0±5.1
(61–73)a | 0.3137 |
| BMI
(kg/m2) | 24.0±2.8
(21–27)a | 22.0±2.2
(20–26)a | 0.3632 |
| Liver biopsy (n) |
|
|
|
| Stage
(F0/F1/F2/F3/F4) | 0/1/2/0/0 | 0/3/2/0/1 | - |
| Grade
(A0/A1/A2/A3) | 0/1/1/1 | 0/3/3/0 | - |
| ALT (IU/l) | 41.0±44.6
(23–282)a | 91.0±38.4
(30–140)a | 0.5445 |
| AST (IU/l) | 48.0±48.2
(30–121)a | 68.0±48.1
(31–173)a | 0.5758 |
| PLT
(×104/ml) | 14.8±3.4
(12.4–19.2)a | 11.5±5.4
(8.7–23.0)a | 0.8118 |
| Hb (g/dl) | 12.5±2.1
(11.7–15.7)a | 13.4±1.3
(10.7–14.5)a | 0.7221 |
| γGTP (IU/l) | 37.0±40.1
(14.0–92.0)a | 41.0±19.4
(31.0–87.0)a | 0.8104 |
| HbA1c (%) | 5.4±0.8
(4.9–6.5)a | 5.1±1.3
(5.1–8.4)a | 0.8604 |
| Fibrinogen
(mg/dl) | 241.4±25.9
(201–249)a | 201.9±33.3
(176–257)a | 0.4007 |
| TC (mg/dl) | 169.0±15.0
(143–169)a | 137.0±29.7
(125–207)a | 0.6485 |
| LDL-C (mg/dl) | 81.0±7.0
(79–92)a | 77.0±17.1
(54–100)a | 0.5397 |
| HDL-C (mg/dl) | 54.0±12.7
(45–63)a | 38.0±11.0
(28–54)a | 0.2184 |
| TG (mg/dl) | 110±34
(77–145)a | 123±79
(77–145)a | 0.5445 |
| HCV RNA (log
IU/ml) |
|
|
|
|
Baseline | 6.4±0.5
(6.2–7.1)a | 7.0±0.6
(5.5–7.1)a | 0.6072 |
| Week
2 | 1.4±0.4
(1.2–2.0)a | 3.9±1.5
(1.8–5.8)a | 0.0315 |
Safety and adverse effects
No serious complications, including a catheter
infection, were observed during extracorporeal circulation and
DFPP. However, one adverse effect of the DFPP therapy was decreased
blood pressure, which was observed in four patients and occurred a
total of six times. However, this adverse effect was managed with
an infusion of ∼100–200 ml physiological saline. In addition, in
certain patients, the fibrinogen level was found to decrease
temporarily to <100 mg/dl at the end of the DFPP treatment;
however, the fibrinogen level was ≥100 mg/dl in all the patients at
the final termination of DFPP.
Discussion
Almost 20 years have elapsed since IFN therapy was
initiated for the treatment of chronic hepatitis C (10). Advances over the past several years
have been marked, such that a SVR is currently obtained in
approximately half of all patients who receive PEG-IFN/RBV
combination therapy, even in patients with genotype 1b HCV and a
high viral load. Direct antiviral agents that target HCV, including
HCV protease inhibitors and polymerase inhibitors, have also been
developed. There are high expectations for these new agents in
significantly improving future treatment outcomes for patients with
chronic hepatitis C. However, there are a number of problems
associated with adverse effects in using these new agents.
Considering the current status of chronic hepatitis C in Japan,
which has a particularly large elderly population, questions of
whether the new agents can be used and whether treatment with these
agents can be delayed until they are approved may be
problematic.
In addition to these pharmaceutical advances, there
are expectations for improved SVR rates with combination therapies
that contribute to virus reduction. DFPP has long been used as a
method for treating diseases by predominantly eliminating
immunoglobulins and other disease-associated proteins. HCV
particles are hypothesized to have diameters of 55–65 nm;
therefore, selective elimination of substances of this size may
enable the efficient elimination of HCV particles. The Cascadeflo
EC-50 W, which is already used in DFPP therapy, has a maximum pore
size of 30 nm. Therefore, theoretically, HCV particles are unable
to pass through this membrane and can be directly eliminated from
extracorporeally circulating blood. In addition, the safety of this
extracorporeal circulation equipment has previously been
established (10).
In the developmental stage, a RVR is expected from
DFPP therapy; thus, DFPP may be performed at the start of IFN
administration. Various other usage methods have also been
considered, including using DFPP as a supplementary treatment in
patients who do not obtain a virological response during IFN
administration. There have also been notable studies investigating
the possibility of preventing HCV reinfection in liver transplant
recipients, and marked effects have been obtained against
post-transplant fibrosing cholestatic hepatitis (11). However, further studies with a
greater sample size are required to investigate whether a SVR can
be obtained with retreatment using a combination of DFPP in
patients who did not achieve a SVR with SOC using PEG-IFN, which is
currently the mainstream treatment.
To date, SVR rates have increased to 40–50% with
SOC. When the timing of the virological response is considered, the
treatment effect has increased to ∼60% with treatment protocols
that extend the treatment duration. Using any treatment method, the
period up to four weeks from the start of administration has been
shown to be important, and the SVR rate in patients in whom a
virological response had previously been obtained is known to be
much higher. Based on these observations, treatment strategies that
aim to eliminate the virus from the blood at an earlier stage are
being tried. Okushin et al reported that an HCV RNA response
was obtained with a high rate early in treatment of administering
IFN-β twice daily at the time of IFN therapy induction places
importance on hepatic migration from venous administration and drug
accumulation with consideration of pharmacokinetics (12).
Fujiwara et al reported a much higher viral
elimination rate in the second week after the start of twice daily
IFN-β therapy compared with a once daily regimen (13). Similarly, Izumi et al reported
a more notable decrease in the level of HCV RNA in the patient
group who received twice daily administration of IFN-β when
compared with the patient group who received once daily
administration (14). These
observations demonstrate that while the half-life of
venously-administered IFN-β in the blood is very short, the blood
levels are maintained in the effective treatment range with twice
daily administration (15). In
addition, Asahina et al analyzed HCV dynamics in serum and
peripheral blood mononuclear cells and demonstrated that twice
daily administration of IFN-β exhibited a strong antiviral effect
until the second phase (16).
Based on these studies, we previously investigated
the efficacy of IFN-β induction therapy for patients with HCV of
genotype 1b with a high viral load (6). In addition, DFPP supplementary
treatment was found to contribute to early virus reduction, even in
patients who relapsed following previous IFN-β induction therapy
(17).
Using IFN-β in combination with RBV has become
possible, and with a combination that also includes DFPP, virus
reduction is hypothesized to occur in a shorter time.
Although the present study had the limitation of a
small number of patients that exhibited a relapse or non-response
with SOC, a SVR of 30% was obtained. There were no statistically
significant differences observed in the background factors between
the patients with and without SVR; however, the extent of virus
reduction alone is hypothesized to be an important factor in
therapeutic efficacy. Various factors are associated with treatment
efficacy in chronic hepatitis C, including drug factors, virus
factors and host factors. Among these, the host factor, interleukin
(IL)-28B, is considered to be one of the most important. However, a
decrease in the level of HCV RNA of ≥4 log IU/ml at week 2 after
the start of induction therapy, obtained through using combination
therapy with DFPP and IFN-β/RBV for patients with genotype 1b and a
high viral load, is also important. If the decrease were to be
<4 log IU/ml, IFN therapy for chronic hepatitis C should aim to
not only produce a SVR, but also improve hepatic function
reserve.
In conclusion, although long-term observations are
required in the future, treatment with DFPP combined with induction
therapy of IFN-β/RBV for chronic hepatitis C was demonstrated to be
a useful induction method. In addition, the results revealed that
the SVR can be predicted from an early decrease in the viral load.
Thus, in addition to IL-28B measurements, the extent of virus
reduction may also be a potential therapeutic diagnostic indicator
in response-guided therapy.
References
|
1
|
Manns MP, McHutchison JG, Gordon SC, et
al: Peginterferon alfa-2b plus ribavirin compared with interferon
alfa-2b plus ribavirin for initial treatment of chronic hepatitis
C: a randomised trial. Lancet. 358:958–965. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kumada H, Toyota J, Okanoue T, Chayama K,
Tsubouchi H and Hayashi N: Telaprevir with peginterferon and
ribavirin for treatment-naïve patients chronically infected with
HCV of genotype 1 in Japan. J Hepatol. 56:78–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chayama K, Hayes CN, Ohishi W and Kawakami
Y: Treatment of chronic hepatitis C virus infection in Japan:
update on therapy and guidelines. J Gastroenterol. 48:1–12. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chopra A, Klein PL, Drinnan T and Lee SS:
How to optimize HCV therapy in genotype 1 patients: management of
side-effects. Liver Int. 33(Suppl 1): 30–34. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cacoub P, Bourlière M, Lübbe J, et al:
Dermatological side effects of hepatitis C and its treatment:
patient management in the era of direct-acting antivirals. J
Hepatol. 56:455–463. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishikawa T, Kubota T, Abe H, et al:
Efficacy of the regimen using twice-daily β-interferon followed by
the standard of care for chronic hepatitis C genotype 1b with high
viral load. Hepatol Res. 42:864–869. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schettler V, Monazahian M, Wieland E, et
al: Reduction of hepatitis C virus load by H.E.L.P.-LDL apheresis.
Eur J Clin Invest. 31:154–155. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Neumann AU, Lam NP, Dahari H, et al:
Hepatitis C viral dynamics in vivo and the antiviral efficacy of
interferon-alpha therapy. Science. 282:103–107. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamashita T, Arai K, Sakai A, et al:
Virological effects and safety of combined double filtration
plasmapheresis (DFPP) and interferon therapy in patients with
chronic hepatitis C: A preliminary study. Hepatol Res. 36:167–175.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strader DB, Wright T, Thomas DL and Seeff
LB: American Association for the Study of Liver Diseases:
Diagnosis, management, and treatment of hepatitis C. Hepatology.
39:1147–1171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taniguchi M, Furukawa H, Shimamura T, et
al: Impact of double-filtration plasmapheresis in combination with
interferon and ribavirin in living donor liver transplant
recipients with hepatitis C. Transplantation. 81:1747–1749. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okushin H, Morii K, Kishi F and Yuasa S:
Efficacy of the combination therapy using twice-a-day IFNβ followed
by IFNα2b in treatment for chronic hepatitis C. Kanzo. 38:11–18.
1997.[In Japanese]. View Article : Google Scholar
|
|
13
|
Fujiwara K, Mochida S, Matsuo S, et al:
Randomized control trial of interferon-β injections at 12-h
intervals as a therapy for chronic hepatitis C. Hepatology Res.
12:240–251. 1998. View Article : Google Scholar
|
|
14
|
Izumi N, Kumada H, Hashimoto N, et al:
Rapid decrease of plasma HCV RNA in early phase of twice daily
administration of 3 MU doses interferon-beta in patients with
genotype 1b hepatitis C infection: a multicenter randomized study.
Dig Dis Sci. 46:516–523. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hilfenhaus J, Damm H, et al:
Pharmacokinetics of human interferon-beta in monkeys. J Interferon
Res. 1:427–436. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Asahina Y, Izumi N, Uchihara M, et al: A
potent antiviral effect on hepatitis C viral dynamics in serum and
peripheral blood mononuclear cells during combination therapy with
high-dose daily interferon alfa plus ribavirin and intravenous
twice-daily treatment with interferon beta. Hepatology. 34:377–384.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ishikawa T, Higuchi K, Kubota T, et al:
Complete early virological response was highly achieved by double
filtration plasmapheresis plus IFN-beta induction therapy for
HCV-1b patients with relapse or no response after previous IFN
therapy. Ther Apher Dial. 15:400–405. 2011.PubMed/NCBI
|