Introduction
The ideal goal of brain tumor surgery treatment is
to maximize the resection of tumor volume, without damaging motion,
sensation, language and other important cognitive functions. The
key in this type of surgery is how to identify the positions of
brain areas associated with important functions during the surgery
accurately and in real time. Accurate positioning may avoid
excessive resection-induced permanent neurological dysfunction, and
also avoid incomplete excision due to excessive carefulness, in
which situation the desired therapeutic effect would be challenging
to achieve (1–3). In the positioning of cerebral primary
functions such as motion and sensation, preoperative positioning
with magnetic resonance imaging (MRI) or intraoperative direct
electrical stimulation (DES) positioning is currently well
developed. However, clinical problems remain in the positioning of
higher cognitive functions, such as language and memory.
Language function is a human-specific high-level
cognitive function; in comparison with motion, sensation and
vision, for example, the language-related cortex is distributed
more widely and in a much more complex manner inside the human
brain. The cortical localization of language varies in different
individuals, particularly in patients with intracranial lesions
where the atypical distribution of the language cortex is
particularly common (4). The ability
to locate the language cortex is the key towards surgery in the
dominant hemisphere, particularly surgery of lesions close to the
language area. How best to perform resection of lesions in the
language area of the brain without inducing a postoperative
language disorder, thus protecting the patient's quality of life,
has become an issue of particular concern in neurosurgery currently
(5–8).
As the most accurate and reliable method of brain
functional area positioning, DES is able to determine in real time
the parts of the brain necessary for such functions as movement,
sensation, language, and even memory. A recent meta-analysis
suggested that it could also improve the degree of resection of
glioma while reducing the incidence of permanent neurological
dysfunction (9).
Investigations of the distribution characteristics
of Chinese language function in the cortex are limited to the
application of functional magnetic resonance in China. Chinese and
English are significantly different languages. Chinese involves the
characteristics of shape, meaning and grammar, and learning it is a
comprehensive study involving calligraphy and pronunciation.
English, however, involves phonetic learning, and a focus on
speaking. Thus, there may be some differences in the language areas
between Chinese- and English-speaking populations. Tan et al
of Hong Kong University found that native Chinese speakers and
native English speakers exhibited differences in anatomical
structures; the left inferior frontal gyrus, middle posterior
frontal gyrus and left anterocentral temporal lobe of native
Chinese speakers were relatively larger than those of native
English speakers (10).
The present authors have carried out resections of
functional area lesions in the awake status of general anesthesia
in China since 2003, with the use of intraoperative DES to position
the functional area cortex (11,12). The
present study summarizes the clinical experience of 91 cases, in
which intraoperative DES was applied during the resection of glioma
in the brain language area, aiming to summarize distribution rules
for the Chinese language cortex and the significance of DES in the
resection of gliomas in the language area.
Materials and methods
Study eligibility
Patients were considered eligible for this
prospective longitudinal study (conducted from January 2003 to
January 2012) if they: i) had suspected neuroepithelial tumors near
or within eloquent areas; ii) were between 16 and 60 years of age;
and iii) were fluent in speaking and understanding the Chinese
language. Patients were excluded from the study if they had severe
preoperative deficits or were mentally disoriented. This study was
conducted in accordance with the Declaration of Helsinki. The study
procedure was approved by the Medical Ethics Committee of
Liuhuaqiao Hospital (Guangzhou, China). Written informed consent
was obtained from all patients.
Preoperative evaluation
Detailed neurological and psychological assessments
were performed for all patients. General cognitive function was
assessed used the mini-mental state examination (MMSE). The
evaluation of handedness judgment was conducted using the Edinburgh
handedness examination (13). Three
days prior to surgery, the psychiatrist responsible for
intraoperative monitoring explained the surgery to the patient, and
screened the intraoperative tasks. Conventional MRI plain and
enhanced scans were performed preoperatively, and patients admitted
after 2006 were examined by functional MRI examination or diffusion
tensor imaging (DTI), magnetic resonance spectroscopy (MRS) and
blood oxygen level dependent-functional magnetic resonance imaging
(BOLD-fMRI).
Surgical treatment: Anesthesia
The intraoperative awake method of general
anesthesia was used (3). Intubation
was performed through a laryngeal mask, and the anesthesia used was
propofol for general anesthesia, bupivacaine or ropivacaine for
local anesthesia of the scalp (including the supraorbital nerve,
auriculotemporal nerve, greater occipital nerve and ear nervelet,
as well as infiltration anesthesia around incision area) and
lidocaine for local anesthesia of the meninges. Following opening
of the meninges, the propofol administration was stopped, the
patient was woken up and the laryngeal mask was unplugged.
Following tumor removal, the patient was anesthetized again and the
laryngeal mask was reapplied.
Anatomical localization of
lesions
Intraoperative B-ultrasound and/or MRI
neuronavigation was performed to determine the location and extent
of the lesions.
Brain area DES method
The DES method was conducted as previously described
(11). Bipolar electrical nerve
stimulation (interval, 5 mm; OSIRIS NeuroStimulator; Inomed
Medizintechnik GmbH, Teningen, Germany) was used. The stimulation
range covered all areas of the exposed cortex and suspicious
subcortical regions; the frequency was 60 Hz, the pulse duration
was 1 msec, and the current was 0.5–10 mA (usually 4–6 mA). The
stimulus duration of every point was 1 sec for motion and sensation
tasks and 4 sec for language tasks.
Observation indices
Positive stimulation of the motion area was assumed
when movements of the contralateral limb or face were induced, with
the concurrent recording of an electromyogram. Positive stimulation
affecting the sensation area was assumed when an abnormal feeling
was induced in the contralateral limb or face. Positive stimulation
of the language area was assumed when the patient exhibited
counting interruption, errors during object naming, language
confusion or other language problems. During the surgical
resection, the patient was asked to repeatedly complete a series of
motion and language tasks. If the patient exhibited weakness of
physical activity, language abnormalities or abnormal sensation,
subcortical DES was performed immediately to confirm the existence
of major conduction tracts. The functional areas as determined by
cortical or subcortical DES were the areas that the surgery was
required not to damage.
Resection
The tumor was resected under the awake status, and
the resection was performed to the greatest extent according to the
real-time monitoring of changes in language, motion and
sensation.
Assessment of postoperative
neurological function and extent of resection
At 1 month (early stage) and 3 months (late stage)
after the surgery, a detailed assessment of cognitive functions,
including nervous system functions and language was performed. The
skull was reviewed within 72 h after the surgery to confirm the
extent of tumor resection. For low-grade glioma, T2 or fluid
attenuation inversion recovery MRI was used as the reference to
determine resection degree, while for high-grade glioma, enhanced
T1 was used. Overall resection was 100% lesion excision;
sub-overall resection was ≥90% and <100% excision, while partial
resection was <90% excision.
Results
Clinical and radiological
findings
The patients' general information, clinical
manifestations and imaging data are shown in Table I. There were 91 cases of brain
function area glioma, including 52 males and 39 females, with an
average age of 38.7 years. The clinical manifestations included 73
cases of epilepsy (80.2%); 13 cases of headache (14.3%) and nine
cases of focal neurological dysfunction (9.9%). The maximum
diameter of the cranial MRI lesions was 2.0–6.0 cm, and all were
located around the lateral fissure. The lesions were all located on
the left, among which 86 cases were right-handed (94.5%), three
cases were left-handed and two cases were ambidextrous. The MMSE
scores of all patients were ≥28 points.
 | Table ISummary of clinical manifestations,
imaging data, pathology, intraoperative positioning, resection
range and postoperative dysfunction of the 91 glioma cases. |
Table I
Summary of clinical manifestations,
imaging data, pathology, intraoperative positioning, resection
range and postoperative dysfunction of the 91 glioma cases.
| Variable | Cases (%) |
|---|
| Gender | |
| Male | 52 (57.1) |
|
Female | 39 (42.9) |
| Symptoms and
signs | |
|
Epilepsy | 73 (80.2) |
| Headache,
increased intracranial pressure | 13 (14.3) |
| Focal
neurological dysfunction | 9 (9.9) |
| DES results | |
| Motion
cortex | 88 (96.7) |
| Language
cortex, summary | 91 (100.0) |
| Language
cortex, counting interruption | 86 (94.5) |
| Language
cortex, anomia | 21 (23.1) |
| Language
cortex, naming error | 5 (5.5) |
| Pathology |
|
| Low-grade
glioma | 66 (72.5) |
|
High-grade glioma | 25 (27.5) |
| Resection degree |
|
|
| Overall
resection | 53 (58.2) |
|
Sub-overall resection | 31 (34.1) |
| Partial
resection | 7 (7.7) |
| Postoperative
neurological dysfunction |
|
| No | 42 (46.2) |
| Early
stage language dysfunction | 39 (42.9) |
| Early
motion dysfunction | 27 (29.7) |
|
Permanent neurological
dysfunction | 1 (1.1) |
Among the patients, 66 cases were of low-grade
glioma (72.5%), including 43 cases of astrocytoma, 17 cases of
oligodendroglioma and six cases of hypo-astrocytoma; and 25 cases
were of high-grade glioma (27.5%), including 15 cases of
glioblastoma, five cases of anaplastic astrocytoma and five cases
of anaplastic oligodendroglioma.
Cortical sites identified by DES
During the awake surgery, when the stimulus reached
a certain intensity (usually 2–4 mA), all the cortical functional
areas were stimulated, at which time 88 cases (125 positive
stimulation points) exhibited facial or hand movement onset, and 91
cases (112 positive stimulation points) exhibited language
disorders or counting interruption, naming error or anomia. The
greatest difference among individuals was in the distribution of
the points that were determined to be in language areas, although
all were concentrated within a range of 1 cm2. Among
these, 86 points were associated with counting interruption, 21
points were associated with anomia and 5 points were associated
with naming errors. The stimulation points that were positive for
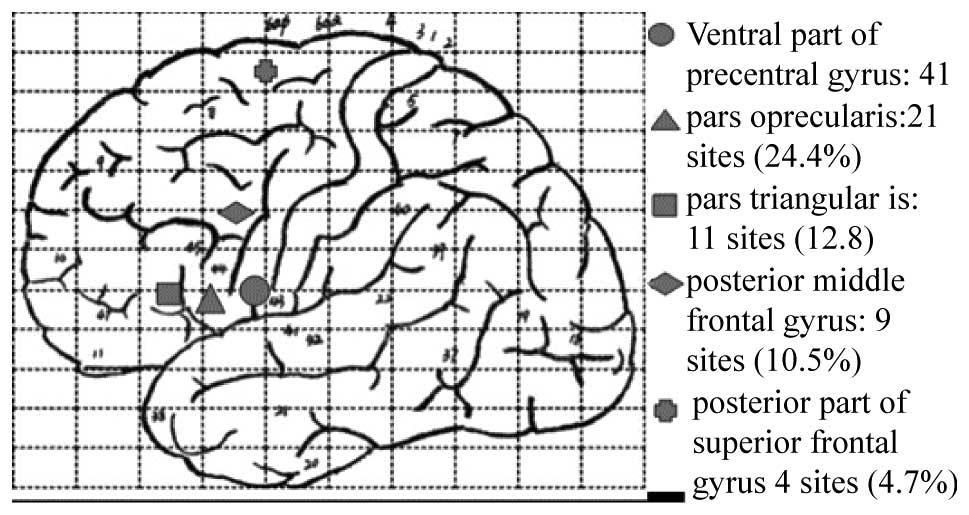
counting interruption were mainly located on the posterior part of
the left anterior central gyrus (41 points, 47.7%), the operculum
of the left inferior frontal gyrus (21 points, 24.4%), the
triangular part of the left inferior frontal gyrus (11 points,
12.8%), the left posterior middle frontal gyrus (9 points, 10.5%)
and the posterior part of the superior frontal gyrus (4 points,
4.7%; Fig. 1).
Extent of resection
Cranial MRI was performed 72 h after the surgery,
and demonstrated that overall resection was achieved in 53 cases
(58.2%), sub-overall resection was performed in 31 cases (34.1%)
and partial resection was performed in seven cases (7.7%).
Intra-operative side-effects
Seven patients exhibited intraoperative electrical
stimulation-induced epileptic seizures, which were controlled by
the use of ice brine to wash the cortex; A total of 21 patients
experienced shivering in the awake procedure prior to the
introduction of a heating blanket to the awake surgeries. With
regard to discomfort, no patient suffered from unendurable pain,
expressed any intraoperative severe pain or had any postoperative
pain memory after waking up. Intraoperative discomfort comprised 38
cases of urine-holding (41.8%), 35 cases of dry mouth (38.5%) and
10 cases of slight pain in the temporalis muscle or meninges
(11.0%).
Postoperative neurological status
Postoperatively, 42 cases (46.2%) had no
postoperative neurologic dysfunction; 39 cases (42.9%) exhibited
brief language dysfunction; 27 cases (29.7%) exhibited a brief limb
motion disorder, which returned to preoperative functional levels
within 2 weeks to 1 month after the surgery; and one case (1.1%)
exhibited permanent neurological dysfunction. In that case,
postoperative cranial MRI indicated that an infarction was present
within the inner vesicle region.
Discussion
Glioma in the language area is a challenging problem
in neurosurgery. The prognosis improves as the amount of glioma
removed increases (1–5); however, the patient's language function
should be retained postoperatively. As gliomas often involve the
language area, the US National Comprehensive Cancer Network glioma
surgery guidelines (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp),
as well as the Chinese consensus on the diagnosis and treatment of
malignant CNS glioma (14), have
proposed that the tumor should be maximally safely resected. This
means that the tumor volume should be maximally resected, while the
language and other important cognitive functions must not be
damaged. The key of this type of surgery is how to position the
language area and lesion range accurately and in real time during
the surgery.
Due to differences between individuals and the
effects caused by the positioning of brain tumors, functional areas
are shifted and remodeled. Thus, the classical anatomical
localization of functional areas is likely to have some errors.
Although new imaging methods, such as positron emission tomography,
fMRI and magnetoencephalography, are able to locate the position of
the motion and sensation cortexes, these methods are not very
accurate towards the positioning of the language area; for example,
the sensitivity of fMRI towards the language area positioning is
59–100%, while the specificity is only 0–97% (4). These methods are not able to achieve
intraoperative real-time monitoring of the brain functional areas,
and cannot perform the positioning of cerebral white matter fibers.
Although they are able to identify the cortical area that is
associated with a certain function, they are not able to determine
which parts are the necessary parts (15). Currently, the most accurate and
reliable method of brain functional area positioning is
intraoperative cortical or subcortical DES. In real time, DES can
determine the parts essential for motion, sensation, language, and
even memory, and is able to identify intraoperatively the positions
of functional areas located towards the cortex and subcortex of the
cerebrum, brainstem and spinal cord (16,17).
In 1870, Fritsch and Hitzig first used this method
to locate the brain areas responsible for motion in a dog (18). In 1874, Bartholow, an American
neurological surgeon, first performed electric stimulation of a
patient's brain, and recorded the associated motion response
(19). Penfield and Flanigin
(20), a Canadian neurosurgeon,
maturely applied this technology, and described in detail the
cerebral positioning areas of language, vision and audition, and
based on these applications, the famous cortical homunculus map was
established. Subsequently, DES spread rapidly throughout Western
countries. Berger et al (21)
and Duffau (22) applied DES to
brain tumor surgeries, particularly low-grade glioma surgeries and
concurrently carried out a large number of clinical studies in
which the DES technology was applied to the location of subcortical
pathways. All the aforementioned efforts have contributed
outstandingly to the promotion of DES, making it one of the
essential techniques in the functional area surgery of neurosurgery
(23–26). In 2002, Wang (Guangzhou, China)
applied DES to the surgery of brain functional area lesions, and
following four periods of nationwide study and training, this
technology has gradually been accepted and promoted by
neurosurgeons in China (11,12).
DES is a safe and reliable positioning method;
histological examination has revealed no inflammation or other
injuries in the stimulated parts, and patient follow-up has also
revealed no significant complications. However, if the stimulation
method is not conducted correctly, it would be likely to cause
false positive or false negative results, or even status
epilepticus. This would affect the surgery, and further cause
postoperative permanent neurological dysfunction. Therefore, it is
particularly important for the correct stimulation method and
stimulation parameters to be used in intraoperative DES (27). The authors of the present study use a
biphasic square wave, as a sine wave would cause adaptive
adjustment of the cell membranes during the stimulation process,
thus increasing the required stimulus current and potentially
causing false positive results or the induction of epileptic
seizures. The use of a biphasic square wave could avoid the
overlaying of current around the cell membranes and the subsequent
ionization hydrolysis and heating of local cerebrospinal fluid
particles, which would be likely to result in nerve cell damage
(7). The stimulation frequency was
60 Hz, and the duration of the stimulating square-wave was 1 msec.
The current was determined based on the appearance of
post-discharge stimulus during electroencephalography monitoring.
The stimulation intensity was usually set as a level lower than the
lowest current level that induced a post potential; for example, if
the current that induced a post potential was 5 mA, the stimulus
current was set at 4 mA. The stimulus current was normally applied
at 1 mA initially, and increased in increments of 1 mA, usually
reaching 2–4 mA. The stimulus duration for the motion and sensation
tasks was ∼1 sec, and that for the language and cognitive tasks was
∼4 sec (28). It has been observed
in certain studies, particularly that regarding patients with
epilepsy, the square wave duration used to stimulate the positioned
functional areas was 0.2 msec; therefore, the stimulus current was
significantly greater than that used in the present study (29). In summary, to prevent the induction
of status epilepticus it is recommended to avoid a high stimulus
frequency, long stimulus duration, excessive stimulus current, post
discharge; and two consecutive positive stimuli.
DES is a reliable and noninvasive method for
cerebral functional area positioning, and provides a new surgical
concept for glioma resection in the language area, which promotes
the modeling of the language area during glioma surgery by a
functional method rather than an anatomical one. The operative
range using DES reaches the functional border rather than the
anatomical border; thus, peritumoral tissues, which may be invaded
by the tumors, may also be resected in certain cases. For low-grade
glioma, the tumor may be resected to the maximum extent under the
premise of retaining the important brain functions, thus prolonging
the survival period. For high-grade glioma, cortical DES avoids
surgery-induced functional deficits, and improve the patients'
survival qualities (30–33). De Witt Hamer et al (9) completed a meta-analysis, which
collected 90 documents and the surgical situations of 8,091 glioma
cases, and found that the rate of long-term severe neurological
dysfunction subsequent to DES was 3.4%, while the long-term severe
disability rate of patients that underwent surgery without DES was
8.2%. Furthermore, for the patients undergoing DES, the overall
resection rate and the rate of involvement of the language
functional area in the resection were significantly increased. The
present study also demonstrated that under the premise of not
reducing the resection extent, although the incidence of early
neurological disorder was high (53.8%), the incidence of long-term
neurological dysfunction was also very low (1.1%), indicating that
the aim of maximum safe resection could be realized.
Furthermore, DES also provides an important research
method for neurosurgeons to understand the higher cognitive
functions of the brain. DES plays an important role in studies of
such higher cognitive functions as hemispheric ignorance,
perceptual awareness, music, calculation, memory and
category-specific naming (12,34–39). In
the present study, it was found that the Broca area of a Chinese
population undergoing DES was mainly concentrated within a
1-cm2 range. However, great differences existed among
individuals. The zones that tested positive for counting
interruption were mainly located on the posterior part of the left
anterior central gyrus (47.7%), the operculum of the left inferior
frontal gyrus (24.4%), the triangular part of the left inferior
frontal gyrus (12.8%), the left posterior middle frontal gyrus
(10.5%) and the posterior part of the superior frontal gyrus
(4.7%).
In summary, cortical DES was found to be a reliable
non-invasive method for cerebral functional area positioning. The
application of this technology in glioma surgery of the language
area may achieve the maximally safe resection of tumors, and
provide assistance towards the cortical positioning of cerebral
functional areas in the Chinese population.
References
|
1
|
Chan-Seng E, Moritz-Gasser S and Duffau H:
Awake mapping for low-grade gliomas involving the left sagittal
stratum: Anatomofunctional and surgical considerations. J
Neurosurg. 120:1069–1077. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanai N and Berger MS: Glioma extent of
resection and its impact on patient outcome. Neurosurgery.
62:753–764. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han SJ and Sughrue ME: The rise and fall
of ‘biopsy and radiate’: A history of surgical nihilism in glioma
treatment. Neurosurg Clin N Am. 23:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giussani C, Roux FE, Ojemann J, Sganzerla
EP, Pirillo D and Papagno C: Is preoperative functional magnetic
resonance imaging reliable for language areas mapping in brain
tumor surgery? Review of language functional magnetic resonance
imaging and direct cortical stimulation correlation studies.
Neurosurgery. 66:113–120. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi BD, Mehta AI, Batich KA, Friedman AH
and Sampson JH: The use of motor mapping to aid resection of
eloquent gliomas. Neurosurg Clin N Am. 23:215–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lubrano V, Draper L and Roux FE: What
makes surgical tumor resection feasible in Broca's area? Insights
into intraoperative brain mapping. Neurosurgery. 66:868–875. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandonnet E, Winkler PA and Duffau H:
Direct electrical stimulation as an input gate into brain
functional networks: Principles, advantages and limitations. Acta
Neurochir (Wien). 152:185–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Duffau H: Awake surgery for nonlanguage
mapping. Neurosurgery. 66:523–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Witt Hamer PC, Robles SG, Zwinderman
AH, Duffau H and Berger MS: Impact of intraoperative stimulation
brain mapping on glioma surgery outcome: a meta-analysis. J Clin
Oncol. 30:2559–2565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tan LH, Spinks JA, Eden GF, Perfetti CA
and Siok WT: Reading depends on writing, in Chinese. Proc Natl Acad
Sci USA. 102:8781–8785. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bai HM, Wang WM, Li TD, et al: Three core
techniques in surgery of neuroepithelial tumors in eloquent areas:
awake anaesthesia, intra-operative direct electrical stimulation
and ultrasonography. Chin Med J (Engl). 124:3035–3041.
2011.PubMed/NCBI
|
|
12
|
Bai HM, Jiang T, Wang WM, Li TD, Liu Y and
Lu YC: Functional MRI mapping of category-specific sites associated
with naming of famous faces, animals and man-made objects. Neurosci
Bull. 27:307–318. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oldfield RC: The assessment and analysis
of handedness: The Edinburgh inventory. Neuropsychologia. 9:97–113.
1971. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou LF, Wang RZ, Bao SD, et al: Chinese
guideline for diagnosis and treatment on central nervous system
tumors. Zhonghua Yi Xue Za Zhi. 92:2309–2313. 2012.[(In
Chinese)].
|
|
15
|
Kim SS, McCutcheon IE, Suki D, et al:
Awake craniotomy for brain tumors near eloquent cortex: correlation
of intraoperative cortical mapping with neurological outcomes in
309 consecutive patients. Neurosurgery. 64:836–845. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sacko O, Lauwers-Cances V, Brauge D, Sesay
M, Brenner A and Roux FE: Awake craniotomy vs surgery under general
anesthesia for resection of supratentorial lesions. Neurosurgery.
68:1192–1198. 2011.PubMed/NCBI
|
|
17
|
De Benedictis A, Moritz-Gasser S and
Duffau H: Awake mapping optimizes the extent of resection for
low-grade gliomas in eloquent areas. Neurosurgery. 66:1074–1084.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fritsch G and Hitzig E: Electric
excitability of the cerebrum (Uber die elektrische Erregbarkeit des
Grosshirns). Epilepsy Behav. 15:123–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartholow R: Experimental investigations
into the functions of the human brain. Am J Med Sci. 66:305–313.
1874. View Article : Google Scholar
|
|
20
|
Penfield W and Flanigin H: Surgical
therapy of temporal lobe seizures. AMA Arch Neurol Psychiatry.
64:491–500. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Berger MS, Stieg PE, Danks RA, Schwartz RB
and Folkerth RD: Lesions in eloquent cortex. Neurosurgery.
40:1059–1063. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duffau H: Surgery of low-grade gliomas:
Towards a ‘functional neurooncology’. Curr Opin Oncol. 21:543–549.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ilmberger J, Ruge M, Kreth FW, Briegel J,
Reulen HJ and Tonn JC: Intraoperative mapping of language
functions: A longitudinal neurolinguistic analysis. J Neurosurg.
109:583–592. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Desmurget M, Reilly KT, Richard N,
Szathmari A, Mottolese C and Sirigu A: Movement intention after
parietal cortex stimulation in humans. Science. 324:811–813. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roux FE, Dufor O, Lauwers-Cances V, et al:
Electrostimulation mapping of spatial neglect. Neurosurgery.
69:1218–1231. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Beez T, Boge K, Wager M, et al: European
Low Grade Glioma Network: Tolerance of awake surgery for glioma: A
prospective European Low Grade Glioma Network multicenter study.
Acta Neurochir (Wien). 155:1301–1308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
De Benedictis A, Sarubbo S and Duffau H:
Subcortical surgical anatomy of the lateral frontal region: Human
white matter dissection and correlations with functional insights
provided by intraoperative direct brain stimulation: laboratory
investigation. J Neurosurg. 117:1053–1069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Duffau H: A new concept of diffuse
(low-grade) glioma surgery. Adv Tech Stand Neurosurg. 38:3–27.
2012.PubMed/NCBI
|
|
29
|
Lüders HO: Symptomatic Areas and
Electrical Cortical StimulationNew York: Churchill Livingstone;
2000
|
|
30
|
Duffau H: Awake surgery for incidental WHO
grade II gliomas involving eloquent areas. Acta Neurochir (Wien).
154:575–584. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Duffau H: Brain mapping in tumors:
Intraoperative or extraoperative? Epilepsia. 54:79–83. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Duffau H: A new philosophy in surgery for
diffuse low-grade glioma (DLGG): Oncological and functional
outcomes. Neurochirurgie. 59:2–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duffau H and Mandonnet E: The
‘onco-functional balance’ in surgery for diffuse low-grade glioma:
Integrating the extent of resection with quality of life. Acta
Neurochir (Wien). 155:951–957. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gras-Combe G, Moritz-Gasser S, Herbet G
and Duffau H: Intraoperative subcortical electrical mapping of
optic radiations in awake surgery for glioma involving visual
pathways. J Neurosurg. 117:466–473. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Klein M, Duffau H and De Witt Hamer PC:
Cognition and resective surgery for diffuse infiltrative glioma: An
overview. J Neurooncol. 108:309–318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Maldonado IL, Moritz-Gasser S, de
Champfleur NM, Bertram L, Moulinié G and Duffau H: Surgery for
gliomas involving the left inferior parietal lobule: new insights
into the functional anatomy provided by stimulation mapping in
awake patients. J Neurosurg. 115:770–779. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pallud J, Mandonnet E and Duffau H:
Diffuse low-grade gliomas and UCSF scores. J Neurosurg.
120:577–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Schucht P, Ghareeb F and Duffau H: Surgery
for low-grade glioma infiltrating the central cerebral region:
location as a predictive factor for neurological deficit,
epileptological outcome and quality of life. J Neurosurg.
119:318–323. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yordanova YN, Moritz-Gasser S and Duffau
H: Awake surgery for WHO Grade II gliomas within ‘noneloquent’
areas in the left dominant hemisphere: toward a ‘supratotal’
resection. Clinical article. J Neurosurg. 115:232–239. 2011.
View Article : Google Scholar : PubMed/NCBI
|