Introduction
Paeoniae Radix is a well-known herb and is
used widely as a component of traditional Chinese prescriptions to
treat certain types of dementia, traumatic injury and inflammation.
Paeoniflorin (PF), a product derived from Paeoniae Radix,
has been reported to exhibit neuroprotective, anti-ischemic,
antioxidative, anti-inflammatory and anticancer effects. The
neuroprotective potential of PF has been demonstrated in animal
models of various neuropathologies (1–4).
Reactive oxygen species (ROS) are produced by
various enzymatic reactions and chemical processes, which are
essential for numerous physiological functions, in addition to
serving as secondary messengers in the human body (5). A number of neurodegenerative diseases,
including Alzheimers, Parkinsons and Huntingtons, are characterized
by severe and/or prolonged oxidative stress (6). The primary outcome of oxidative stress
is the irreversible damage of macromolecules by ROS (7). The association between oxidative stress
and inflammation is due to the activation of nuclear factor (NF)-κB
and activator protein-1, and the inhibition of nuclear factor
(erythroid-derived 2)-like 2, peroxynitrite-mediated endothelial
dysfunction, altered nitric oxide levels and macrophage migration
(8). Previous studies have indicated
that PF protects neurons against ischemia-reperfusion injury by
reducing the expression levels of intracellular adhesion molecule 1
and tumor necrosis factor α (TNF-α), resulting in reduced
inflammation in infarcted brain regions, and PF prevents chronic
cognitive damage by downregulating the expression of NF-κB in
hippocampal astrocytes (4,9). The present study investigated the
neuroprotective effect of PF following
H2O2-induced injury in PC12 cells and the
possible signaling pathways involved.
Materials and methods
Reagents and cell line
PF (purity, 98.5%) was purchased from Nanjing Zelang
Medical Technology Co., Ltd. (Nanjing, China). The PC12 cell line
was obtained from the American Type Culture Collection (Manassas,
VA, USA).
MTT cell proliferation assay
Cell viability was measured using an MTT assay as
described in a previous study (9).
The PC12 cells received different treatments, including no
treatment (control), 200 µM H2O2 alone or 200
µM H2O2 in combination with 20, 40 or 80 µM
PF. Briefly, the cells were seeded into 96-well plates
(3.0×103/well) and cultured for 6 h. MTT solution (5
mg/ml; Sigma-Aldrich, St. Louis, MO, USA) was added to each well
and incubated for 4 h. Next, 150 µl dimethyl sulfoxide (DMSO;
Sigma-Aldrich) was added to dissolve the formazan precipitate.
Absorbance was then measured at 570 nm using a ThermoMax microplate
reader (Molecular Devices LLC, Sunnyvale, CA, USA). Cell viability
is expressed as a percentage relative to the untreated control.
Lactate dehydrogenase (LDH) release
assay
The rate of cell death was further assessed by
measuring the leakage of LDH into the surrounding medium, as
described in a previous study (6).
Briefly, following treatment of the PC12 cells, the supernatants of
each group were collected. The quantity of LDH released was
determined using a Neutral Red LDH Cytotoxicity Assay Kit according
to the manufacturers instructions (Beyotime Institute of
Biotechnology, Wuhan, China). Optical absorbance was measured at
440 nm using the ThermoMax microplate reader.
Measurement of intracellular ROS
levels
Intracellular H2O2 and
low-molecular weight peroxides are able to oxidize
2,7-dichlorofluorescin diacetate (DCFH-DA) to dichlorofluorescein
(DCF), which is highly fluorescent under absorption analysis. A
DCFH-DA fluorescent probe from a Reactive Oxygen Species Assay kit
(Beyotime Institute of Biotechnology) was used to measure ROS
generation, as previously reported (6). Following treatment, cells were
incubated with 10 mM DCFH-DA for 30 min at 37°C and washed twice
with phosphate-buffered saline. Subsequently, the DCF fluorescence
was measured using the ThermoMax microplate spectrofluorometer at
excitation and emission wavelengths of 485 and 530 nm,
respectively.
Hoechst 33258 staining
PC12 cells at the logarithmic-growth phase were
seeded into 96-well plates (1×104/well). The cells were
cultured in H2O2 alone or with 80 µM PF. A
third group of cells received no treatment and was used as a
control group. Next, the cells were fixed with 3.7%
paraformaldehyde for 30 min at room temperature, then washed and
stained with Hoechst 33258 (Sigma-Aldrich) for 30 min at 37°C. PC12
cells were observed under a Nikon 80i fluorescence microscope
equipped with a UV filter (Nikon Corporation, Tokyo, Japan).
Western blot analysis
PC12 cells were seeded in 6-well plates
(3.0×105/well) and pretreated with 200 µM
H2O2 alone or 200 µM
H2O2 + 80 µM PF for 6 h. A third group of
cells received no treatment and was used as a control group. After
incubation the culture medium was collected for detection of the
levels of TNF-α and interleukin (IL)-1β. Cells were collected and
lysed in a buffer containing 50 mM HEPES (pH 7.4), 150 mM NaCl,
0.1% Triton X-100, 1.5 mM MgCl2, 1 mM EDTA, 2 mM sodium
orthovanadate, 4 mM sodium pyrophosphate, 100 mM NaF and protease
inhibitor mixture (1:500; Sigma-Aldrich) for cell lysates. Cell
lysates were subjected to 10% SDS-polyacrylamide gel (Invitrogen,
Thermo Fisher Scientific, USA) electrophoresis, then transferred
onto polyvinylidine fluoride membranes (EMD Millipore, Billerica,
MA, USA). The membranes were subsequently probed with antibodies,
including rabbit polyclonal caspase-3 (#9662), cleaved
poly(ADP-ribose) polymerase (PARP; #9541), B-cell lymphoma 2
(Bcl-2; #2872) and Bcl-2-associated X (Bax; #2772) antibodies
purchased from Cell Signaling Technology, Inc. (1:1,000; Danvers,
MA, USA). Mouse monoclonal NF-κB-p65RelA (1:800; sc-8008) and
rabbit polyclonal p-NF-κB Ser536 (1:500; sc-33020) antibodies were
obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA)
and mouse monoclonal β-actin antibody (1:10,000; #ab6276) from
Abcam (Cambridge, MA, USA). Immunoblots were developed using
horseradish peroxidase (HRP)-conjugated secondary antibodies. Bound
antibodies were visualized using Immobilon Western Chemiluminescent
HRP substrate (EMD Millipore) and quantified by densitometry using
a ChemiDoc XRS system (Bio-Rad Laboratories Inc., Berkeley, CA,
USA). Densitometric analyses of bands were adjusted against
β-actin, which functioned as a loading control. The percentage
increase or reduction in protein expression levels was estimated by
comparison to a vehicle control. Experiments were performed in
triplicate, separately.
TNF-α and IL-1β assays
The culture medium was collected in microcentrifuge
tubes and subjected to centrifugation for 10 min. The supernatants
were separated out and the expression levels of TNF-α and IL-1β
were detected using Human TNF-alpha Quantikine (DTA00C) and Human
IL-1 beta/IL-1F2 Quantikine ELISA kits (R&D Systems Inc.,
Minneapolis, MN, USA) according to the manufacturers
instructions.
Statistical analysis
Statistical analysis was performed using SPSS
software for Windows, version 18.0 (SPSS, Inc., Chicago, IL, USA).
Data are presented as the mean ± standard deviation and were
analyzed by one-way analysis of variance. Multiple comparisons
between groups were performed using the Student-Newman-Keuls method
and P<0.05 was considered to indicate a statistically
significant difference.
Results
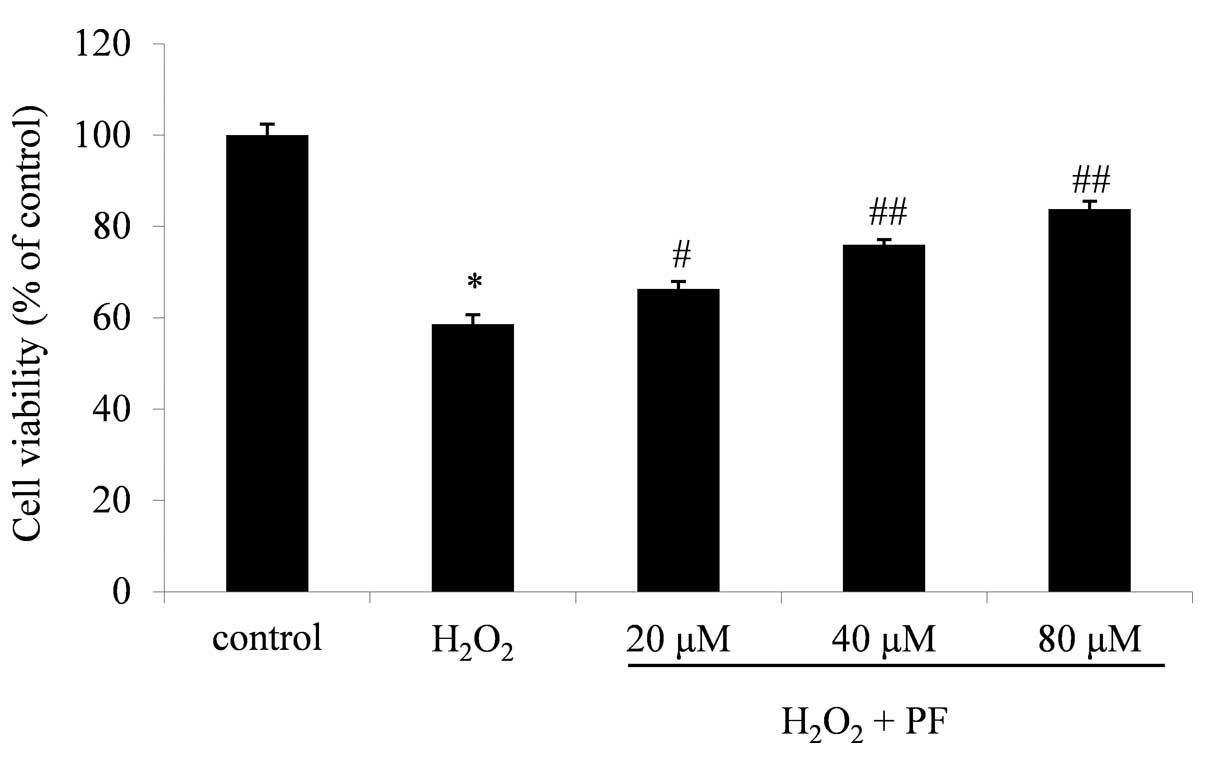
Effect of PF on cell viability
The viability of cells incubated with 200 µM
H2O2 was 58.6±2.4% of the control value
(P<0.01; Fig. 1). The viabilities
of cells treated with 200 µM H2O2 + 20, 40 or
80 µM PF were increased in a dose-dependent manner to 66.3±1.6
(P<0.05 vs. H2O2), 75.9±1.1 and 83.4±1.7%
(P<0.01 vs. H2O2) of the control values,
respectively (n=3; Fig. 1). These
results clearly indicate that PF attenuated the
H2O2-induced cytotoxicity in the PC12
cells.
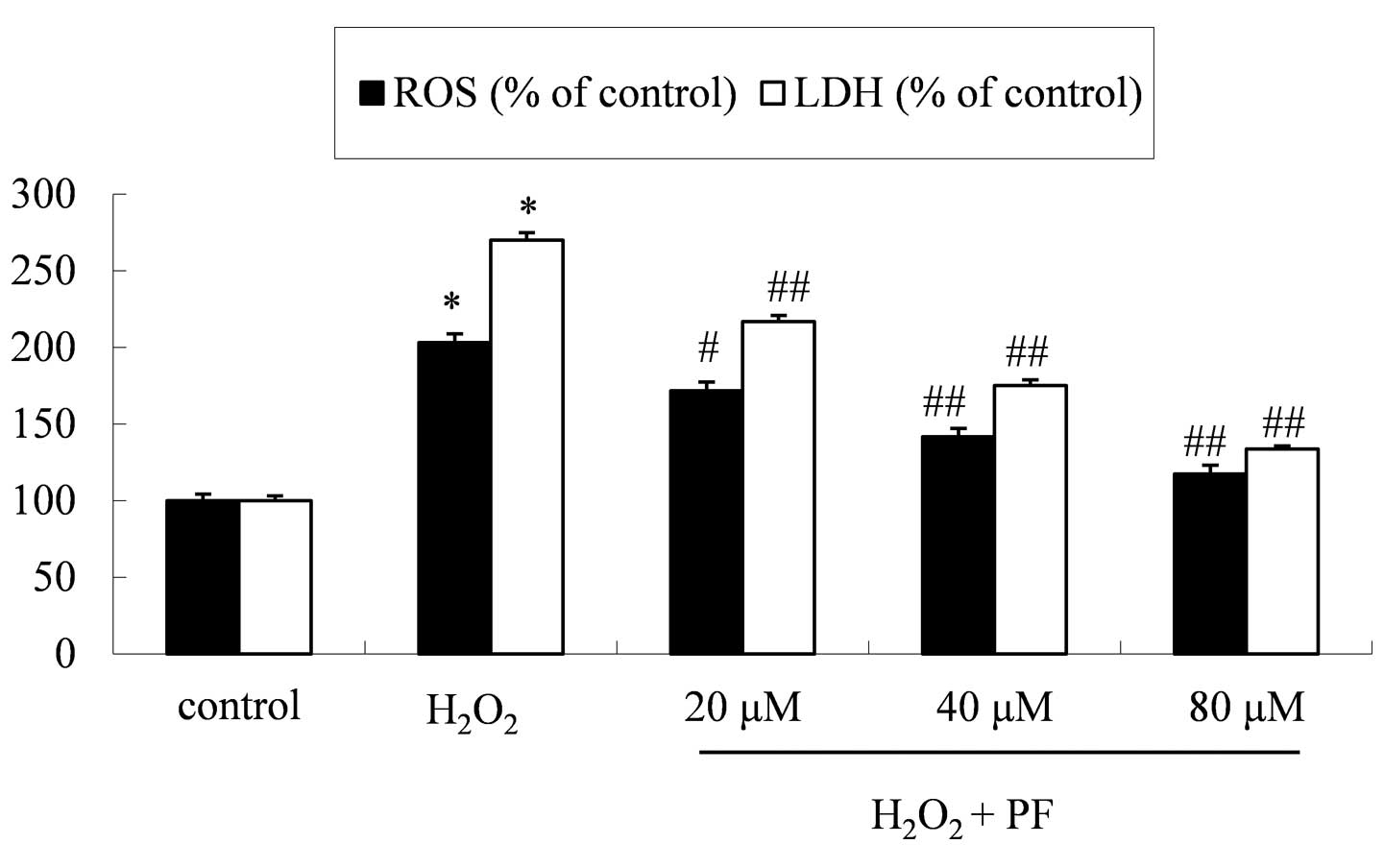
Effect of PF on LDH and ROS
levels
The neuroprotective effect of PF was further
investigated by measuring ROS accumulation and levels of LDH
release following treatment. Pretreatment with PF attenuated the
H2O2-induced increase in levels of ROS and
LDH release (Fig. 2).
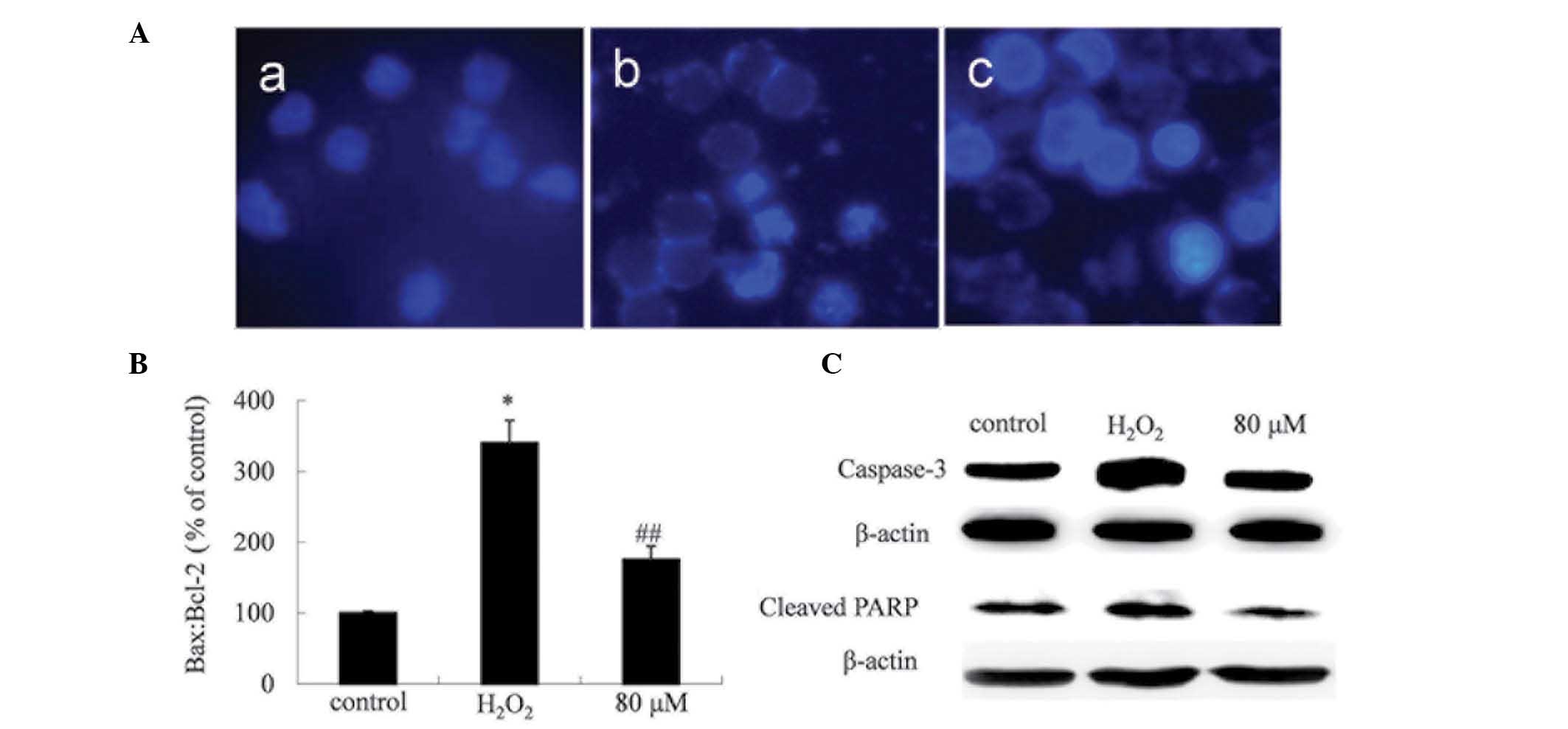
PF protects PC12 cells against
H2O2-induced apoptosis
Alterations of cellular morphology were assessed
using Hoechst 33258 staining in order to characterize the degree of
H2O2-induced PC12 cell death (Fig. 3A). The nuclei of the PC12 cells
treated with H2O2 appeared fragmented,
indicating that apoptosis affected the morphology of the cells.
However, treatment with 80 µM PF for 6 h clearly reduced the
percentage of necrotic and apoptotic cells.
Expression levels of the
anti-apoptotic protein Bcl-2 and the pro-apoptotic protein Bax were
measured using western blot analysis
H2O2 was observed to increase
the Bax:Bcl-2 ratio in the PC12 cells, while the PF treatment
produced an opposite effect (Fig.
3B). Furthermore, the H2O2-induced
elevation of the expression levels of caspase-3 and cleaved PARP
appeared significantly reduced in cells treated with PF (Fig. 3).
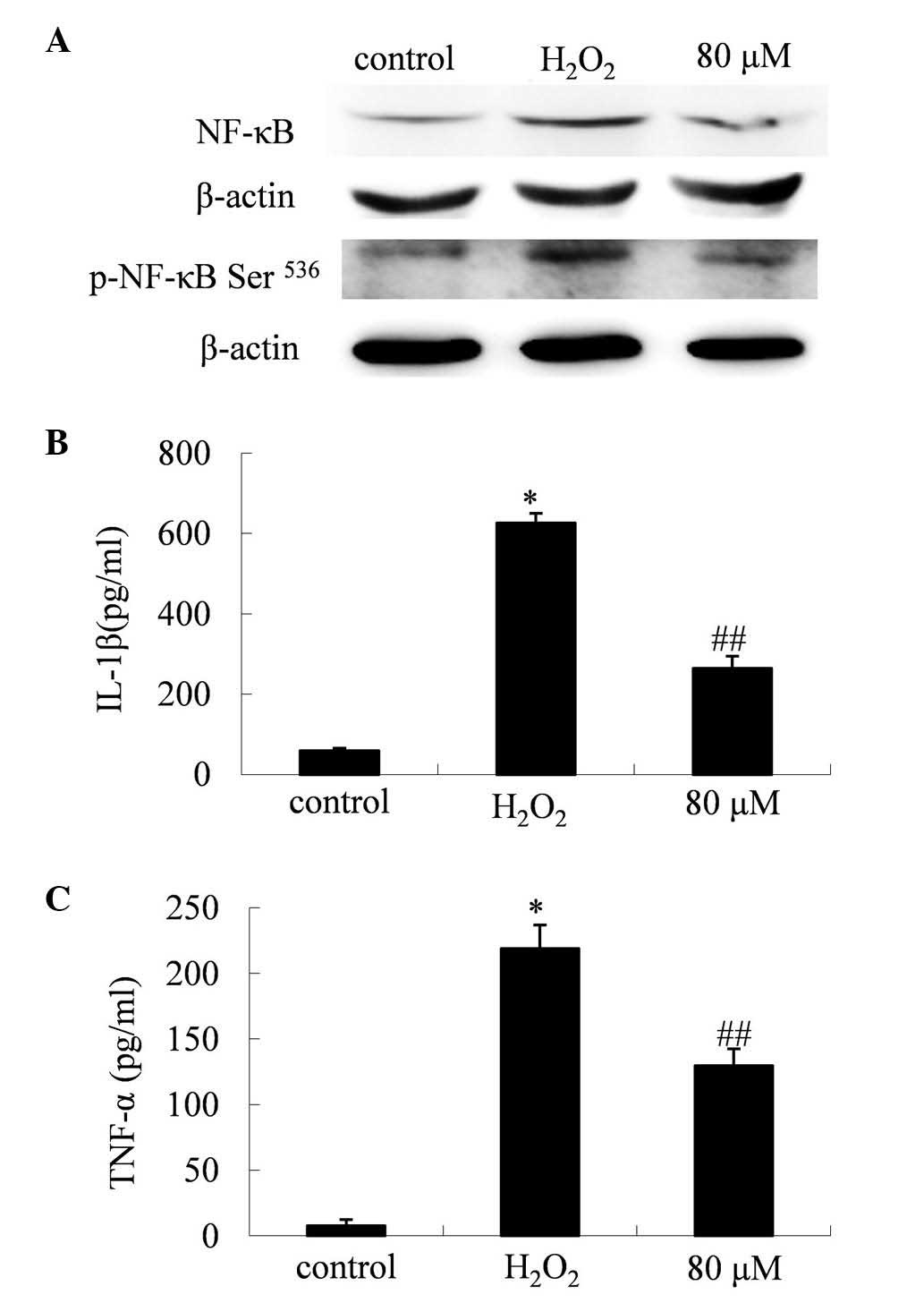
PF suppresses the expression levels of
NF-κB and its associated inflammatory factors
Western blot analysis indicated that the expression
levels and activity of NF-κB were elevated in cells treated with
H2O2 alone. Treatment with PF appeared to
significantly reduce this H2O2-induced NF-κB
activity (Fig. 4A). The levels of
total NF-κB protein displayed a marked reduction in cells treated
with PF (P<0.01). Further analysis indicated that the levels of
p-NF-κB (Ser536), the active form of NF-κB, were significantly
reduced by the PF treatment (P<0.01).
Inhibitory effect of PF on the
expression levels of TNF-α and IL-1β
Western blot analysis indicated that treatment with
PF reversed the H2O2-induced elevation of the
expression levels of IL-1β (P<0.01; Fig. 4C) and TNF-α (P<0.01; Fig. 4B) in the PC12 cells.
Discussion
PF is the main component of Paeoniae Radix
used in traditional Chinese medicine, and has been reported to
exhibit numerous pharmacological effects. The results of the
present study indicated that PF may protect PC12 cells from
H2O2-induced oxidative injury. PF was
observed to regulate H2O2-induced oxidative
stress, indicated by the modulation of LDH and ROS levels in PC12
cells. PF was also demonstrated to reduce
H2O2-induced apoptosis and promote overall
cell survival. Furthermore, the results indicated that PF
downregulated H2O2-induced neuroinflammation
by regulating NF-κB-associated inflammatory signals.
Oxidative stress has been extensively implicated in
the pathophysiology of cerebral ischemia and stroke (10). Hypoxia is a crucial initiator of the
loss of neurocytes and apoptosis is considered to be a pivotal
source of damage to neurocytes during this process (11). The accumulation of ROS may lead to
various forms of oxidative modification of proteins, lipids and
DNA, resulting in cellular damage (12). The present study demonstrated that
200 µM H2O2 was able to significantly
stimulate the accumulation of ROS and the release of LDH (203.1 and
270.1% of control values, respectively) in PC12 cells. Furthermore,
the cell survival rate in the H2O2 group was
58.6±2.4% of the control value.
PF treatment appeared to markedly improve these
oxidative conditions. The ROS levels in the 20, 40 and 80 µM PF
treatment groups were 171.8, 141.6 and 117.4% of the control group,
respectively. The LDH expression levels of the 20, 40 and 80 µM PF
treatment groups were 217.0, 175.1 and 133.8% of the control group,
respectively. These results indicated that the PF treatment
produced a significant reduction in
H2O2-induced toxicity and oxidative stress in
the PC12 cells.
ROS are widely recognized to be key mediators of
cell survival, proliferation, differentiation and apoptosis
(5,13,14).
Previous studies have demonstrated that proteins of the Bcl-2
family, including Bax and Bcl-2, are associated with apoptosis
induced by ROS-generating agents (Ji BS, Renaud, Pan). In addition,
ROS may activate caspase-3, which results in the cleavage of PARP,
a 116-kDa nuclear poly (ADP-ribose) polymerase, which appears to be
involved in DNA repair in response to environmental stress. PARP
may be cleaved by numerous caspase-1-like caspases in vitro
and is one of the primary cleavage targets of caspase-3 in
vivo. Furthermore, ROS may activate caspase-3, which results in
the cleavage of PARP into an 89-kDa fragment (6,15–17,20).
In the present study, a Hoechst 33258 staining assay indicated that
treatment with 200 µM H2O2 alone induced
notable cell apoptosis in PC12 cells, while 80 µM PF produced a
reduction in the extent of apoptosis-associated nuclear
fragmentation (Fig. 3A).
Furthermore, H2O2-induced apoptosis was
associated with an increase in the Bax:Bcl-2 ratio and with the
activation of caspase-3. Treatment with PF was observed to
downregulate the expression of the pro-apoptotic protein Bax, and
to upregulate the anti-apoptotic protein Bcl-2. The results of the
present study also demonstrated that caspase-3 and cleaved PARP
were modulated by PF treatment.
Oxidative stress-induced neuroinflammation has been
reported to be a vital factor in nerve injury and associated
diseases (5,8,21).
Numerous studies have suggested that chronic inflammation is
implicated in neurodegenerative disease and injury (1,4,21–23). A
number of well-established inflammatory target proteins, including
matrix metalloproteinase-9, cyclooxygenase-2, inducible nitric
oxide synthase and certain adhesion molecules have been associated
with ROS generation, which is also induced by proinflammatory
cytokines, peptides, peroxidants and infection (5,13,24,25).
Increasing inflammatory stress has been reported to correlate with
oxidative stress during the progression of neurodegenerative
disease (5,19,26).
NF-κB, a proinflammatory transcription factor, functions as the
‘first responder’ to various generators of cellular stress,
including free radicals and pro-inflammatory cytokines (e.g. TNF-α)
and bacterial biomolecules) (27).
In the present study, H2O2 was observed to
induce an inflammatory response involving NF-κB and its associated
signals. Following H2O2 treatment, the levels
of NF-κB and its active form, p-NF-κB (Ser536), were elevated, as
were the levels of TNF-α and IL-1β. However, cells cocultured with
80 µM PF exhibited reduced levels of these inflammatory factors,
indicating that PF modified the apoptotic process, in addition to
correcting the abnormal inflammatory signals induced by
H2O2.
In conclusion, PF treatment significantly reduced
H2O2-induced apoptosis and ROS accumulation,
promoted cell survival and downregulated neuroinflammation in PC12
cells. Thus, PF may serve as a protective agent against oxidative
stress and scavenger of intracellular ROS, and may offer a novel
pharmacological preventative or palliative treatment for ischemic
cerebral injury.
References
|
1
|
Liu HQ, Zhang WY, Luo XT, Ye Y and Zhu XZ:
Paeoniflorin attenuates neuroinflammation and dopaminergic
neurodegeneration in the MPTP model of Parkinsons disease by
activation of adenosine A1 receptor. Br J Pharmacol. 148:314–325.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu DZ, Xie KQ, Ji XQ, Ye Y, Jiang CL and
Zhu XZ: Neuroprotective effect of paeoniflorin on cerebral ischemic
rat by activating adenosine A1 receptor in a manner different from
its classical agonists. Br J Pharmacol. 146:604–611. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang LM, Liu IM and Cheng JT: Stimulatory
effect of paeoniflorin on adenosine release to increase the glucose
uptake into white adipocytes of Wistar rat. Planta Med. 69:332–336.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kapoor S: Neuroprotective effects of
paeoniflorin: An emerging concept in neurology. Folia Neuropathol.
51:922013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsieh HL and Yang CM: Role of redox
signaling in neuroinflammation and neurodegenerative diseases.
Biomed Res Int. 2013:4846132013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia BS and Gao Y: Protective effect of
trihexyphenidyl on hydrogen peroxide-induced oxidative damage in
PC12 cells. Neurosci Lett. 437:50–54. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dasgupta A, Zheng J and Bizzozero OA:
Protein carbonylation and aggregation precede neuronal apoptosis
induced by partial glutathione depletion. ASN Neuro. 4:e000842012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sandireddy R, Yerra VG, Areti A,
Komirishetty P and Kumar A: Neuroinflammation and oxidative stress
in diabetic neuropathy: Futuristic strategies based on these
targets. Int J Endocrinol. 2014:6749872014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Jin DZ, Xiao L and Zhu XZ:
Paeoniflorin attenuates chronic cerebral hypoperfusion-induced
learning dysfunction and brain damage in rats. Brain Res.
1089:162–170. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li RC, Morris MW, Lee SK, Pouranfar F,
Wang Y and Gozal D: Neuroglobin protects PC12 cells against
oxidative stress. Brain Res. 1190:159–166. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vilalta A and Brown GC: Deoxyglucose
prevents neurodegeneration in culture by eliminating microglia. J
Neuroinflammation. 11:582014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Giorgio M, Trinei M, Migliaccio E and
Pelicci PG: Hydrogen peroxide: A metabolic by-product or a common
mediator of ageing signals? Nat Rev Mol Cell Biol. 8:722–728. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dröge W: Free radicals in the
physiological control of cell function. Physiol Rev. 82:47–95.
2002.PubMed/NCBI
|
|
14
|
von Bernhardi R and Eugenín J: Alzheimers
disease: Redox dysregulation as a common denominator for diverse
pathogenic mechanisms. Antioxid Redox Signal. 16:974–1031. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang R, Zhang HY and Tang XC: Huperzine A
attenuates cognitive dysfunction and neuronal degeneration caused
by beta-amyloid protein-(1–40) in rat. Eur J Pharmacol.
421:149–156. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SY, Ha TY, Son DJ, Kim SR and Hong JT:
Effect of sesaminol glucosides on β-amyloid-induced PC12 cell death
through antioxidant mechanisms. Neurosci Res. 52:330–341. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shelton SN, Shawgo ME, Matthews SB, Lu Y,
Donnelly AC, Szabla K, Tanol M, et al: KU135, a novel
novobiocin-derived C-terminal inhibitor of the 90-kDa heat shock
protein, exerts potent antiproliferative effects in human leukemic
cells. Mol Pharmacol. 76:1314–1322. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Renaud J, Bournival J, Zottig X and
Martinoli MG: Resveratrol protects DAergic PC12 cells from high
glucose-induced oxidative stress and apoptosis: Effect on p53 and
GRP75 localization. Neurotox Res. 25:110–123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan C, Giraldo GS, Prentice H and Wu JY:
Taurine protection of PC12 cells against endoplasmic reticulum
stress induced by oxidative stress. J Biomed Sci. 17:(Suppl 1).
S172010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cheung HH, Lynn Kelly N, Liston P and
Korneluk RG: Involvement of caspase-2 and caspase-9 in endoplasmic
reticulum stress-induced apoptosis: A role for the IAPs. Exp Cell
Res. 312:2347–2357. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Di Napoli M and Shah IM: Neuroinflammation
and cerebrovascular disease in old age: A translational medicine
perspective. J Aging Res. 2011:8574842011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo RB, Wang GF, Zhao AP, Gu J, Sun XL and
Hu G: Paeoniflorin protects against ischemia-induced brain damages
in rats via inhibiting MAPKs/NF-κB-mediated inflammatory responses.
PLoS One. 7:e497012012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bettcher BM and Kramer JH: Inflammation
and clinical presentation in neurodegenerative disease: A volatile
relationship. Neurocase. 19:182–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee IT and Yang CM: Role of NADPH
oxidase/ROS in pro-inflammatory mediators-induced airway and
pulmonary diseases. Biochem Pharmacol. 84:581–90. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiurchiù V and Maccarrone M: Chronic
inflammatory disorders and their redox control: From molecular
mechanisms to therapeutic opportunities. Antioxid Redox Signal.
15:2605–2641. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Urrutia PJ, Mena NP and Núñez MT: The
interplay between iron accumulation, mitochondrial dysfunction, and
inflammation during the execution step of neurodegenerative
disorders. Front Pharmacol. 5:382014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu HP, Gao ZH, Cui SX, Sun DF, Wang Y,
Zhao CR, Lou HX and Qu XJ: Inhibition of intestinal adenoma
formation in APC(Min/+) mice by Riccardin D, a natural product
derived from liverwort plant Dumortiera hirsuta. PLoS One.
7:e332432012. View Article : Google Scholar : PubMed/NCBI
|