Introduction
Primary liver cancer, also known as hepatocellular
carcinoma (HCC), is the third leading cause of cancer-associated
mortalities worldwide with ∼600,000 cases reported annually
(1). Chronic liver diseases, viral
hepatitis and alcoholism, as well as dietary carcinogens, including
aflatoxins and nitrosoamines, are known etiologies (2). In China, the incidence rate of HCC is
particularly high due to the large number of patients with
cirrhosis caused by chronic hepatitis following viral hepatitis B
and C infections (3–5). Postoperative HCC recurrence rates are
as high as 50% (6) and at present,
therapies primarily include surgery, local ablation, interventional
and radiation therapies, in addition to medication. Superior
curative effects may be achieved for HCC treatments when
chemotherapy is combined with interventional traditional Chinese
medicine (TCM), including Curcuma oil (microsphere) perfusion
embolization. This intubation technique exhibited similar effects
to western medicine using hepatic arterial transcatheter
chemoembolization with regard to tumor size and survival time, but
was also found to have a less severe effect on peripheral white
blood cells and liver damage (7). A
variety of TCMs combined with interventional hepatic artery
chemotherapy embolization for toxicity reduction and enhancing the
efficacy have been investigated for the treatment of HCC. There are
two main advantages for TCM treatments of HCC: Firstly, the
cytotoxic effects of the ingredients contained in the active
natural anticancer drugs directly inhibit the growth of tumor cells
(8–11), and secondly, the improvement of the
immune system (12). Cidan capsules
are a formula containing more than ten types of plant extracts,
including Rhizoma Curcumae (19%), Astragalus (19.6%),
Cremastra appendiculata (9.8%), Salvia miltiorrhiza
(9.8%), hive (9.8%) and Bombyx batryticatus (9.8%). Cidan
has been clinically used for >10 years as a safe and nontoxic
antitumor drug. A number of studies have investigated the clinical
application of TCMs for HCC (13,14),
demonstrating that β-elemene, which is present in Rhizoma Curcumae
and the main component of cidan, may inhibit the proliferation of
HepG2 cells in a time- and dose-dependent manner. The results
indicated that β-elemene exhibited positive effects on apoptosis
and induced the cell cycle arrest of HepG2 cells in the G2/M phase,
while Fas and Fas ligand expression levels were markedly increased
(15,16). In addition, a meta-analysis demonstrated that β-elemene
improved the effect of lung cancer chemotherapy as an adjunctive
treatment (14). In the present
study, the outcomes of postoperative HCC medications with and
without cidan were compared. In addition, the effects of cidan on
human HCC cells transplanted in mice were investigated, as well as
the effects of cidan on in vitro cultivated liver cancer
cell proliferation and invasion capabilities and cyclooxygenase-2
(COX-2) and vascular endothelial growth factor (VEGF) expression
levels.
Patients and methods
Patients
In total, 372 patients diagnosed with primary liver
cancer via surgery and pathological examination were included in
the study. A total of 92 patients comprised the cidan group, while
the additional 280 patients were controls. The diagnosis and
inclusion criteria were based on clinical and pathological
observations, which were as follows: (i) AFP ≥400 µg/ml and
exclusion of pregnancy, embryonic derived gonad tumors and active
or metastatic liver cancer; could feel a swelling, hard, and have
large nodular tumor of the liver or clear liver space occupying
lesions by imaging examination; (ii) AFP <400 µg/ml and
exclusion of pregnancy, embryonic derived gonad tumors and active
or metastatic liver cancer, but have the characteristics of liver
space-occupying lesions by two types of imaging examinations, or
have a positive expression of at least two liver cancer markers
(DCP, GGTII, AFU, CAl9-9 or others) and the characteristic of liver
space-occupying lesions by one imaging examination; (iii) clear
clinical appearance and positive extrahepatic metastasis lesions,
including visible hemorrhagic ascites or cancer cells found in the
lesion, and exclusion of metastatic liver cancer.
Treatments
The study was prospective, but non-randomized since
the treatment group consisted of patients who agreed with the
offered cidan treatment. In the treatment group, patients were
postoperatively administered 1.35 g cidan capsules (Weida
Pharmaceutical Co., Ltd., Beijing, China) three times a day in
addition to the conventional liver protective drugs, polyene and
phosphatidyl choline, whilst the control group received only
conventional liver protective drugs without cidan. Administration
of cidan capsules was continued for three months and long-term
follow-up was continued with visits every two months. Patients in
the two groups received routine treatments, including transarterial
chemoembolization, when recurrence occurred. Any other anticancer
drugs were discontinued in the treatment process.
Criteria of curative efficacy
Following the tumor resection, the two-year overall
survival (OS) and disease-free survival (DFS) times of the patients
were monitored. DFS was defined as the number of days from the
first day following surgery until tumor recurrence. During the
two-year follow-up period, all the patients routinely received
general health examinations, blood, urinary and stool analyses, as
well as liver and kidney function tests and cardiograms. Efficacy
monitoring included: (i) Associated symptoms and signs; (ii) liver
function tests analyzing the levels of total bilirubin, direct
bilirubin, glutamic-pyruvic transaminase, glutamic oxalacetic
transaminase, albumin, prealbumin and bile acid; (iii) enzyme
analyses measuring the levels of ALP, GGT and lactic dehydrogenase
isoenzyme; (iv) analysis of AFP; and (v) quality of life.
In vivo animal experiment
In total, 90 C57BL/6 mice (age, 5 weeks) were
purchased from the Shanghai Laboratory Animal Center (Shanghai,
China) and inoculated subcutaneously in the dorsal area with
Hepa1-6 cells, diluted to 2×106 cells/mouse. After seven
days, the mice with cancerous tumors of >5 mm in diameter were
divided into three groups, which included the blank control, cidan
high-dose (4.80 mg/kg) and cidan low-dose (1.92 mg/kg) groups. The
blank control group comprised 30 mice, while the Hepa1-6
inoculation model groups included 10 mice per group. Reagents were
infused into the stomach once per day, while the blank control
group were administered distilled water. At days 7, 14 and 21
following the start of treatment, the mice were sacrificed by
cervical dislocation and the tumors were isolated and measured in
order to calculate the growth inhibition ratios. The C57BL/6 mice
were housed under specific pathogen-free conditions and animal
treatments were conducted in accordance with the Principle of
Laboratory Animal Care. The experimental procedures were performed
with approval from the Committee of Experimental Animal
Administration of the Second Military Medical University Laboratory
(Shanghai, China) and written informed consent was provided by all
the participating patients at admission.
Cell lines and culture conditions
Hepa1-6 cells were obtained from the Cell Bank of
Shanghai Institute of Biochemistry and Cell Biology and cultured in
high glucose Dulbecco's modified Eagle's medium supplemented with
10% heat-inactivated fetal bovine serum (FBS), 100 U/ml
benzylpenicillin and 100 µg/ml streptomycin in a humidified
atmosphere with 5% CO2 at 37°C, as previously described
(17). The human hepatoma cell
lines, SMMC-7721 and CSQT-1, were established in the laboratory
(18). Cells were grown in RPMI-1640
medium supplemented with 10% heat-inactivated FBS, 100 U/ml
benzylpenicillin and 100 µg/ml streptomycin in a humidified
atmosphere with 5% CO2 at 37°C. SMMC-7721 and CSQT-1
cells were seeded in 6-cm culture plates and treated with various
concentrations of cidan and the saline control (10, 20 and 40
µg/ml) for different time courses. Each measurement was performed
with three culture plates.
Cytotoxic activity assay
Cytotoxicity assays were performed according to the
MTT method, as previously described (19). MTT was purchased from Sigma-Aldrich
(St. Louis, MO, USA). Briefly, the cells were washed twice with
phosphate-buffered saline (PBS) and cultured at a density of
5×104 cells/ml in flat-bottomed 96-well microtiter
plates in 100 µl RPMI-1640 medium. Several dilutions of the tested
compounds, in 100 µl RPMI-1640 medium with 10% FBS, were added to
the wells. The final concentration of dimethyl sulfoxide used for
MTT solubilization was 0.2% (v/v). Following incubation for 48 h,
100 µl medium was removed from each well and 20 µl MTT solution (5
mg/ml PBS) was added. Following incubation for 4 h, the optical
density was evaluated at 570 nm. Growth inhibition rates were
calculated as a percentage of the parallel negative controls. Each
experiment was performed three times.
Cell Matrigel invasion assays
Tumor cell invasion through a reconstituted basement
membrane (Matrigel; Sigma-Adrich, Carlsbad, CA, USA) was assayed as
previously described (20). In
24-well Transwell cell culture chambers (Chemicon, Temecula, CA,
USA), polycarbonate filters (pore size, 8 µm) were precoated with 1
µg fibronectin on the lower surface, following which 5 µg/10 µl
Matrigel was applied to the upper surface of the filters. Uncoated
wells were used as a negative control. The filters were first dried
and washed in PBS, and following rehydration, the SMMC-7721 and
CSQT-1 (5×105) cells were suspended in RPMI-1640
containing 0.1% bovine serum albumin. The filters were then
pretreated with the tested samples for 30 min on ice, added to the
upper chamber, and then incubated at 37°C for 12 h. Following
incubation, the cells that had invaded the lower chamber and
attached to the lower surface of the filter were stained with
calcein and quantified at excitation and emission wavelengths of
495 and 515 nm, respectively. Four high-power fields were analyzed
for each well.
Quantitative polymerase chain reaction
(qPCR)
Cells were collected at specified times and total
RNA was extracted using TRIzol reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA), in accordance with the
manufacturer's instructions. cDNA was constructed as a template to
perform qPCR with COX-2, VEGF and β-actin specific primers. The
qPCR analyses for VEGF and COX-2 transcription were performed in
single microcapillary tubes using a LightCycler™ (Roche
Diagnostics, Basel, Switzerland) and SYBR® Green Tag ReadyMix™
(Sigma-Aldrich). Cycling parameters were optimized as follows:
Denaturation at 94°C (10 sec), annealing at 55°C (5 sec), extension
at 72°C (24 sec) and detection at 80°C (1 sec). Each microcapillary
tube contained 7.1 µl nuclease-free H2O, 10 µl SYBR
reagent, 0.5 µl template cDNA, 1.6 µl MgCl2 (25 mM) and
0.8 µl primer mixture (25 pmol/µl). Cycler software was used to
quantify COX-2 and VEGF mRNA expression levels.
Cell cycle analysis using flow
cytometery
Treated (40 µg/ml cidan for 24 h) and untreated
CSQT-1 cells were harvested, washed with PBS and suspended
(106/ml) in 1.5 ml hypotonic fluorochromic solution [50
mg/ml propidium iodide (PI) in 0.1% sodium citrate plus 0.1% Triton
X-100; Sigma-Aldrich] for 60 min at 48°C in the dark. PI
fluorescence was analyzed using a FACScaliber flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA) with ModFIT cell cycle
analysis software version 2.01.2 (BD Biosciences). The experiments
were repeated three times for each condition.
Statistical analysis
Data were analyzed using the unpaired t-test with
two-tailed P-values or analysis of variance (for multiple
comparisons) to calculate the statistical significance between the
control and treatment groups. Levels of total bilirubin, direct
bilirubin and albumin, the ratio of albumin to globin, prothrombin
time, percentage of globin in protein electrophoresis and
α-L-fucosidase were compared between the groups using analysis of
variance. The Wilcoxon rank sum test was used for comparisons of
AFP, carcinoembryonic antigen, carbohydrate antigen 19-9, alanine
aminotransferase, aspartate aminotransferase, ALP and hepatitis B
virus-DNA/1,000 between the groups. Additional categorical
variables were compared between the groups using Fisher's exact
test. The results are presented as the mean ±standard error of the
mean, and P<0.05 was considered to indicate a statistically
significant difference.
Results
No significant variation in the basic
characteristics of the cidan and control patients
Although statistically significant differences [AFP,
hepatitis B e antigen positive, anti-hepatitis C virus positive and
portal clamping duration] were observed in the basic
characteristics between the two groups prior to surgery, the
majority of the parameters did not significantly vary (Table I). The pathological data at day 2
following surgery indicated that there were no statistically
significant differences between the cidan treatment and control
groups, despite a reduction in the occurrence of incomplete tumor
capsules in the cidan treatment group (Table II).
 | Table I.Preoperative comparison of patient
characteristics between the two treatment groups. |
Table I.
Preoperative comparison of patient
characteristics between the two treatment groups.
| Variable | Control group
(n=280) | Treatment group
(n=92) | P-value |
|---|
| Age, years | 49.16±10.824 | 50.64±10.553 | 0.252 |
| Male, n (%) | 226 (80.7) | 84 (91.3) | 0.018 |
| Female, n (%) | 54 (19.3) | 8 (8.7) |
|
| AFP, µg/l | 559.413±554.8254 | 367.926±501.8287 | 0.002 |
| AFU, U/l | 33.614±1.0493 | 33.391±0.9514 | 0.866 |
| ALP, U/l |
114.964±101.4081 |
107.315±58.3713 | 0.493 |
| ALT, U/l | 50.748±50.7809 |
58.627±122.0881 | 0.382 |
| AST, U/l | 54.581±46.0912 |
64.865±140.9475 | 0.289 |
| CA19-9, U/ml | 38.078±84.1362 | 25.833±22.6981 | 0.169 |
| CEA, µg/l | 3.201±6.7797 | 3.242±3.7395 | 0.956 |
| GGT, U/l |
116.793±120.5559 |
123.686±126.3800 | 0.639 |
| Albumin, g/l | 41.173±3.9815 | 41.974±4.1169 | 0.098 |
| Ratio of albumin
and globin | 1.341±0.2238 | 1.384±0.2637 | 0.131 |
| Size of tumor,
cm | 9.1350±4.74699 | 9.2489±4.91545 | 0.843 |
| Prealbumin,
mg/l |
220.018±57.9002 |
228.902±56.9922 | 0.201 |
| Total bilirubin,
µmol/l | 15.426±6.4219 | 14.754±5.4803 | 0.368 |
| Direct bilirubin,
µmol/l | 5.730±3.0187 | 5.351±2.1050 | 0.265 |
| Prothrombin time,
sec | 12.202±.9329 | 12.065±.9718 | 0.228 |
| Hepatic portal
blocking time, min | 16.98±11.048 | 13.60±7.507 | 0.001 |
| Intraoperative
bleeding, ml | 463.11±616.957 | 373.37±412.807 | 0.115 |
| Intraoperative
blood transfusion, ml |
358.07±1479.115 | 158.70±460.328 | 0.204 |
| Globin in protein
electrophoresis, % |
20.3164±4.29137 |
20.1241±4.66117 | 0.715 |
| Anti-HBcAg, n
(%) |
|
|
|
|
Positive | 269 (96.1) | 91 (98.9) | 0.308 |
|
Negative | 11 (3.9) | 1 (1.1) |
|
| Anti-HBeAg, n
(%) |
|
|
|
|
Positive | 175 (62.5) | 62 (67.4) | 0.581 |
| Negative | 105 (37.5) | 30 (32.6) |
|
| HBeAg, n (%) |
|
|
|
|
Positive | 108 (38.6) | 24 (26.1) | 0.030 |
|
Negative | 172 (61.4) | 68 (73.9) |
|
| Anti-HCV, n
(%) |
|
|
|
|
Positive | 4 (1.4) | 6 (6.5) | 0.017 |
|
Negative | 276 (98.6) | 86 (93.5) |
|
| Microscopic
vascular invasion, n (%) |
|
|
|
|
Yes | 53 (18.9) | 15 (16.3) | 0.572 |
| No | 227 (81.1) | 77 (83.7) |
|
| Portal vein tumor
thrombus, n (%) |
|
|
|
|
Yes | 38 (13.6) | 15 (16.3) | 0.515 |
| No | 242 (86.4) | 77 (83.7) |
|
| Satellite nodules,
n (%) |
|
|
|
|
Yes | 70 (25.0) | 16 (17.4) | 0.133 |
| No | 210 (75.0) | 76 (82.6) |
|
| Tumor size, n
(%) |
|
|
|
| ≤5
cm | 69 (24.6) | 25 (27.2) | 0.628 |
| >5
cm | 211 (75.4) | 67 (72.8) |
|
| Tumor size, n
(%) |
|
|
|
| ≤10
cm | 176 (62.9) | 54 (58.7) | 0.476 |
| >10
cm | 104 (37.1) | 38 (41.3) |
|
| Tumor number, n
(%) |
|
|
|
| Single
tumor | 231 (82.5) | 83 (90.2) | 0.077 |
|
Multiple tumors | 49 (17.5) | 9 (9.8) |
|
| Rupture of tumor, n
(%) |
|
|
|
|
Yes | 5 (1.8) | 3 (3.3) | 0.414 |
| No | 275 (98.2) | 89 (96.7) |
|
 | Table II.Comparison of postoperative
pathological determinations between the two groups of patients. |
Table II.
Comparison of postoperative
pathological determinations between the two groups of patients.
| Variable | Liver protection
group | Cidan group | P-value |
|---|
| Cell type, n
(%) |
|
|
|
| Coarse
trabecular | 190 (67.9) | 54 (58.7) |
|
| Coarse
trabecularpseudo ductular | 2 (0.7) | 1 (1.1) |
|
| Coarse
trabecularhyaline | 3 (1.1) | - |
|
| Super
fat | 1 (0.4) | - |
|
|
Mixed | 2 (0.7) | - |
|
| Pseudo
ductular | 4 (1.4) | 8 (8.7) |
|
|
Others | 2 (0.7) | - |
|
|
Hyaline | 8 (2.9) | 2 (2.2) |
|
| In
groups and sheets | 8 (2.9) | 10 (10.9) |
|
| In
groups and sheetshyaline | - | 1 (1.1) |
|
| Fine
trabecular | 56 (20.0) | 16 (17.4) |
|
| Fine
trabecularsuper fat | 1 (0.4) | - |
|
| Fine
trabecularpseudo ductular | 3 (1.1) | - |
|
| Tumor capsule, n
(%) |
|
|
|
|
Total | 280 (100.0) | 92 (100.0) |
|
|
Incomplete | 89 (31.8) | 19 (20.7) | 0.0119 |
|
Complete | 95 (33.9) | 47 (51.1) | 0.0598 |
| Without | 96 (34.3) | 26 (28.3) | 0.4658 |
| Satellite nodules,
n (%) |
|
|
|
|
Total | 280 (100.0) | 92 (100.0) |
|
|
Without | 210 (75.0) | 76 (82.6) | 0.1547 |
|
With | 70 (25.0) | 16 (17.4) | 0.2609 |
| Microscopic
vascular invasion, n (%) |
|
|
|
|
Total | 280 (100.0) | 92 (100.0) |
|
|
Without | 227 (81.1) | 77 (83.7) | 0.6427 |
|
With | 53 (18.9) | 15 (16.3) | 0.7587 |
| Cell grading, n
(%) |
|
|
|
|
Total | 280 (100.0) | 92 (100.0) |
|
| I | 1 (0.4) | 1 (1.1) | 0.5843 |
| II | 63 (22.5) | 22 (23.9) | 0.8896 |
|
III | 214 (76.4) | 68 (73.9) | 0.9269 |
| IV | 2 (0.7) | 1 (1.1) | 0.5759 |
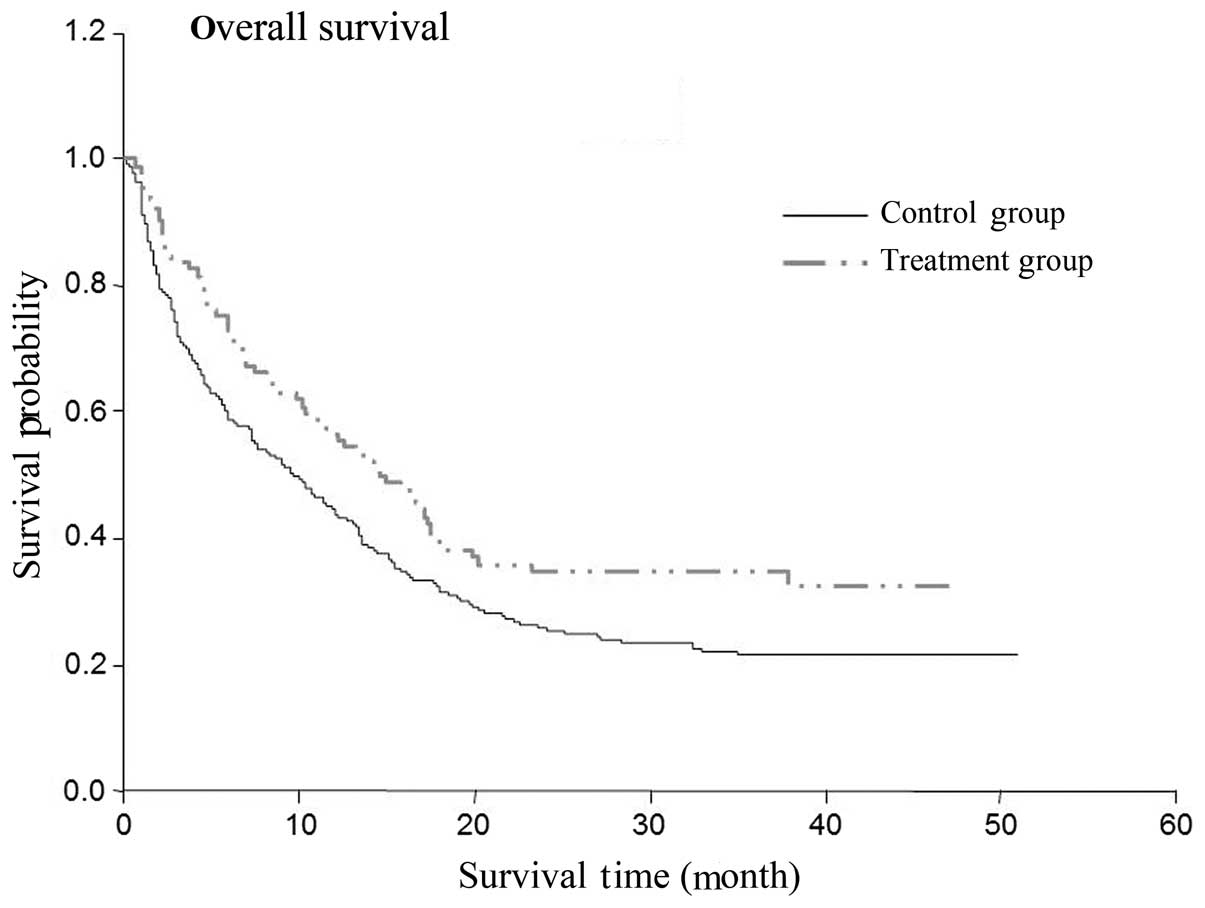
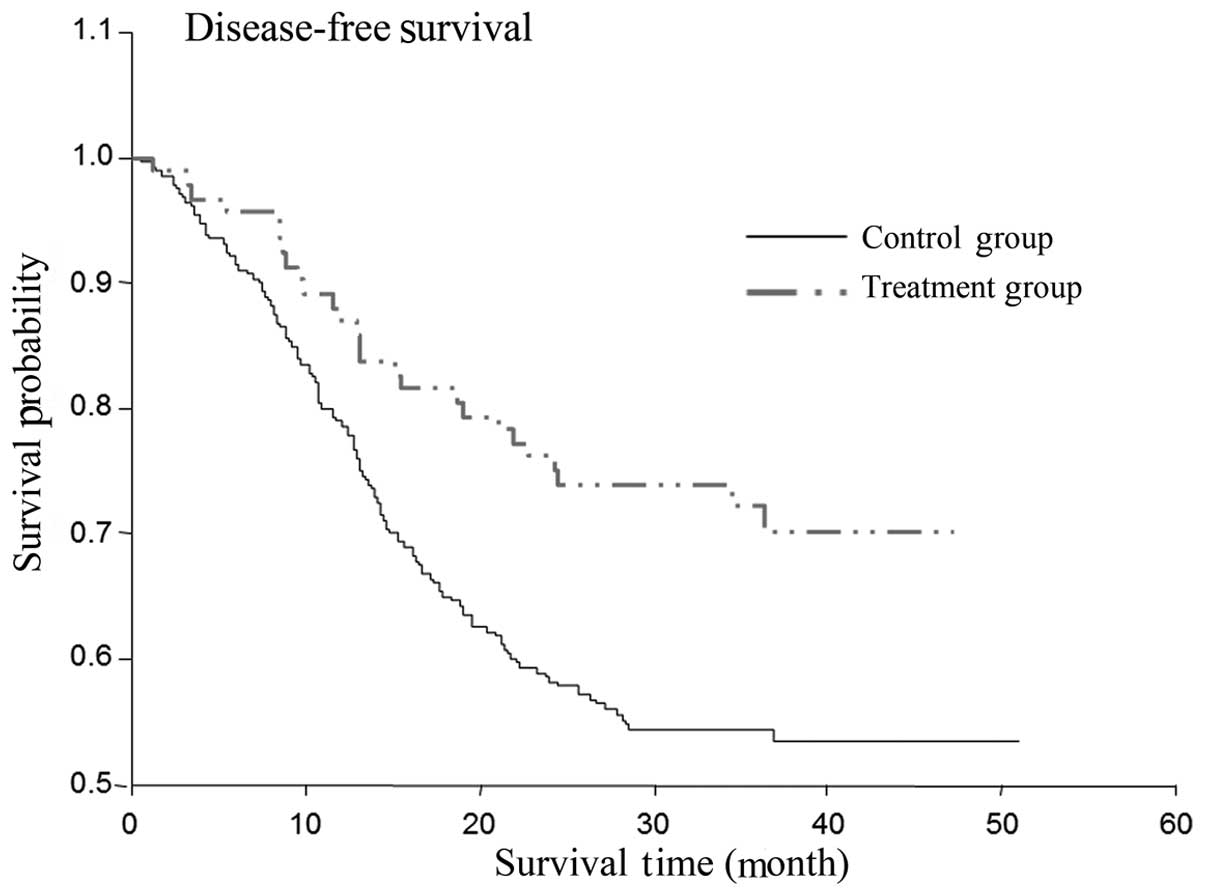
Differences in the OS and DFS times
between the cidan and control groups
The two-year OS rate of the patients that received
postoperative cidan as adjuvant therapy was 77%, while the OS rate
of the solely hepatoprotective drug treatment group was 58%
(Table III; P=0.0031). The
two-year DFS rate in the cidan treatment group was 36%, while in
the control group, the DFS was 24% (Table IV; P=0.006). A Cox regression model
was used to analyze the effect of postoperative adjunctive cidan
medication on the OS and DFS rates of patients with HCC following
surgery (Figs. 1 and 2), which demonstrated that the use of
adjuvant cidan therapy reduced tumor recurrence and improved the
survival times.
 | Table III.Effect of antitumor medication
(cidan) on the prognosis of patients (OS rate). |
Table III.
Effect of antitumor medication
(cidan) on the prognosis of patients (OS rate).
| Subgroup | HR | 95% CI lower | 95% CI upper | P-value |
|---|
| Cidan (+) vs.
(-) | 0.476 | 0.292 | 0.778 | 0.0031 |
 | Table IV.Effect of antitumor medication
(cidan) on the prognosis of patients (DFS time). |
Table IV.
Effect of antitumor medication
(cidan) on the prognosis of patients (DFS time).
| Subgroup | HR | 95% CI lower | 95% CI upper | P-value |
|---|
| Cidan (+) vs.
(−) | 0.646 | 0.473 | 0.882 | 0.0060 |
Anticancer effect of cidan in the
Hepa1-6 implanted male C57BL/6 mouse model
In order to further analyze the effect of cidan on
hepatic tumor cells, a mouse HCC model was used. Following
culturing, 2×106 mouse Hepa1-6 hepatoma cells were
injected into the subcutaneous tissue of C57BL/6 mice. After seven
days, the mice were treated with high (4.80 mg/kg) or low (1.92
mg/kg) doses of cidan, or with distilled water as a control. The
tumors were removed from the mice after one, two or three weeks
following the start of treatment. The results revealed that the
average weight of the tumors after two weeks of high-dose cidan
treatment was 50% lower when compared with the control group.
Low-dose cidan treatment reduced the tumor size to a lesser extent
when compared with the high-concentration cidan treatment. The same
trend was observed after three weeks of treatment (Table V).
 | Table V.Inhibition of Hepa1-6 cell xenograft
tumor growth in mice by cidan treatment. |
Table V.
Inhibition of Hepa1-6 cell xenograft
tumor growth in mice by cidan treatment.
| Treatment | Cidan dose,
mg/kg | Animals, n | Animal weight,
g | Tumor weight,
g | Inhibition ratio,
% |
|---|
| 1 week |
|
|
|
|
|
|
Control | - | 10 | 28.8±3.5 | 2.0±0.7 | - |
| Cidan
low | 1.92 | 10 | 28.7±2.3 | 1.8±0.7 | 9.0 |
| Cidan
high | 4.80 | 10 | 28.2±4.2 |
1.6±0.5a | 22.0 |
| 2 weeks |
|
|
|
|
|
|
Control | - | 10 | 29.0±3.1 | 2.2±0.8 | - |
| Cidan
low | 1.92 | 10 | 27.4±2.5 | 2.1±0.7 | 15.4 |
| Cidan
high | 4.80 | 10 | 27. 8±4.3 |
1.1±0.3b | 30.2 |
| 3 weeks |
|
|
|
|
|
|
Control | - | 10 | 28.2±3.0 | 3.2±0.9 | - |
| Cidan
low | 1.92 | 10 | 28.4±2.3 | 1.8±0.5 | 18.0 |
| Cidan
high | 4.80 | 10 | 27.8±4.2 |
1.1±0.4b | 35.0 |
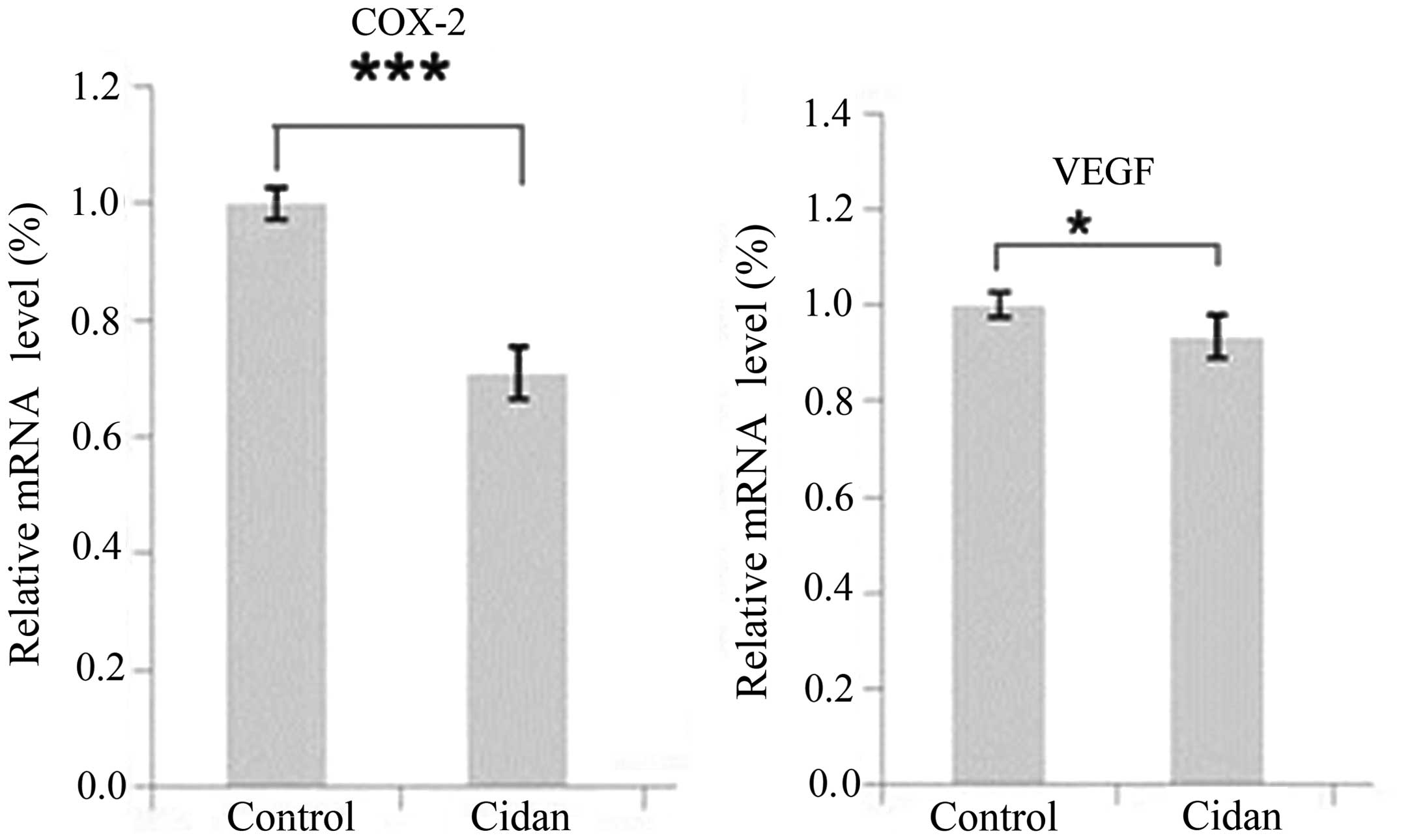
Cidan inhibits COX-2 and VEGF
expression, is toxic in the long-term and inhibits the cell
invasion capacities of hepatic tumor cells
SMMC-7721 tumor cells were cultured and treated with
cidan, following which the transcription levels of COX-2 and VEGF
were analyzed and changes in the tumor cell growth rates were
determined. As shown in Fig. 3, the
mRNA expression levels of COX-2 and VEGF in the cells treated with
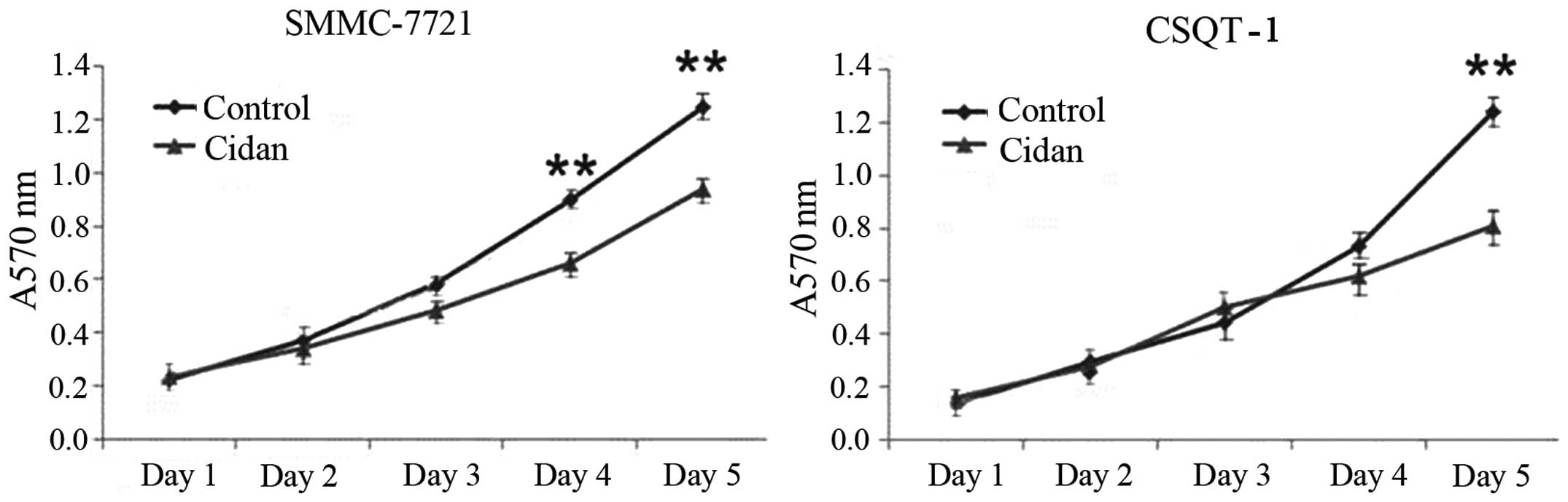
cidan were significantly decreased compared with the control. MTT
cytotoxicity assays were then performed to determine the effect of
cidan on the viability of SMMC-7721 and CSQT-1 cell lines. Cidan
exhibited statistically significant cytotoxic effects on SMMC-7721
cells after four and five days (P<0.01), and on CSQT-1 cells
after five days (P<0.01), when applied at a dosage of 40 µg/ml
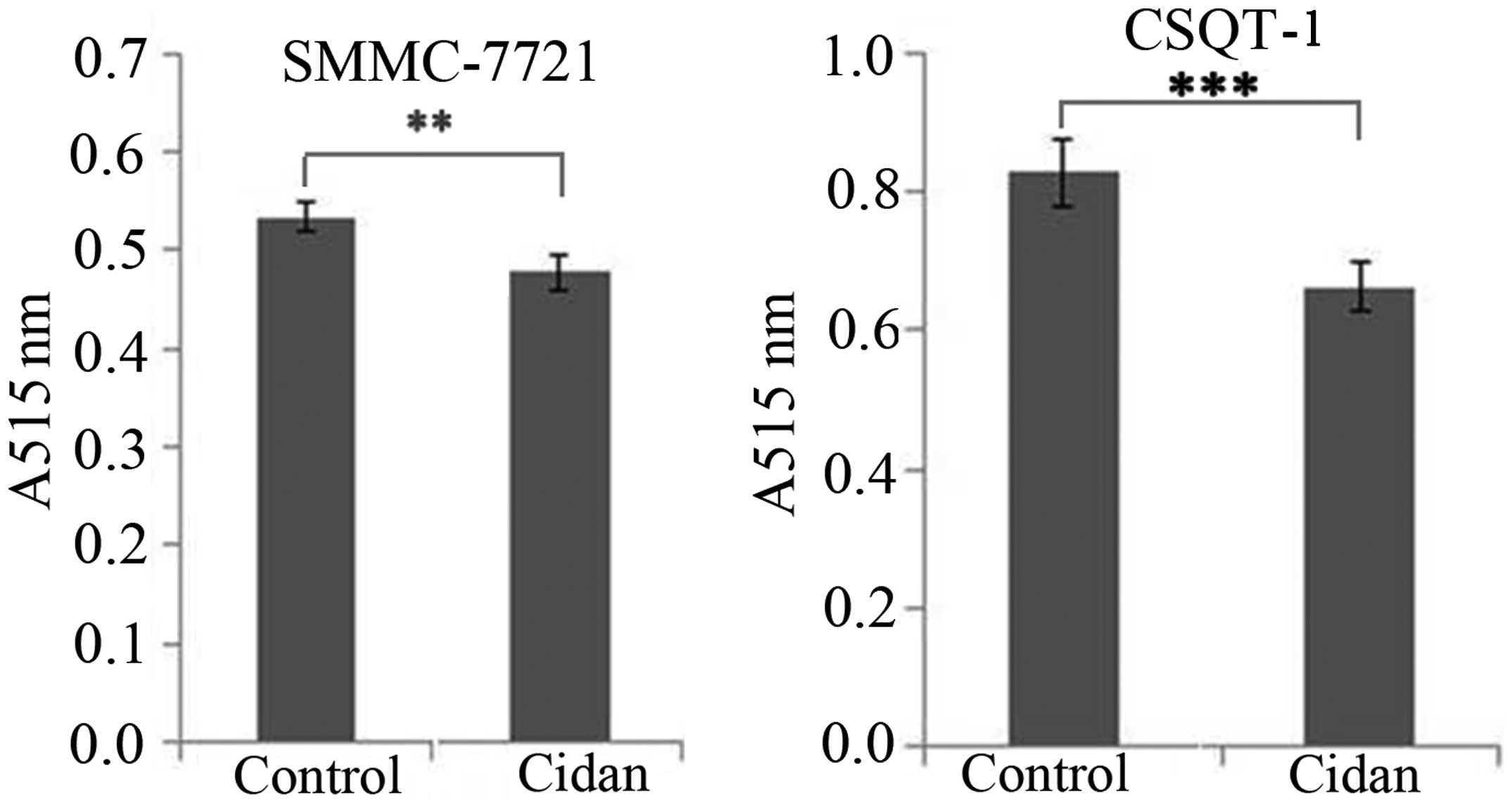
(Fig. 4). In addition, Matrigel cell
invasion capacity analysis using a Transwell chamber assay revealed
that cidan (40 µg/ml) significantly suppressed SMMC-7721
(P<0.01) and CSTQ (P<0.001) cell invasion through
Matrigel-coated filters (Fig.
5).
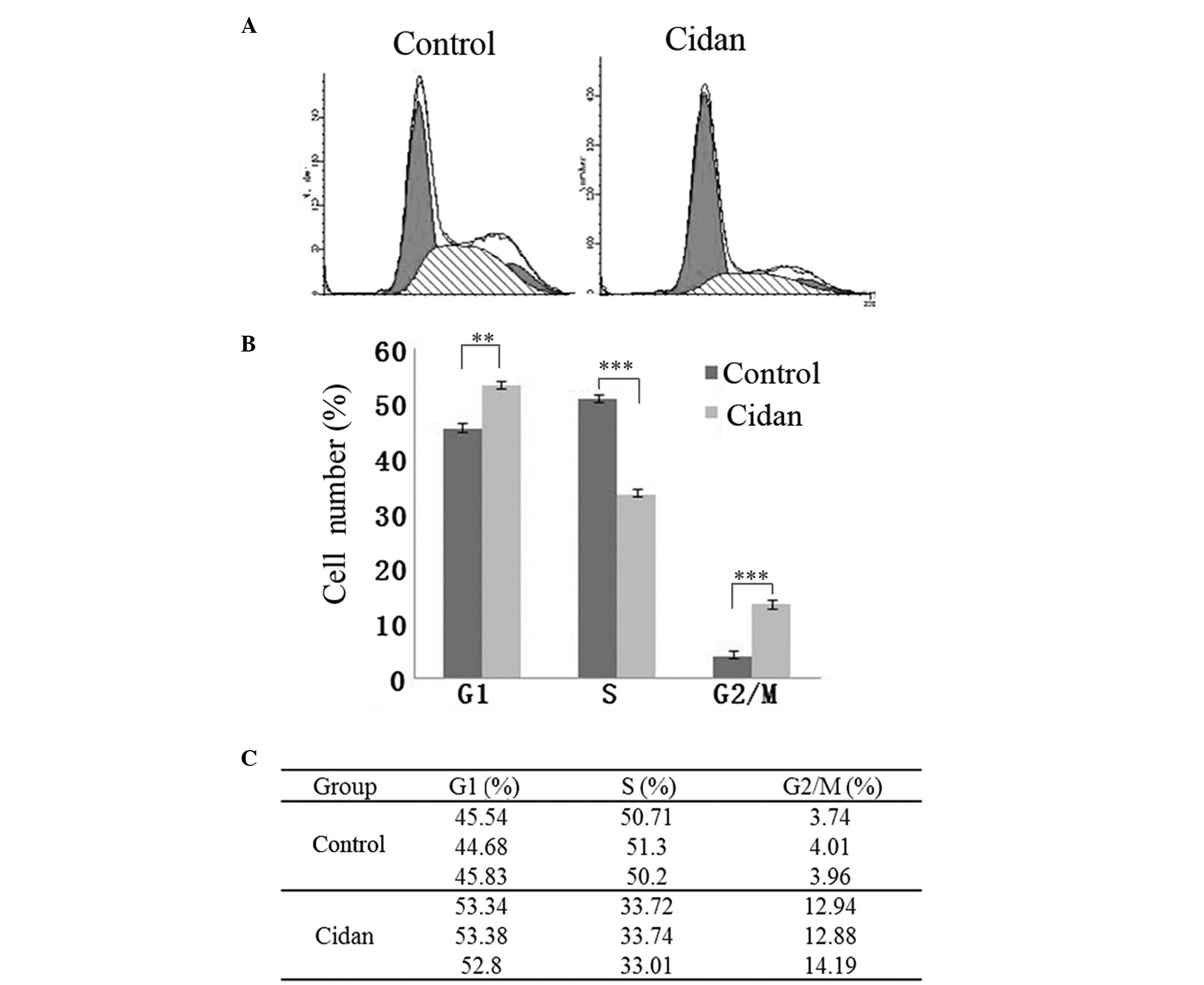
Cidan induces pronounced G2/M cell
cycle arrest in CSQT-1 cells
As shown in Fig. 6,
40 µg/ml cidan application for 24 h increased the number of cells
in the G1 and G2/M cell cycle phases. In addition, the number of
cells in the S cell cycle phase was reduced, indicating that the
hepatic cancer cell proliferation rate was reduced.
Therefore, the results revealed that cidan
positively affected the DFS and OS rates of patients with HCC.
Cidan also reduced mouse model hepatic tumor cell growth in a
dose-dependent manner, reduced COX-2 and VEGF expression levels and
exhibited cytotoxic effects in hepatic cancer cells. In addition,
cidan induced G2/M and G1 cell cycle arrests in vitro.
Discussion
In the present study, cidan was demonstrated to
improve the postoperative DFS and OS rates in patients with HCC
(Figs. 1 and 2), as well as reduce the growth of
subcutaneously implanted HCC tumors in mouse models in a
dose-dependent manner (Table V).
Cidan controls the progression of HCC effectively via a number of
oxidative stress and inflammatory reactions, where several
associated cytokines and signaling pathways have been confirmed to
affect the pathogenesis of HCC (21,22). A
number of phytochemicals exhibit anti-inflammatory effects, such as
curcumin, a polyphenolic compound derived from rhizomes of
Curcuma. Curcumin mediates the suppression of nuclear
factor-κB (NF-κB), the master switch in the inflammatory cascade
(23). NF-κB activation is known to
regulate several key inflammatory mediators, including cytokines,
chemokines and kinases, which have been shown to play critical
roles in the pathogenesis of the majority of chronic illnesses
(24) The curcumin-mediated
attenuation of the NF-κB-activated inflammatory cascade is a
critical mechanism of its therapeutic effects (25). Although cidan is the most widely
known and effective curcuminoid present in China, this drug also
contains more than three additional polyphenolic curcuminoids.
Elemene is the anticancer component extracted from Rhizoma
Curcumae, which has been demonstrated to have an anticancer effect
by inducing apoptosis in tumor cells (26,27).
Recently, an additional study demonstrated that curcumin induced
G2/M cell cycle arrest via targeting the anaphase-promoting
complex/cyclosome protein, Cdc27, and inducing enhanced apoptosis
rates (28). The results of the
present study were in accordance with these observations, and
marked G2/M phase cell cycle arrest was observed in the CSQT-1
cells following incubation with cidan (Fig. 6). In addition to the enhanced G2/M
cell cycle arrest of CSQT-1 cells, cidan was shown to inhibit COX-2
and VEGF expression levels and prevent hepatic cancer cell invasion
activity (Figs. 3 and 5). These observations indicated that there
was a synergistic cell growth inhibition of SMMC-7721 and CSQT-1
cells, accompanied with an increase in apoptosis rates and a
decrease in NF-κB activation with concomitant lowering of COX-2
expression levels, as demonstrated in a previous study (29). Tanshinone, the effective component
extracted from Salvia miltiorrhiza, is also found in cidan
and has known anticancer properties. This compound has been
demonstrated to dissolve fibrin wrapped on the surface of cancer
cells, increase the immunogenicity of tumors, induce cell
differentiation and gradually reduce the malignancy of cancer cells
(30). Furthermore, cidan capsules
contain Brucea javanica oil, extracted from Brucea
javanica, which has been shown to induce apoptosis and decrease
cell proliferation; thus, demonstrating favorable antitumor
activity (31). Additional
ingredients include Cirrhopetalanthrin, a type of chemical
composition extracted from Cremastrae appendiculata, which
exhibits anticancer activity by inhibiting mitosis, and Astragalus,
which promotes the body to monitor tumor cells and produce
interferon, increasing lymphokine-activated killer and natural
killer cell activity and strengthening the phagocytic function of
the reticuloendothelial system to kill cancer cells (32). Furthermore, a low therapeutic cidan
concentration in patients has been cited as a reason for the lack
of sufficient success in clinical trials. In the present in
vivo study, this hypothesis was supported since tumor growth
was shown to diminish in the mouse model in a dose-dependent manner
(Table V).
In conclusion, the present study demonstrated that
cidan effectively inhibited proliferation and was toxic to hepatoma
cells. Furthermore, COX-2 and VEGF expression levels were found to
be downregulated following cidan administration. Thus, cidan
therapy may effectively reduce tumor recurrence and improve the OS
time when applied as postoperative treatment for HCC. However,
further studies are required to clarify the exact mechanisms
involved in the antitumor effects of cidan, with particular focus
on the activity of the individual ingredients.
Acknowledgements
The authors thank Chao Xu from Shanghai Changzheng
Hospital for their assistance and support. The study was supported
by grants from the China National Funds for Distinguished Young
Scientists (no. 81125018), the National Natural Science Fund (no.
81101511), the New Excellent Talents Program (no. XBR2011025),
General Program from Shanghai Municipal Health Bureau (no.
20124301), the New Excellent Talents Program (no. 10XD1405800) and
the Shanghai Rising-star Program from the Shanghai Science and
Technology Committee (no. 13QA1404900) and National Major
Scientific and Technological Special Project for ‘Significant New
Drugs Development’ during the Twelfth Five-year Plan Period (no.
2013ZX09104006).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: a review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li W, Gao Z, Yang C, et al: The estimation
of prevalence, incidence, and residual risk of
transfusion-transmitted human hepatitis B infection from blood
donated at the Anhui blood center, China, from 2009 to 2011. PloS
One. 8:e734722013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su Y, Norris JL, Zang C, Peng Z and Wang
N: Incidence of hepatitis C virus infection in patients on
hemodialysis: a systematic review and meta-analysis. Hemodial Int.
17:532–541. 2013.PubMed/NCBI
|
|
5
|
Chen WQ, Zheng RS and Zhang SW: Liver
cancer incidence and mortality in China, 2009. Chin J Cancer.
32:162–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng JH, Chang G and Wu WY: A controlled
clinical study between hepatic arterial infusion with embolized
curcuma aromatic oil and chemical drugs in treating primary liver
cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 21:165–167. 2001.[(In
Chinese)]. PubMed/NCBI
|
|
8
|
Wang WX, Li TX, Ma H, et al: Tumoral
cytotoxic and antioxidative phenylpropanoid glycosides in Smilax
riparia A. DC. J Ethnopharmacol. 149:527–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang QL, Dai CC, Jiang JH, Tang YP and
Duan JA: A new cytotoxic casbane diterpene from Euphorbia
pekinensis. Fitoterapia. 80:514–516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang R, Houghton PJ and Hylands PJ:
Cytotoxic effects of compounds from Iris tectorum on human cancer
cell lines. J Ethnopharmacol. 118:257–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan JA, Wang L, Qian S, Su S and Tang Y:
A new cytotoxic prenylated dihydrobenzofuran derivative and other
chemical constituents from the rhizomes of Atractylodes lancea DC.
Arch Pharm Res. 31:965–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin H, Liu J and Zhang Y: Developments in
cancer prevention and treatment using traditional Chinese medicine.
Front Med. 5:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong HY, Shao JW, Wang T, Guo YH and Yan
LY: Effects on the activities and mRNA expression of CYP3A in rat's
liver by four kinds of extracts from anti-cancer traditional
Chinese medicines. Zhong Yao Cai. 31:68–71. 2008.[(In Chinese)].
PubMed/NCBI
|
|
14
|
Wang B, Peng XX, Sun R, et al: Systematic
review of β-elemene injection as adjunctive treatment for lung
cancer. Chin J Integr Med. 18:813–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai ZJ, Tang W, Lu WF, et al:
Antiproliferative and apoptotic effects of β-elemene on human
hepatoma HepG2 cells. Cancer Cell Int. 13:272013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae - a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Luan W, Goz V, Burakoff SJ and
Hiotis SP: Non-invasive in vivo imaging for liver tumour
progression using an orthotopic hepatocellular carcinoma model in
immunocompetent mice. Liver Int. 31:1200–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu HS, Cheng SQ, Shi J, et al:
Establishment and characterization of a human hepatocellular
carcinoma cell line CSQT-1 derived from portal vein tumor thrombus.
Di Er Jun Yi Da Xue Xue Bao. 30:1–4. 2009.
|
|
19
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
20
|
Bauer JS, Schreiner CL, Giancotti FG,
Ruoslahti E and Juliano RL: Motility of fibronectin
receptor-deficient cells on fibronectin and vitronectin:
collaborative interactions among integrins. J Cell Biol.
116:477–487. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pang R, Tse E and Poon RT: Molecular
pathways in hepatocellular carcinoma. Cancer Lett. 240:157–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong CM and Ng IO: Molecular pathogenesis
of hepatocellular carcinoma. Liver Int. 28:160–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh S and Aggarwal BB: Activation of
transcription factor NF-kappa B is suppressed by curcumin
(diferuloylmethane) [corrected]. J Biol Chem. 270:24995–25000.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Libby P: Inflammatory mechanisms: the
molecular basis of inflammation and disease. Nutr Rev.
65:S140–S146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Tharakan ST, Sung B and Anand P: Potential of spice-derived
phytochemicals for cancer prevention. Planta Med. 74:1560–1569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae - a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu WS, Dang YY, Guo JJ, et al: Furanodiene
induces endoplasmic reticulum stress and presents antiproliferative
activities in lung cancer cells. Evid Based Complement Alternat
Med. 2012:4265212012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SJ and Langhans SA: Anaphase-promoting
complex/cyclosome protein Cdc27 is a target for curcumin-induced
cell cycle arrest and apoptosis. BMC Cancer. 12:442012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Notarbartolo M, Poma P, Perri D, Dusonchet
L, Cervello M and D'Alessandro N: Antitumor effects of curcumin,
alone or in combination with cisplatin or doxorubicin, on human
hepatic cancer cells. Analysis of their possible relationship to
changes in NF-κB activation levels and in IAP gene expression.
Cancer Lett. 224:53–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tung YT, Chen HL, Lee CY, et al: Active
component of Danshen (Salvia miltiorrhiza Bunge), tanshinone I,
attenuates lung tumorigenesis via inhibitions of VEGF, cyclin A,
and cyclin B expressions. Evid Based Complement Alternat Med.
2013:3192472013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie YL, Liu KX, Mao XY, Li YL, Li J and
Zhang MM: Effect of injection of Brucea javanica oil emulsion plus
chemoradiotherapy for lung cancer: a review of clinical evidence. J
Evid Based Med. 5:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XY: Immunomodulating components from
chinese medicines. Pharm Biol 38 (Suppl 1). 33–40. 2000. View Article : Google Scholar
|