Introduction
Cardiovascular diseases have become the leading
cause of disability and mortality worldwide (1). Cardiac fibrosis is an important
pathological and physiological response to various diseases (at the
middle and end stages), including heart failure, hypertrophic
cardiomyopathy, myocardial infarction and malignant arrhythmias,
and it is a challenge in current clinical therapy (1). A growing body of evidence indicates
that cardiac fibroblasts (CFs) are the main cells responsible for
the cardiac fibrosis process. Under the impact of a variety of cell
stimulating factors, CFs promote an increasing influx of external
ions. This leads to pathologic hyperplasia and pathological
differentiation, the formation of cellular matrix-like collagen and
the initiation of the tissue fibrosis process. Therefore, the
identification of methods to enable early intervention in the
function of CFs, in order to block the onset and delay the
development of cardiac fibrosis, has become a key issue for study
in recent decades (2,3).
A type of Ca2+-permeable channel with a
high expression level of transient receptor potential melastatin 7
(TRPM7) has been found in the cell membranes of Sprague-Dawley (SD)
rats (4,5). Previous studies have shown that TRPM7
is the only calcium channel expressed on the cell membrane of CFs
(6) and that Ca2+
signaling is closely associated with the initiation of fibrosis
(7–9). These findings strongly suggest that
TRPM7 may be the one of the most potent fibrosis factors, playing
an important role in the molecular mechanism and pathological and
physiological processes by which angiotensin II (Ang II) induces
cardiac fibrosis. Therefore, it is hypothesized that TRPM7 is
expressed at high levels when stimulated by Ang II. Ca2+
signaling on the cell membrane of CFs may be mediated by the
stimulatory effect of Ang II on TRPM7, causing collagen synthesis
in the CFs to increase and leading to the fibrosis of cardiac
interstitial tissue.
Materials and methods
Subjects
SD rats (<3 days old) were purchased from Wuhan
University Center for Animal Experiment/Animal Biological Safety
Level-3 Laboratory (Wuhan, China). The study was approved by the
Medical Ethics Committee of Renmin Hospital of Wuhan
University.
Cell culture
The tissue method of Runnels et al (10) was adopted to separate and collect the
rat CFs. Following resuspension in the culture solution [Dulbecco's
modified Eagle's medium (DMEM)-F12 (Invitrogen Life Technologies,
Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Santa
Cruz Biotechnology, Inc., Dallas, TX, USA)], CFs were collected
using the method of differential sedimentation and inoculated in a
culture dish. The cells were then synchronized by the starvation
method in fetal bovine serum-free medium and placed in an incubator
for subculture at 37°C and 5% CO2. The culture solution
(containing mycillin and DMEM-F-12] was replaced once every 36 h.
When the cell density was ≥90%, subculture was conducted by
digesting cells with 0.25% trypsin and 0.5 mM EDTA. One agar plate
was retained for the Ang II treatment group and the establishment
of the normal control group.
Cell interventions: Determination of
the concentration of Ang II having the optimal fibrosis-inducing
effect
i) Treatment. The cultured CFs were separated into
six groups, and DMEM culture solution (containing 10% fetal calf
serum) was added along with 0, 1, 5, 10, 100 or 1,000 nmol/l Ang
II. After 12 h (37°C), the expression levels of collagen I and
collagen III proteins in each group were measured by western
blotting and the group in which the concentration of Ang II had the
greatest inducement effect for fibrosis was selected.
ii) Western blotting. Western blotting technology
was adopted to evaluate the expression levels of collagen I and
collagen III proteins in each group. The total cellular protein was
extracted using a protein extraction kit in accordance with the
manufacturer's instructions (Beyotime Institute of Biotechnology,
Wuhan, China). The Bradford method was used to determine the
protein concentration and equalize the protein concentrations in
the samples of the different groups through adjustment.
Electrophoresis by the SDS-PAGE technique was conducted based on 50
µg total protein/lane loading using electrophoresis equipment from
Beijing Liuyi Instrument Factory (Beijing, China) and an SDS-PAGE
gel preparation regulator from Wuhan Huge Biotechnology Co., Ltd.
(Wuhan, China). Following the electrophoresis, the protein on the
gel was transferred onto a polyvinylidene fluoride membrane. The
gel was sealed with 5% skimmed milk powder [prepared with 0.5%
Tris-buffered saline-Tween® (TBST)] at room temperature for 1 h.
Rabbit polyclonal antibodies (1:500; Abcam, Cambridge, UK) against
collagen I (cat. no. ab34710) and collagen III (cat. no. ab7778)
were added following membrane rinsing and the membrane was
incubated at 4°C overnight. The membrane was then incubated at room
temperature for 30 min with goat anti-rabbit secondary antibody
(1:3,000; cat. no. 074–1506; KPL, Inc., Gaithersburg, MD, USA) and
washed three times (5 min each time) in a gyratory shaker at room
temperature with TBST. Finally, fixation and color development with
3,3′-diaminobenzidine was conducted in a dark chamber. The
conditions of exposure were adjusted based on the luminous
intensity; the photographic film was scanned and filed; and
AlphaEaseFC software (Alpha Innotech, San Leandro, CA, USA) was
used to analyze the optical density of the blots.
Recording the CF TRPM7 current
i) Treatment. The cultured CFs were separated into
five groups and treated for 0, 6, 12, 24 and 48 h with the optimal
concentration of Ang II for inducing fibrosis. The TRPM7 current
was determined for the five time-points as described below. In
addition, the collagen and TRPM7 protein levels of the cells were
determined using the previously described western blotting
technique (anti-TRPM7; cat. no. ab109438; Abcam).
ii) Electrophysiological recording. The Axopatch
200B amplifier (Molecular Devices, LLC, Sunnyvale, CA, USA) was
used to record the whole-cell currents of the CFs. The ramp
stimulus procedure was adopted with a stimulating voltage of
120±100 mV. The internal fluid (11)
in the electrode included: 145 mmol/l cesium mesilate; 8 mmol/l
sodium chloride; 10 mmol/l ethylene glycol tetra-acetic acid and 10
mmol/l hydroxyethylpiperazine ethane sulfonic acid (Sinopharm,
Shanghai, China). The pH value was adjusted to 7.2 with sodium
hydroxide solution. In certain experiments, 3 mmol/l
Mg2+ was added to the internal fluid (calculated by
MaxChelator software; http://maxchelator.stanford.edu). The standard
extracellular Tyrode solution was composed of 140 mmol/l sodium
chloride, 5 mmol/l potassium chloride, 2 mmol/l calcium chloride,
20 mmol/l hydroxyethylpiperazine ethane sulfonic acid and 10 mmol/l
glucose (Sinopharm). The pH value was adjusted to 7.4 with sodium
hydroxide solution.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons among groups were conducted with a Student's t-test on
two independent samples. Statistical analysis was conducted using
SPSS software (version 16.0; SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Microscopic examination of the
cultured CFs
During microscopic examination, the CFs were
observed to be elongated and spindle-like. They grew with a
homogeneous distribution and arranged themselves into garland-like
or roller forms. No cardiac impulse was observed (Fig. 1).
Protein expression levels in CFs
following treatment for 12 h with Ang II at various
concentrations
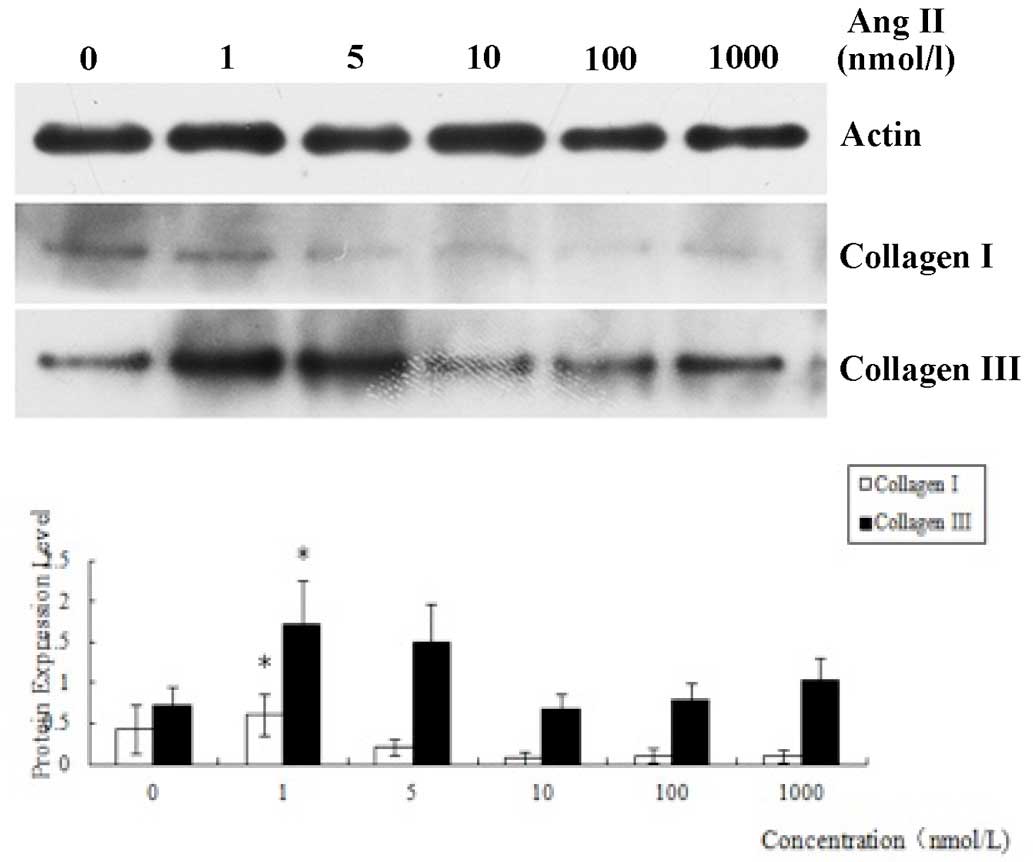
The expression levels of collagen I and collagen III
in the CFs treated for 12 h with Ang II at different concentrations
were determined by western blotting. Analysis of the results
indicated that the expression levels of the two types of collagen
induced by Ang II (1 nmol/l) were increased significantly compared
with those in the other groups (P<0.05), which indicated that
the concentration of Ang II having the best inducement effect for
fibrosis was 1 nmol/l (Fig. 2).
Protein expression levels of collagen
I, collagen III and TRPM7 in CFs following treatment with Ang II
for different time periods
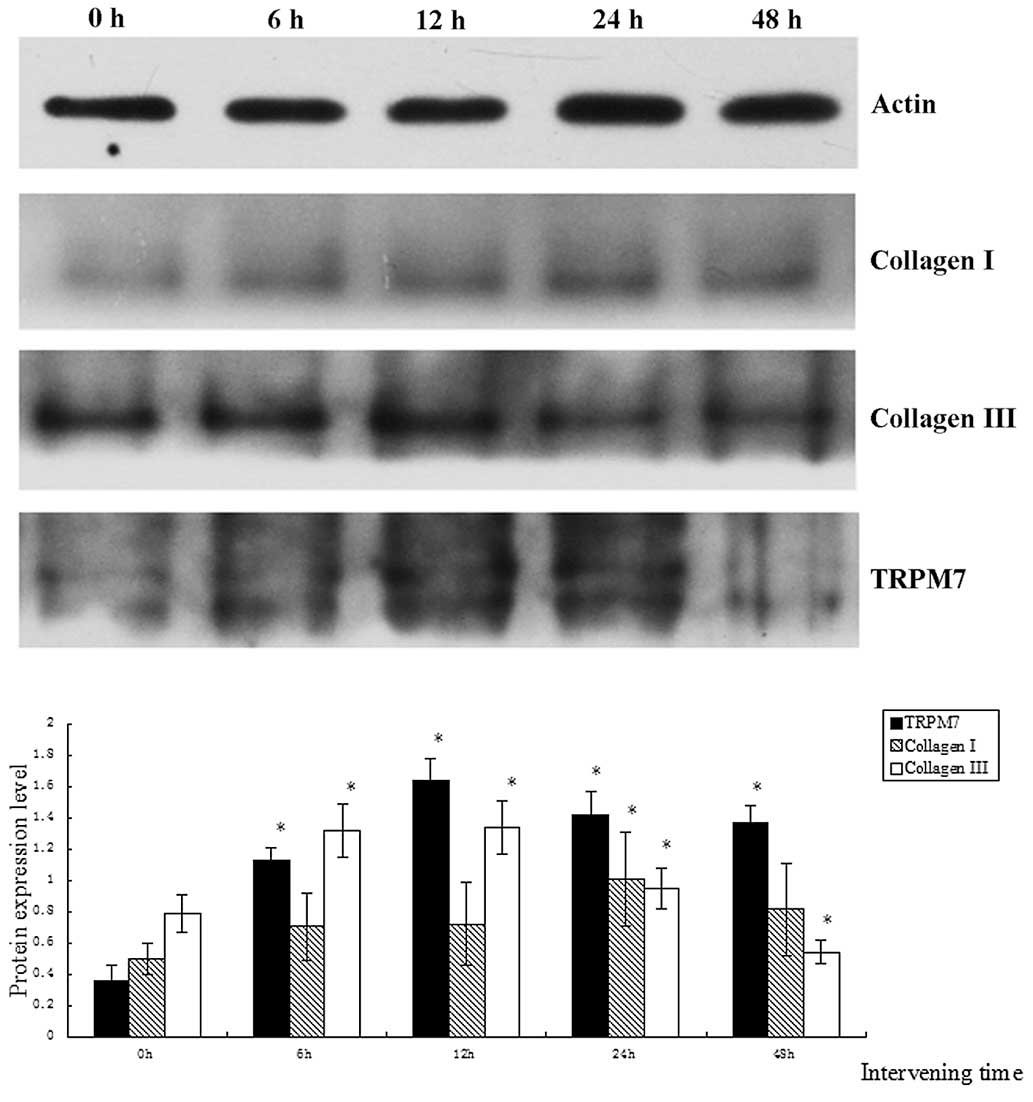
The protein expression levels of collagen III and
TRPM7 gradually increased over time and reached a maximum when the
duration of treatment was 12 h, gradually decreasing subsequently
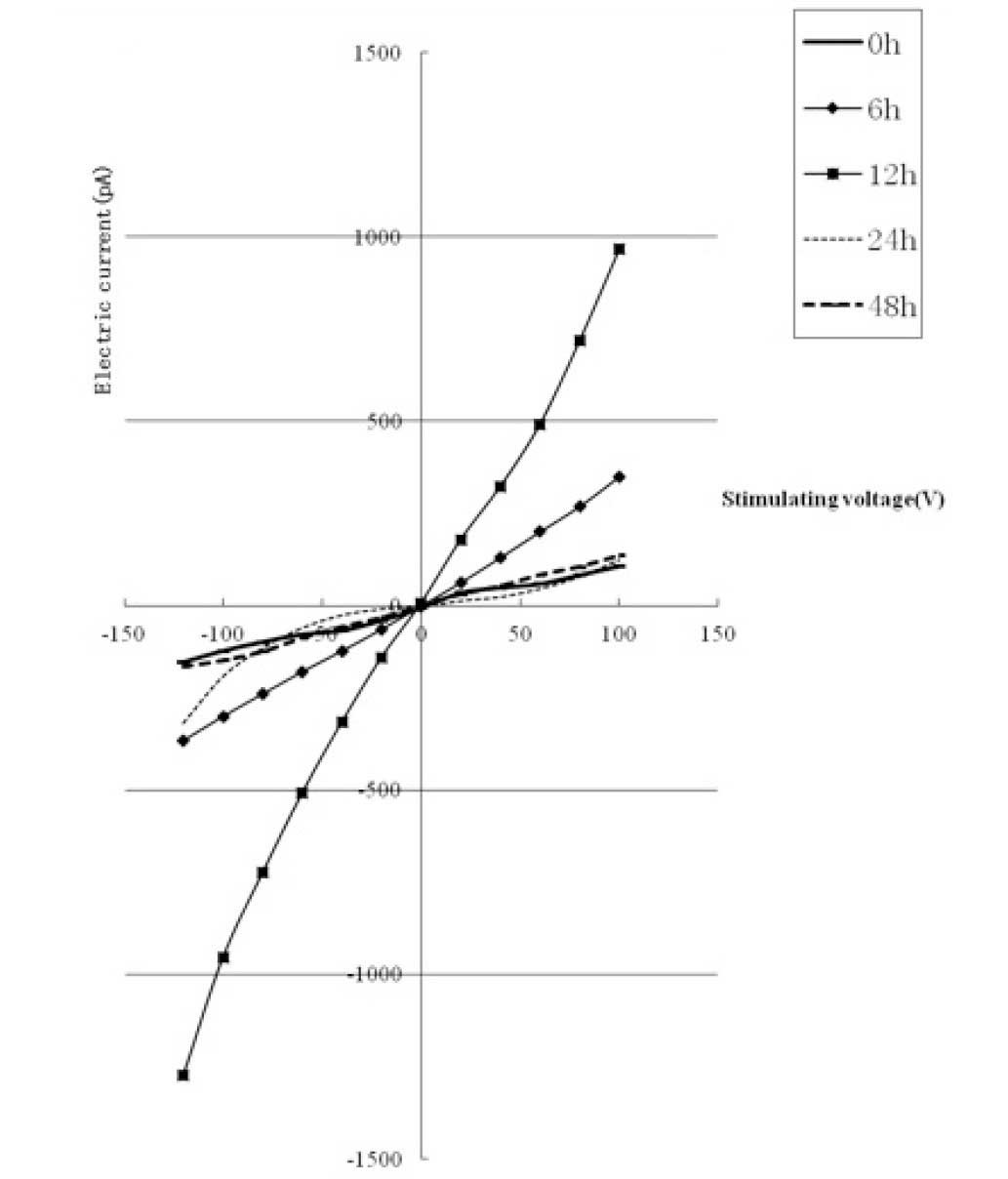
(Fig. 3). The TRPM7 current
underwent corresponding changes (gradually increasing and then
decreasing with time) and reached a maximum (8-fold higher than
that of the 0 h group) when the duration of treatment was 12 h (as
shown in Fig. 4). The protein
expression level of collagen III was lower than that of the control
group when the duration of intervention was 48 h (P<0.05). The
protein expression level of TRPM7 remained higher than that of the
control group (P<0.05). The protein expression level of collagen
I reached a maximum when the duration of intervention was 24 h, and
was significantly different from that in the other groups
(P<0.05).
Discussion
TRPM7, belonging to the TRP superfamily, is an ion
channel with dual channel and kinase activity (10). Its expression enables inward
rectifier currents of Ca2+ to permeate cell membranes.
The current amplitude rises with the consumption of Mg2+
inside the cell and is inhibited by other bivalent ions. TRPM7 is
activated by protein kinase A and deactivated by
phosphatidylinositol 4,5-bisphosphate (10). Previous studies have shown that TRPM7
plays a significant role in the growth and apoptosis of
histiocytes, is activated by mechanical stress and is associated
with vasodilation (12–14). It has been reported that the
expression of TRPM7 by vascular smooth muscle cells is
significantly upregulated when activated by transforming growth
factor (TGF)-β1 and fluid flow (15). The expression of TRPM7 in CFs has
previously been verified by immune cytochemistry (16). Two characteristics of TRPM7 channels,
specifically, that they are non-voltage-gated and have
Ca2+ permeability, suggest that they may have
significant pathological and physiological functions in various
cells, particularly in non-excitable cells such as CFs.
Preliminary research suggested that the TRPM7 ion
channel is the molecular basis of the TRPM currents observed in our
previous studies (4,5). It also suggested that the resting
membrane potential level of CFs is −25 mV, under which all
voltage-gated channels are in the deactivation state. This further
supports TRPM7 being the only channel for the inward flow of
Ca2+ in CFs. Under the impact of endogenous and
exogenous stimulating factors, TRPM7 channels would open, leading
to a considerable flow of extracellular Ca2+ into the
cell. This flow is likely to cause collagen synthesis to increase,
leading to fibrosis. Furthermore, the considerable inward flow of
Ca2+ would activate signaling pathways such as the
mitogen-activated protein kinase (MAPK) and TGF-β/Smad pathways,
causing inflammation, apoptosis and cellular differentiation
leading to an acceleration of fibrosis.
During one preliminary clinical study, it was found
that the myocardial tissues of patients suffering from atrial
fibrillation had a certain degree of fibrosis (17); another preliminary clinical study
showed that in the myocardial tissues of patients suffering from
atrial fibrillation due to rheumatic heart disease, the mRNA and
protein expression levels of TRPM7 rose significantly compared with
those of individuals with sinus rhythm (18). These studies suggest that under the
impact of abnormal stimulation, such as an increased volume and
pressure load, TRPM7, the only Ca2+ channel protein on
CFs would be activated and its expression increased. This would
increase the internal flow of extracellular Ca2+ to the
cell to activate and launch biological signaling cascades leading
to the pathological transfer, differentiation and secretion of a
large quantity of extracellular matrix, finally giving rise to
cardiac interstitial fibrosis.
The primary causes of fibrosis include the
disequilibrium between pro-fibrogenic cell growth factors and
anti-fibrogenic cell growth factors. Studies on myocardial fibrosis
have gradually focused on cell growth factors, which include TGF,
connective tissue growth factor, basic fibroblast growth factor and
hepatocyte growth factor (8,19,20). Ang
II, frequently researched in recent years, is the pro-fibrogenic
cell growth factor (8). Ang II plays
a significant role in the occurrence and development of organ
fibrosis. Through protein quantification, the present study
verified that the concentration of Ang II having the greatest
inducement effect for fibrosis was 1 nmol/l. The results also
showed that Ang II at various concentrations had different effects
on the growth of CFs: the effect of Ang II on CFs was
concentration-dependent. The effect on the growth of CFs decreased
as the concentration continued to rise; this may be associated with
an increase in the induction of apoptosis and necrosis of the
cells. The protein expression levels of TRPM7 and collagen III
obtained by treating CFs for 0, 6, 12, 24 and 48 h with the optimum
concentration of Ang II increased at first and reached a maximum
when the duration of intervention was 12 h (P<0.05) and then
decreased gradually. The fact that the inward and outward currents
of TRPM7 recorded on the CF cell membranes also took on
corresponding trends in changes (first increasing and then
decreasing) showed that the influence of Ang II on the cell is
time-dependent to some extent. In the present study, the protein
expression of collagen I also took on the same change trend (first
increasing and then decreasing), but it reached a maximum when the
duration of intervention was 24 h, and then the expression
decreased and took on the same change trend as was observed for the
other two proteins.
In conclusion, the present study demonstrated that
under the effect of Ang II at the concentration that had the
maximum inducement effect on fibrosis, the protein expression level
of TRPM7 by the CFs increased, the internal flow of Ca2+
increased and collagens I and III were expressed, promoting
fibrosis. When the exposure to Ang II was prolonged, the induction
of CFs to undergo apoptosis and necrosis increased, which may have
given rise to various inflammatory reactions and further promoted
fibrosis. The limitation of this study lies in that the experiment
was conducted in vitro; therefore, it is necessary to
conduct in vivo experiments with CFs to further verify the
hypothesis that Ang II leads to cardiac fibrosis by mediating TRPM7
on the CF cell membrane.
Acknowledgements
This study was financially supported by the National
Natural Science Grant of China (No. 81170085). The authors are
grateful to the staff members at their institutions for their
valuable comments.
References
|
1
|
Brown RD, Ambler SK, Mitchell MD and Long
CS: The cardiac fibroblast: therapeutic target in myocardial
remodeling and failure. Annu Rev Pharmacol Toxicol. 45:657–687.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Camelliti P, Borg TK and Kohl P:
Structural and functional characterisation of cardiac fibroblasts.
Cardiovasc Res. 65:40–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pawlinski R, Fernandes A, Kehrle B, et al:
Tissue factor deficiency causes cardiac fibrosis and left
ventricular dysfunction. Proc Natl Acad Sci USA. 99:15333–15338.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jiang J, Li M and Yue L: Potentiation of
TRPM7 inward currents by protons. J Gen Physiol. 126:137–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li M, Jiang J and Yue L: Functional
characterization of homo- and heteromeric channel kinases TRPM6 and
TRPM7. J Gen Physiol. 127:525–537. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang YH, Sun HY, Chen KH, Du XL, Liu B,
Cheng LC, Li X, Jin MW and Li GR: Evidence for functional
expression of TRPM7 channels in human atrial myocytes. Basic Res
Cardiol. 107:2822012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
González A, López B and Díez J: Fibrosis
in hypertensive heart disease: role of the
renin-angiotensin-aldosterone system. Med Clin North Am. 88:83–97.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olson ER, Shamhart PE, Naugle JE and
Meszaros JG: Angiotensin II-induced extracellular signal-regulated
kinase 1/2 activation is mediated by protein kinase Cdelta and
intracellular calcium in adult rat cardiac fibroblasts.
Hypertension. 51:704–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manabe I, Shindo T and Nagai R: Gene
expression in fibroblasts and fibrosis: Involvement in cardiac
hypertrophy. Circ Res. 91:1103–1113. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Runnels LW, Yue L and Clapham DE: The
TRPM7 channel is inactivated by PIP (2) hydrolysis. Nat Cell Biol.
4:329–336. 2002.PubMed/NCBI
|
|
11
|
Jiang J, Li M and Yue L: Potentiation of
TRPM7 inward currents by protons. J Gen Physiol. 126:137–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baldoli E, Castiglioni S and Maier JA:
Regulation and function of TRPM7 in human endothelial cells: TRPM7
as a potential novel regulator of endothelial function. PLoS One.
8:e598912013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen YF, Chen YT, Chiu WT and Shen MR:
Remodeling of calcium signaling in tumor progression. J Biomed Sci.
20:232013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Simon F, Varela D and Cabello-Verrugio C:
Oxidative stress-modulated TRPM ion channels in cell dysfunction
and pathological conditions in humans. Cell Signal. 25:1614–1624.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park HS, Hong C, Kim BJ and So I: The
pathophysiologic roles of TRPM7 channel. Korean J Physiol
Pharmacol. 18:15–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yue Z, Zhang Y, Xie J, Jiang J and Yue L:
Transient receptor potential (TRP) channels and cardiac fibrosis.
Curr Top Med Chem. 13:270–282. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yi X, Li M, Ma L, et al: Relationship of
CTGF, HGF and atrial fibrosis in rheumatic heart disease patients
with atrial fibrillation. Wuhan Da Xue Xue Bao. Yi Xue Ban.
33:824–828. 2012.[(In Chinese)].
|
|
18
|
Li M, Yi X, Ma L, et al: Relationship of
TRPM7 and atrial fibrosis in rheumatic heart disease patients with
atrial fibrillation. Wuhan Da Xue Xue Bao. Yi Xue Ban. 33:875–878.
2012.[(In Chinese)].
|
|
19
|
Yi X, Li X, Zhou Y, et al: Hepatocyte
growth factor regulates the TGF-β1-induced proliferation,
differentiation and secretory function of cardiac fibroblasts. Int
J Mol Med. 34:381–390. 2014.PubMed/NCBI
|
|
20
|
Kinoshita T, Ishikawa Y, Arita M, et al:
Antifibrotic response of cardiac fibroblasts in hypertensive hearts
through enhanced TIMP-1 expression by basic fibroblast growth
factor. Cardiovasc Pathol. 23:92–100. 2014. View Article : Google Scholar : PubMed/NCBI
|