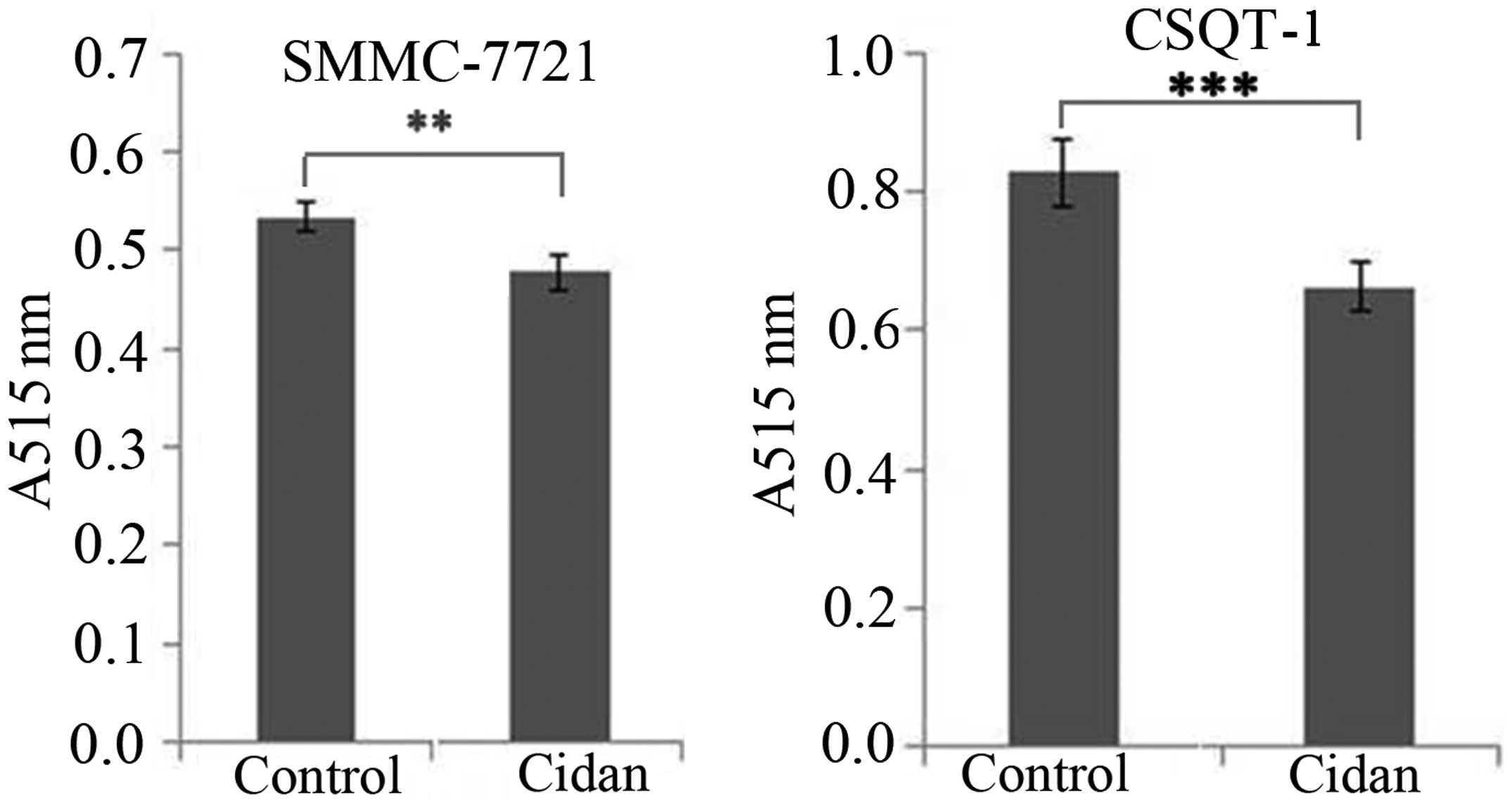
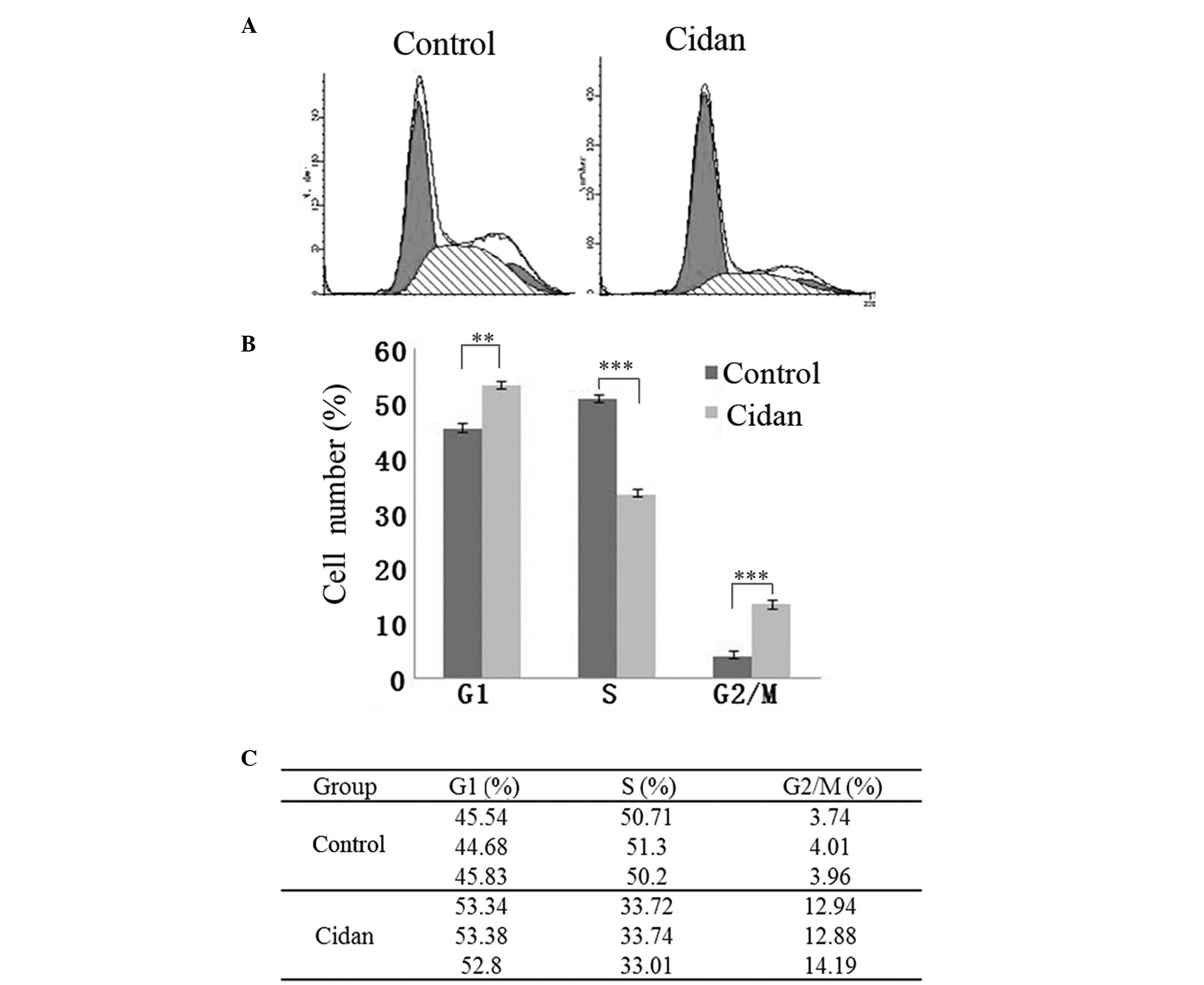
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Darvesh AS, Aggarwal BB and Bishayee A:
Curcumin and liver cancer: a review. Curr Pharm Biotechnol.
13:218–228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li W, Gao Z, Yang C, et al: The estimation
of prevalence, incidence, and residual risk of
transfusion-transmitted human hepatitis B infection from blood
donated at the Anhui blood center, China, from 2009 to 2011. PloS
One. 8:e734722013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Su Y, Norris JL, Zang C, Peng Z and Wang
N: Incidence of hepatitis C virus infection in patients on
hemodialysis: a systematic review and meta-analysis. Hemodial Int.
17:532–541. 2013.PubMed/NCBI
|
|
5
|
Chen WQ, Zheng RS and Zhang SW: Liver
cancer incidence and mortality in China, 2009. Chin J Cancer.
32:162–169. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Llovet JM, Burroughs A and Bruix J:
Hepatocellular carcinoma. Lancet. 362:1907–1917. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cheng JH, Chang G and Wu WY: A controlled
clinical study between hepatic arterial infusion with embolized
curcuma aromatic oil and chemical drugs in treating primary liver
cancer. Zhongguo Zhong Xi Yi Jie He Za Zhi. 21:165–167. 2001.[(In
Chinese)]. PubMed/NCBI
|
|
8
|
Wang WX, Li TX, Ma H, et al: Tumoral
cytotoxic and antioxidative phenylpropanoid glycosides in Smilax
riparia A. DC. J Ethnopharmacol. 149:527–532. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liang QL, Dai CC, Jiang JH, Tang YP and
Duan JA: A new cytotoxic casbane diterpene from Euphorbia
pekinensis. Fitoterapia. 80:514–516. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fang R, Houghton PJ and Hylands PJ:
Cytotoxic effects of compounds from Iris tectorum on human cancer
cell lines. J Ethnopharmacol. 118:257–263. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duan JA, Wang L, Qian S, Su S and Tang Y:
A new cytotoxic prenylated dihydrobenzofuran derivative and other
chemical constituents from the rhizomes of Atractylodes lancea DC.
Arch Pharm Res. 31:965–969. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin H, Liu J and Zhang Y: Developments in
cancer prevention and treatment using traditional Chinese medicine.
Front Med. 5:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong HY, Shao JW, Wang T, Guo YH and Yan
LY: Effects on the activities and mRNA expression of CYP3A in rat's
liver by four kinds of extracts from anti-cancer traditional
Chinese medicines. Zhong Yao Cai. 31:68–71. 2008.[(In Chinese)].
PubMed/NCBI
|
|
14
|
Wang B, Peng XX, Sun R, et al: Systematic
review of β-elemene injection as adjunctive treatment for lung
cancer. Chin J Integr Med. 18:813–823. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai ZJ, Tang W, Lu WF, et al:
Antiproliferative and apoptotic effects of β-elemene on human
hepatoma HepG2 cells. Cancer Cell Int. 13:272013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae - a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Q, Luan W, Goz V, Burakoff SJ and
Hiotis SP: Non-invasive in vivo imaging for liver tumour
progression using an orthotopic hepatocellular carcinoma model in
immunocompetent mice. Liver Int. 31:1200–1208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hu HS, Cheng SQ, Shi J, et al:
Establishment and characterization of a human hepatocellular
carcinoma cell line CSQT-1 derived from portal vein tumor thrombus.
Di Er Jun Yi Da Xue Xue Bao. 30:1–4. 2009.
|
|
19
|
Carmichael J, DeGraff WG, Gazdar AF, Minna
JD and Mitchell JB: Evaluation of a tetrazolium-based semiautomated
colorimetric assay: assessment of chemosensitivity testing. Cancer
Res. 47:936–942. 1987.PubMed/NCBI
|
|
20
|
Bauer JS, Schreiner CL, Giancotti FG,
Ruoslahti E and Juliano RL: Motility of fibronectin
receptor-deficient cells on fibronectin and vitronectin:
collaborative interactions among integrins. J Cell Biol.
116:477–487. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pang R, Tse E and Poon RT: Molecular
pathways in hepatocellular carcinoma. Cancer Lett. 240:157–169.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wong CM and Ng IO: Molecular pathogenesis
of hepatocellular carcinoma. Liver Int. 28:160–174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh S and Aggarwal BB: Activation of
transcription factor NF-kappa B is suppressed by curcumin
(diferuloylmethane) [corrected]. J Biol Chem. 270:24995–25000.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Libby P: Inflammatory mechanisms: the
molecular basis of inflammation and disease. Nutr Rev.
65:S140–S146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aggarwal BB, Kunnumakkara AB, Harikumar
KB, Tharakan ST, Sung B and Anand P: Potential of spice-derived
phytochemicals for cancer prevention. Planta Med. 74:1560–1569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lu JJ, Dang YY, Huang M, Xu WS, Chen XP
and Wang YT: Anti-cancer properties of terpenoids isolated from
Rhizoma Curcumae - a review. J Ethnopharmacol. 143:406–411. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu WS, Dang YY, Guo JJ, et al: Furanodiene
induces endoplasmic reticulum stress and presents antiproliferative
activities in lung cancer cells. Evid Based Complement Alternat
Med. 2012:4265212012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee SJ and Langhans SA: Anaphase-promoting
complex/cyclosome protein Cdc27 is a target for curcumin-induced
cell cycle arrest and apoptosis. BMC Cancer. 12:442012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Notarbartolo M, Poma P, Perri D, Dusonchet
L, Cervello M and D'Alessandro N: Antitumor effects of curcumin,
alone or in combination with cisplatin or doxorubicin, on human
hepatic cancer cells. Analysis of their possible relationship to
changes in NF-κB activation levels and in IAP gene expression.
Cancer Lett. 224:53–65. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tung YT, Chen HL, Lee CY, et al: Active
component of Danshen (Salvia miltiorrhiza Bunge), tanshinone I,
attenuates lung tumorigenesis via inhibitions of VEGF, cyclin A,
and cyclin B expressions. Evid Based Complement Alternat Med.
2013:3192472013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nie YL, Liu KX, Mao XY, Li YL, Li J and
Zhang MM: Effect of injection of Brucea javanica oil emulsion plus
chemoradiotherapy for lung cancer: a review of clinical evidence. J
Evid Based Med. 5:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li XY: Immunomodulating components from
chinese medicines. Pharm Biol 38 (Suppl 1). 33–40. 2000. View Article : Google Scholar
|