Introduction
The incidence of ovarian cancer is gradually rising
and its mortality rate ranks first among the gynecological
malignant tumors; the majority of cases are epithelial ovarian
cancer (EOC) derived from the ovarian epithelial tissue (1). The pathogenesis and development of
ovarian cancer have been closely linked to inflammatory processes
associated with several cytokines (2,3), such as
vascular endothelial growth factor (4) interleukin-6 (5) and interleukin-12 (6). Among the already established prognostic
indicators, ovarian tumor stage and postoperative residual tumor
mass at primary cytoreductive surgery have been shown to most
reliably predict the outcome of patients with ovarian cancer
(7). Clinical decisions regarding
adjuvant therapies are largely based on the International
Federation of Gynecologists and Obstetricians (FIGO) stage and
tumor grade, particularly in the early stages of the disease
(8). With the progress in the
surgical techniques, management of the perioperative period and
range of combined treatments, the survival rate of patients with
ovarian cancer has been increased significantly; however, the high
recurrence rate following treatment remains the primary reason for
poor prognoses (9). Reliable
indicators for the identification of those patients that are at
high risk of recurrence or mortality in the early stages of the
disease would be beneficial for the evaluation of the prognosis of
patients with ovarian cancer and for the development of specific
measures to counteract unfavorable outcomes.
C-reactive protein (CRP) is an acute-phase protein
expressed as an acute response to infections and tissue injuries,
and increases in its expression are often associated with multiple
system and organ diseases (10). At
present, the theory of a causal association between inflammation
and the innate immune system regarding cancer is widely accepted.
Numerous malignant tumors occur at sites of infection, chronic
irritation and inflammation, and 15–20% of human tumors are
associated with inflammations (11).
Several studies have shown that patients with cancer have higher
circulating CRP levels than healthy controls prior to clinical
diagnosis (3–5 years) (12–15). In addition, the increase in serum CRP
has been reported to be associated with poor prognosis in
esophageal, liver, colon, lung and cervical cancer (16–20).
Among the various gynecological tumor markers, such as
α-fetoprotein and human epididymis protein-4 (21), cancer antigen 125 (CA125) is the most
specific and sensitive marker for EOC detection (22), particularly for recurrent tumors
(21,22). It has been reported that there are
considerable ethnic differences regarding the five-year survival
rate of patients with EOC; in Asian females, the five-year survival
rate was found to be almost double that of Caucasians in a survey
performed in the USA between 1992 and 1997 (25). There are few reports, however,
investigating whether the serum CRP concentration of patients with
ovarian cancer in China complies with that in European patients and
those from the USA; therefore, the aim of the present study was to
investigate a correlation between the serum CRP concentrations and
clinicopathological parameters of Chinese patients with EOC, using
CA125 as a positive control marker.
Materials and methods
Characteristics of patients
Between January 2006 and March 2010, 107 patients
with EOC underwent surgery at the Departments of Obstetrics and
Gynecology of the Provincial Hospital Affiliated to Shandong
University (Jinan, China; n=71) and the Haimen City People's
Hospital (Haimen, China; n=36). Diagnoses were confirmed by the
pathology results. All the patients were classified by FIGO (2000)
standard staging (26), and the age
of the patients at the first visit was 34–79 years (mean,
55.28±10.34 years). The control group comprised females with benign
ovarian tumors [n=32; age, 32–72 years (mean age, 51.38±9.05
years)], including simple ovarian cyst, benign serous or mucinous
cystadenoma, and healthy adult females [n=12; age, 38–61 years
(49.50±6.53 years)]. There was no significant age difference
between the groups.
Enrollment criteria
For enrollment in the study, patients had to meet
the following criteria: i) Ovarian cancer without surgical
contraindication and EOC confirmed by postoperative pathology; and
ii) normal white blood cell count and neutrophil ratios, as shown
by routine blood examination on admission. Patients were excluded
due to infection, trauma, coronary heart disease, hypertension and
connective tissue diseases, as these conditions could result in
elevated CRP concentrations. The study protocol was approved by the
Ethics Committee of Haimen City People's Hospital and the
Provincial Hospital Affiliated to Shandong University, and all
participants provided their written informed consent to participate
in the study.
Measurement methods
On the second day after admission, 6 ml morning
fasting blood was collected from each patient and the serum of 3 ml
was used for CRP quantification via nephelometry rates
(High-Sensitivity CRP kit; Beckman Coulter, Miami, FL, USA). With
the development of a high-sensitivity assay method, the
associations between inflammation and disease risk allow a wide
spectrum of CRP levels, particularly in the range of concentrations
previously widely considered ‘normal’, i.e. levels <10 mg/l. Due
to the intra-assay variability (1.64–3.34%), CRP serum levels ≤8
mg/l were defined as the normal range in the two hospitals in the
present study. The serum of another 3 ml blood was used to
determine the content of CA125 via electrochemiluminescence (Roche
Diagnostics GmbH, Manneheim, Germany), and the CA125 normal
reference value was set to 0–35 U/ml.
Surgery and postoperative adjunctive
therapy
A total of 69 patients received surgery without
residual tumor or residual lesions <2 cm; 38 patients received
subtotal tumor resections with residual lesions ≥2 cm. Sixty-five
cases underwent pelvic and para-aortic lymph node dissection: 21
patients had lymph node metastasis and 44 cases were without
metastasis. Intraperitoneal combined with intravenous chemotherapy
or simple intravenous chemotherapy was started 7 days after surgery
once every 4 weeks, and regular follow-ups were performed for 11–74
months with a mean duration of 28.5 months.
Statistical analysis
SPSS 17.0 statistical software was used for the
statistical analyses, and the measurement data are presented as the
mean ± standard deviation or median. Comparisons of measurement
data were performed using the Student's t-test, rank sum test or
analysis of variance, and count data were compared using the
χ2 test. Single or multiple factors were compared using
logistic regression analyses. The correlation of CRP with CA125 was
analyzed by a Spearman rank correlation analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients' characteristics
According to the FIGO staging criteria, 29 cases
were in stage I (27.10%), 14 cases in stage II (13.08%), 44 cases
in stage III (41.12%) and 20 cases in stage IV (18.69%). The
analysis of pathological type revealed that 43 cases were serous
cystadenocarcinoma (40.19%); 38 cases were mucinous
cystadenocarcinoma (35.51%) and 35 cases were other types of
epithelial cancers (32.71%), including endometrial cancer, clear
cell carcinoma, transitional cell carcinoma and epithelial cancer
that could not be classified due to tissue necrosis. Ascites
occurred in 42 cases (39.25%), and 65 cases (60.75%) were without
ascites. For histological cell grading, 28 cases were in the G1
phase (26.17%), 44 cases in the G2 phase (41.12%) and 35 cases in
the G3 phase (32.71%).
Preoperative serum CRP and CA125
concentrations of patients with EOC
In the present study, 62% of the patients (66/107)
were positive for CRP (CRP >8 mg/l) in the EOC group, while no
positive results were found in the control group. The mean
preoperative serum CRP concentration of the patients with EOC was
14.32 mg/l (1.02–163.00 mg/l), which was ∼6.6-fold that of the
ovarian benign tumor group [2.175 mg/l (1.00–4.59 mg/l)]
(P<0.001), as well as ∼9.7-fold the serum concentration of the
healthy adult females [1.47 mg/l (1.00–3.16 mg/l)] (P<0.001).
The serum concentration of CA125 in the EOC group was 475.90 U/l
(16.26–5,000 U/l), whereas in the benign ovarian tumor group it was
23.85 U/l (7.74–61.31 U/l) and in the healthy adult females it was
8.77 U/l (1.35–18.33 U/l) (P<0.05). The CA125 serum
concentration in the EOC group was ∼20-fold that of the benign
ovarian tumor group and 54-fold that of the healthy adult females.
A rank correlation analysis of preoperative serum CRP and CA125
concentrations in the EOC group revealed that the Spearman
correlation coefficient (rs) was 0.603 (P<0.001, two-way),
suggesting that CRP was positively correlated with CA125; however,
no significant correlation between the CRP and CA125 concentrations
existed in the control group (rs=-0.095, P=0.708).
Correlation of EOC serum CRP
concentrations with clinical and pathological parameters
It was next analyzed whether elevated preoperative
serum CRP concentrations correlated with the clinicopathological
parameters of the patients with EOC. As shown in Table I, the serum concentrations of CRP
correlated with histological grading, FIGO staging, ascites, tumor
size and lymph node metastasis, whereas only positive serum CRP did
not correlate with histological grading. No significant difference
was found in the serum CRP concentrations between different ages
and different pathological tumor types.
 | Table I.Correlation of serum CRP concentration
with the clinicopathological parameters of patients with epithelial
ovarian cancer. |
Table I.
Correlation of serum CRP concentration
with the clinicopathological parameters of patients with epithelial
ovarian cancer.
| Clinicopathological
parameter | n | CRP level, mg/l | P-valuea, ANOVA | CRP-positive
rateb, n (%) | P-valuea, ANOVA |
|---|
| Age, years |
|
|
>50 | 63 | 22.24±36.39 | 0.283 | 35 (56) | 0.081 |
| ≤50 | 44 | 29.27±23.93 |
| 31 (54) |
| FIGO stage |
|
| I | 29 | 4.61±3.06 | <0.001 | 4 (14) | <0.001 |
| II | 14 | 10.81±4.48 |
| 10 (71) |
| III | 44 | 35.48±36.98 |
| 35 (91) |
| IV | 20 | 51.04±33.23 |
| 17 (80) |
| Histological
grading |
|
| G1 | 28 | 12.03±14.21 | 0.005 | 11 (39) | 0.165 |
| G2 | 44 | 26.75±29.70 |
| 30 (68) |
| G3 | 35 | 38.66±41.58 |
| 25 (71) |
| Type |
|
|
Pathological serous
cystadenocarcinoma | 43 | 29.93±35.93 | 0.089 | 28 (65) | 0.118 |
| Mucinous
cystadenocarcinoma | 38 | 13.24±10.93 |
| 22 (58) |
| Other
types of epithelial carcinoma | 26 | 32.19±34.18 |
| 16 (62) |
| Ascites |
|
| With | 42 | 36.01±34.92 | 0.014 | 36 (86) | 0.026 |
|
Without | 65 | 20.36±29.63 |
| 30 (46) |
| Tumor resection |
|
| Residual
tumor diameter <2 cm | 69 | 21.14±27.43 | 0.006 | 39 (57) | 0.004 |
|
Residual tumor diameter ≥2
cm | 38 | 40.04±39.98 |
| 27 (71) |
| Lymph node
metastasis |
|
|
Positive | 21 | 30.79±36.99 | 0.002 | 17 (81) | 0.004 |
|
Negative | 44 | 10.14±15.31 |
| 15 (34) |
Logistic regression analyses of single
and multiple factors affecting CRP serum concentrations
Based on the results of single factor analyses, a
positive serum CRP value was correlated with FIGO tumor staging,
histological grading, presence of ascites and lymph node metastasis
(Table II), while CA125 had no
effect on CRP-positive rates. When the factors that correlated with
CRP in the single factor analyses as independent variables were
used in a multiple factor logistic regression analysis it was found
that the FIGO tumor staging, presence of ascites and lymph node
metastasis were correlated with CRP-positive rates (Table III).
 | Table II.Single factor logistic regression
analysis for C-reactive protein expression. |
Table II.
Single factor logistic regression
analysis for C-reactive protein expression.
|
| 95% CI of OR |
|---|
|
|
|
|---|
| Factor | B | SE | Wald | df | P-value | OR | Lower | Upper |
|---|
| Histological
grading | -1.151 | 0.794 | 3.147 | 1 | 0.047 | 0.163 | 0.537 | 2.517 |
| Pathological
type | 0.360 | 0.366 | 0.964 | 1 | 0.326 | 1.433 | 0.699 | 2.938 |
| Ascites | -1.732 | 0.805 | 4.633 | 1 | 0.031 | 0.177 | 0.037 | 0.857 |
| Residual tumor | -0.296 | 0.661 | 0.201 | 1 | 0.654 | 0.744 | 0.204 | 2.716 |
| CA125 | -0.633 | 0.958 | 0.436 | 1 | 0.509 | 0.531 | 0.081 | 3.473 |
| FIGO stage | -1.287 | 0.412 | 9.771 | 1 | 0.002 | 0.276 | 0.123 | 0.619 |
| Lymph node | 2.144 | 0.663 | 10.468 | 1 | 0.001 | 8.537 | 2.329 | 31.298 |
| Age | 0.383 | 0.583 | 0.431 | 1 | 0.511 | 1.467 | 0.468 | 4.599 |
 | Table III.Multiple factor logistic regression
analysis for C-reactive protein expression. |
Table III.
Multiple factor logistic regression
analysis for C-reactive protein expression.
|
| 95% CI of OR |
|---|
|
|
|
|---|
| Factor | B | SE | Wald | df | P-value | OR | Lower | Upper |
|---|
| FIGO stage | 1.381 | 0.412 | 11.227 | 1 | 0.001 | 3.980 | 1.774 | 8.928 |
| Ascites | 1.767 | 0.826 | 4.571 | 1 | 0.033 | 5.852 | 1.158 | 29.559 |
| Lymph node
metastasis | -2.087 | 0.646 | 10.420 | 1 | 0.001 | 0.124 | 0.035 | 0.441 |
Correlation of CRP expression with the
five-year survival rate of patients with EOC
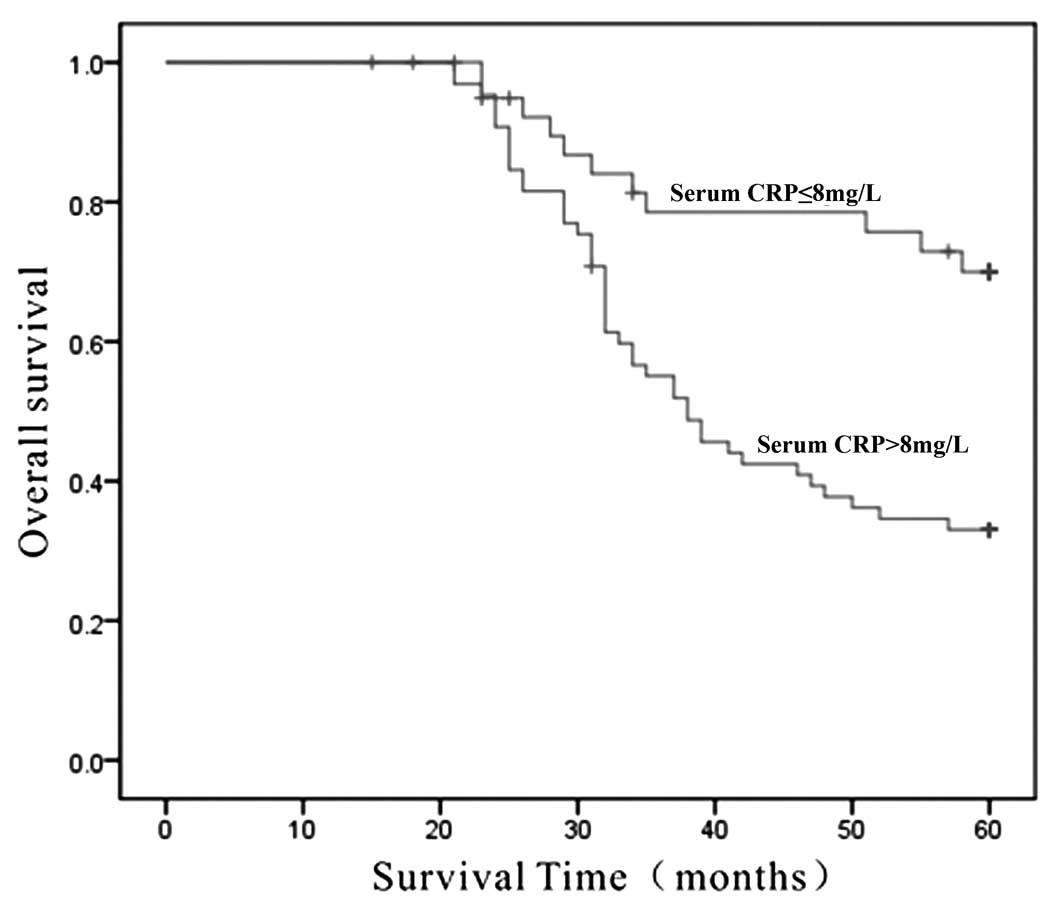
The overall five-year survival rate of patients with
serum CRP ≤8 mg/l was 75.8%, compared with a survival rate of 33.3%
for patients with CRP concentrations >8 mg/l (P<0.001)
(Fig. 1).
Discussion
McSorley et al (27) found that the serum concentration of
CRP in females with ovarian cancer was enhanced, indicating chronic
inflammation during the development of ovarian cancer. Other
gynecological inflammatory diseases, such as pelvic inflammation,
endometriosis and polycystic ovarian syndrome, may progress into
ovarian cancer, which indirectly supports the interaction of
inflammation and cancer (28).
Ovulation is basically an inflammatory process, which includes
repair cycles of the ovarian cortex wound with concomitant healing.
It has been suggested that persistent ovulation is a potential
inducer of ovarian cancer; this is supported by the fact that
anovulatory factors, including oral contraceptives, pregnancy and
lactation, can greatly reduce the risk of ovarian cancer (29). Cancer induces nonspecific
inflammation, leading to the release of a variety of
pro-inflammatory mediators and factors (30). These nonspecific inflammatory
reactions produced by tumor tissue necrosis and/or local tissue
injury can induce the liver cells to synthesize CRP, which is then
released into the serum. Several reports have confirmed that serum
CRP levels are elevated in patients with malignant tumors, and the
elevation is associated with the malignancy degree, increase in
tumor metastasis rate and decrease in postoperative survival rate
(16–20,31). In
the present study it was demonstrated that preoperative serum CRP
concentrations correlated with tumor FIGO staging, lymph node
metastasis and ascites formation among the 107 patients with EOC,
which is in line with the previous reports (32–34).
Furthermore, it was found in this study that the average CRP
concentration in Chinese patients with EOC was 14.32 mg/l, which
was lower than the value reported for Caucasian cases (36 mg/l)
(32), which may reflect ethnical
variations; however, the same study (32) also reported that enhanced serum CRP
concentrations correlated with an unfavorable prognosis, which has
been confirmed by the present finding that CRP-positive patients
with EOC had significantly lower five-year survival rates. In the
107 cases of EOC in the present study, the mean preoperative serum
CA125 concentration was 475.90 U/ml, and the value in the EOC group
was significantly higher than that in the benign ovarian tumor
group (23.85 U/ml) (P<0.001). Based on a rank correlation
analysis of serum CRP and CA125 concentrations, a positive
correlation was found (P<0.001), which is also in contrast to
previous reports (32,33).
In conclusion, the CRP serum concentrations of
patients with EOC were significantly enhanced and correlated with
FIGO staging, lymph metastases and the occurrence of ascites, as
well as with CA125 serum concentrations. Preoperative CRP serum
concentration analyses could be a useful parameter for evaluating
the severity of EOC and treatment adjustments, and could be
utilized to monitor the treatment success in combination with the
measurement of serum CA125 concentration.
Acknowledgements
The authors would like to thank Mr. Xiancai Ni, Mr.
Qiongcheng Yan and Ms. Jumei Gu from the Haimen City People's
Hospital for coordinating the study and for their assistance with
data collection.
References
|
1
|
Wong KH, Mang OW, Au KH and Law SC: .
Incidence, mortality, and survival trends of ovarian cancer in Hong
Kong, 1997 to 2006: a population-based study. Hong Kong Med J.
18:466–474. 2012.PubMed/NCBI
|
|
2
|
Helzlsouer KJ, Erlinger TP and Platz EA:
C-reactive protein levels and subsequent cancer outcomes: results
from a prospective cohort study. Eur J Cancer. 42:704–707. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balkwill F and Mantovani A: Inflammation
and cancer: back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hefler LA, Zeillinger R, Grimm C, et al:
Preoperative serum vascular endothelial growth factor as a
prognostic parameter in ovarian cancer. Gynecol Oncol. 103:512–517.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tempfer C, Zeisler H, Sliutz G, et al:
Serum evaluation of interleukin 6 in ovarian cancer patients.
Gynecol Oncol. 66:27–30. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeimet AG, Widschwendter M, Knabbe C, et
al: Ascitic interleukin-12 is an independent prognostic factor in
ovarian cancer. J Clin Oncol. 16:1861–1868. 1998.PubMed/NCBI
|
|
7
|
Friedlander ML: Prognostic factors in
ovarian cancer. Semin Oncol. 25:305–314. 1998.PubMed/NCBI
|
|
8
|
Winter-Roach BA, Kitchener HC and Lawrie
TA: Adjuvant (post-surgery) chemotherapy for early stage epithelial
ovarian cancer. Cochrane Database Syst Rev.
3:CD0047062012.PubMed/NCBI
|
|
9
|
Marchetti CI, Pisano C, Facchini G, et al:
First-line treatment of advanced ovarian cancer: current research
and perspectives. Expert Rev Anticancer Ther. 10:47–60. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Marnell L, Mold C and Du Clos TW:
C-reactive protein: ligands, receptors and role in inflammation.
Clin Immunol. 117:104–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Allavena P, Garlanda C, Borrello MG, Sica
A and Mantovani A: Pathways connecting inflammation and cancer.
Curr Opin Genet Dev. 18:3–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lundin E, Dossus L, Clendenen T, et al:
C-reactive protein and ovarian cancer: a prospective study nested
in three cohorts (Sweden, USA, Italy). Cancer Causes Control.
20:1151–1159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Siemes C, Visser LE, Coebergh JW, et al:
C-reactive protein levels, variation in the C-reactive protein
gene, and cancer risk: the Rotterdam Study. J Clin Oncol.
24:5216–5222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Il'yasova D, Colbert LH, Harris TB, et al:
Circulating levels of inflammatory markers and cancer risk in the
health aging and body composition cohort. Cancer Epidemiol
Biomarkers Prev. 14:2413–2418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trichopoulos D, Psaltopoulou T, Orfanos P,
Trichopoulou A and Boffetta P: Plasma C-reactive protein and risk
of cancer: a prospective study from Greece. Cancer Epidemiol
Biomarkers Prev. 15:381–384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gockel I, Dirksen K, Messow CM and
Junginger T: Significance of preoperative C-reactive protein as a
parameter of the perioperative course and long-term prognosis in
squamous cell carcinoma and adenocarcinoma of the oesophagus. World
J Gastroenterol. 12:3746–3750. 2006.PubMed/NCBI
|
|
17
|
Hashimoto K, Ikeda Y, Korenaga D, et al:
The impact of preoperative serum C-reactive protein on the
prognosis of patients with hepatocellular carcinoma. Cancer.
103:1856–1864. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McMillan DC, Canna K and McArdle CS:
Systemic inflammatory response predicts survival following curative
resection of colorectal cancer. Br J Surg. 90:215–219. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brown DJ, Milroy R, Preston T and McMillan
DC: The relationship between an inflammation-based prognostic score
(Glasgow Prognostic Score) and changes in serum biochemical
variables in patients with advanced lung and gastrointestinal
cancer. J Clin Pathol. 60:705–708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Polterauer S, Grimm C, Tempfer C, et al:
C-reactive protein is a prognostic parameter in patients with
cervical cancer. Gynecol Oncol. 107:114–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anastasi E, Marchei GG, Viggiani V, et al:
HE4: a new potential early biomarker for the recurrence of ovarian
cancer. Tumour Biol. 31:113–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klug TL, Bast RC Jr, Niloff JM, Knapp RC
and Zurawski VR Jr.: Monoclonal antibody immunoradiometric assay
for an antigenic determinant (CA 125) associated with human
epithelial ovarian carcinomas. Cancer Res. 44:1048–1053.
1984.PubMed/NCBI
|
|
23
|
Schilthuis MS, Aalders JG, Bouma J, et al:
Serum CA 125 levels in epithelial ovarian cancer: relation with
findings at second-look operations and their role in the detection
of tumour recurrence. Br J Obstet Gynaecol. 94:202–207. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Santillan A, Garg R, Zahurak ML, et al:
Risk of epithelial ovarian cancer recurrence in patients with
rising serum CA-125 levels within the normal range. J Clin Oncol.
23:9338–9343. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howe HL, Tung KH, Coughlin S,
Jean-Baptiste R and Hotes J: Race/ethnic variations in ovarian
cancer mortality in the United States, 1992–1997. Cancer. 97(10
Suppl): 2686–2693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benedet JL, Bender H, Jones H III, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McSorley MA, Alberg AJ, Allen DS, et al:
C-reactive protein concentrations and subsequent ovarian cancer
risk. Obstet Gynecol. 109:933–941. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ness RB: Endometriosis and ovarian cancer:
thoughts on shared pathophysiology. Am J Obstet Gynecol.
189:280–294. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tung KH, Wilkens LR, Wu AH, et al: Effect
of anovulation factors on pre- and postmenopausal ovarian cancer
risk: revisiting the incessant ovulation hypothesis. Am J
Epidemiol. 161:321–329. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shacter E and Weitzman SA: Chronic
inflammation and cancer. Oncology (Williston Park). 16:217–226.
2002.PubMed/NCBI
|
|
31
|
McMillan DC, Elahi MM, Sattar N, et al:
Measurement of the systemic inflammatory response predicts
cancer-specific and non-cancer survival in patients with cancer.
Nutr Cancer. 41:64–69. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hefler LA, Concin N, Hofstetter G, et al:
Serum C-reactive protein as independent prognostic variable in
patients with ovarian cancer. Clin Cancer Res. 14:710–714. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hefler-Frischmuth K, Hefler LA, Heinze G,
et al: Serum C-reactive protein in the differential diagnosis of
ovarian masses. Eur J Obstet Gynecol Reprod Biol. 147:65–68. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu Y, Yin F and Chen Z: Prognostic value
of preoperative serum C-reactive protein in patients with
epithelial ovarian cancer. Progress inObstetrics and Gynecology.
17:489–492. 2008.
|