Introduction
Current therapeutic approaches for ischemic stroke
mainly focus on the re-canalization of occluded blood vessels in
the brain; however, reperfusion following thrombolytic therapy
induces a cascade of pathological events, including an inflammatory
response and blood-brain barrier (BBB) breakdown (1,2). BBB
injury is the key event that leads to vascular edema and hemorrhage
transformation, two high-risk factors for intracranial hypertension
and brain death (1,3,4). Thus,
preventing BBB breakdown is a promising strategy to halt the
progression of ischemic stroke.
In Traditional Chinese Medicine, Ligusticum
wallichii Franchat. (Chuan Xiong) is widely used in the
treatment of cardiovascular and neurovascular diseases.
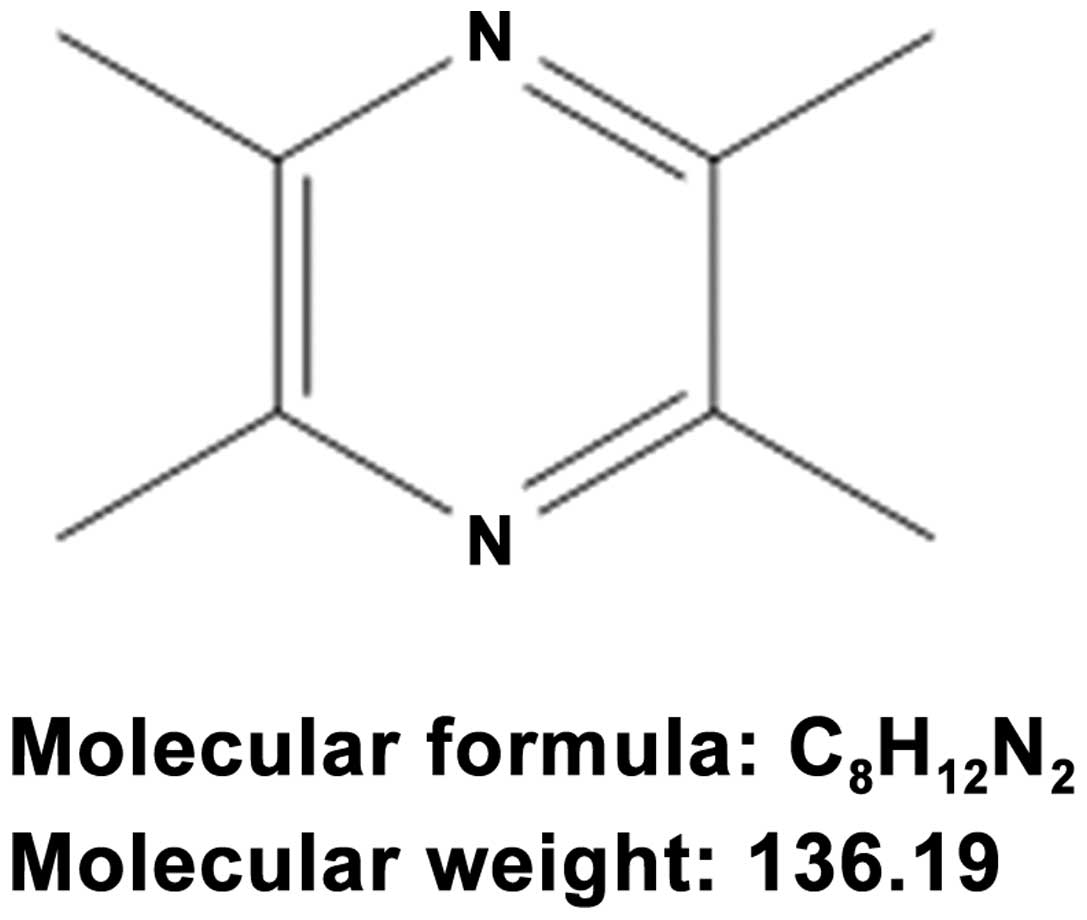
Ligustrazine (Fig. 1), also known as
2,3,5,6-tetramethylpyrozine (TMP), is one of the most important
active ingredients of Chuan Xiong (5). The compound can penetrate BBB and can
be found throughout the brain. Chuan Xiong and TMP are widely
applied by Chinese herbalists to treat ischemic stroke (6). Several animal studies have demonstrated
that TMP is capable of reducing the infarct volume, neurological
score and brain edema in the model of permanent and temporal
cerebral ischemia injuries (7–11). TMP
also reduces cellular inflammatory responses that follow brain
ischemia (11) and enhances
neurogenesis and spatial cognition during ischemic injury (8). The neuroprotective effect of TMP has
been confirmed by multiple in vitro studies (12,13). TMP
has been shown to have neuroprotective effects against Parkinson's
disease (14), neuropathic pain
(15) and spinal cord ischemia
(16).
With regard to the mechanism underlying the
TMP-induced neuroprotection, TMP has been reported to possess
diverse pharmacological properties, including free radical
scavenging and the inhibition of cytokine production and release
(17–19). The inflammatory response and free
radical production during cerebral ischemia and reperfusion are the
main causes of BBB breakdown. Thus, it is possible that TMP could
protect the BBB during cerebral ischemia and reperfusion, due to
its association with free radical scavenging and inflammatory
inhibition. In the present study, a classical brain ischemia and
reperfusion model was utilized to investigate the neuroprotective
and BBB-protective role of TMP. The relevant mechanisms involved in
the effects of TMP were additionally investigated.
Materials and methods
Ethics statement
Male adult Sprague Dawley rats (250–270 g) were
obtained from the Animal Center of the Guangzhou University of
Chinese Medicine (Guangzhou, China). The present study was
conducted in strict accordance with the international ethical
guidelines and the Guide for the Care and Use of Laboratory Animals
by the National Institutes of Health. All animal experiments were
approved by the Institutional Animal Care and Use Committee of
Guangzhou University of Chinese Medicine. Every effort was made to
minimize the number and suffering of the animals used.
Middle cerebral artery occlusion
(MCAO) and drug treatment
MCAO was selected as the model for cerebral
ischemia/reperfusion (20). Briefly,
anesthesia was induced in the rats through the inhalation of 5%
isoflurane and maintained with 2% isoflurane in a mixture of 70%
N2O and 30% O2. Following vessel isolation, a
3-0 monofilament nylon suture (Johnson & Johnson, New
Brunswick, NJ, USA) was inserted into the external carotid artery
and then threaded forward into the internal carotid artery and
anterior cerebral artery to occlude the MCA. Following the surgery,
the rats were transferred to an intensive care incubator where the
temperature was maintained at 37°C until the animals regained
consciousness. The suture was removed following a 1.5-h occlusion
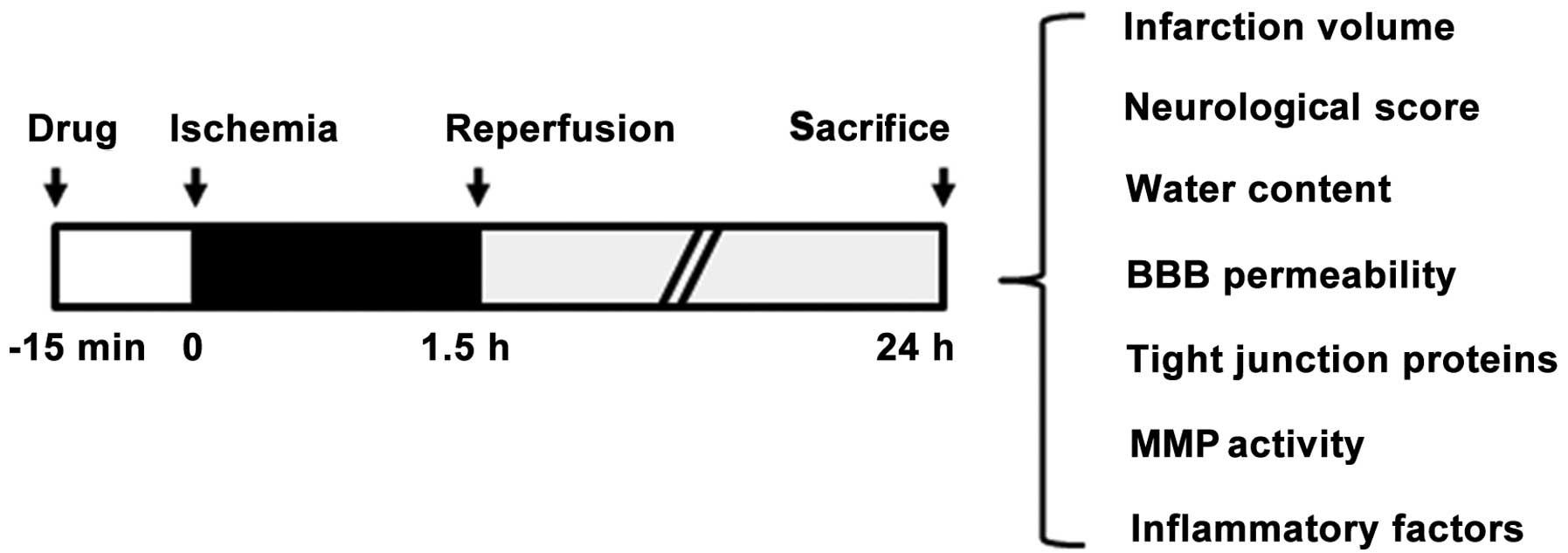
period, in order to induce reperfusion. TMP (20 mg/kg; Forever
Biotech, Shanghai, China) (Fig. 1)
or a saline vehicle was intraperitoneally injected into the rats 15
min before suture insertion. Following 22.5 h of reperfusion, each
rat brain was isolated and subjected to infarct volume, BBB
permeability and brain edema measurement, as well as protein
preparation for western blotting and the determination of matrix
metalloproteinase (MMP) activity (Fig.
2).
Measurement of infarct volume, water
content and neurological score
Following reperfusion, each rat brain was isolated
and sliced into 5 coronal sections (2-mm), which were stained with
2,3,5-triphenyltetrazolium chloride (Sigma, St. Louis, MO, USA).
The evaluation of the infarct area was accomplished by calculating
the hemispheric lesion area with ImageJ-1.38x software (National
Institutes of Health, Bethesda, MD, USA). The relative infarct
volume percentage (RIVP) was calculated using the following
formula: RIVP = IVA - TA × 100% (IVA, total infracted area of the 5
coronal sections; TA, total area of the 5 sections).
Neurological evaluation was performed at 3 h after
occlusion and scored as follows: 0, no neurological deficit; 1,
difficulty in fully extending the left forepaw; 2, unable to extend
the left forepaw; 3, mild circling to the left; 4, severe circling
to the left; and 5, falling to the left.
In order for the brain water content to be
determined, the isolated brain samples were dried in an oven at
110°C for 24 h, and the water content of these samples was
subsequently evaluated using the following formula: Water content
(%) = [wet weight - dry weight] - wet weight × 100.
BBB permeability evaluation
BBB permeability was evaluated by measuring the
Evans blue dye extravasation in accordance with a previously
described method with certain modifications (21). Briefly, 2% Evans blue was injected
intravenously (2 ml/kg, Sigma) 0.5 h before sacrifice; transcardial
saline perfusion was then performed for the removal of all
intravascular dye from the vessels, until the drainage was
colorless. The ipsilateral hemisphere was then removed and
incubated for 24 h in N,N′-dimethyl formamide (Sigma) in a water
bath at 60°C, and a spectrophometer (Analytik Jena AG, Jena,
Germany) was used to measure the Evans blue content in the
supernatants at a wavelength of 632 nm. Rats were euthanized after
22.5 h of reperfusion, by an intraperitoneal injection of
pentobarbitone (200 mg/ml).
In situ zymography
The kit used for the analysis of the gelatinolytic
activities of MMP-2 and -9 in cryosections from brain tissues by
in situ zymography was the EnzChek® collagenase kit
(Invitrogen Life Technologies, Carlsbad, CA, USA); the
manufacturer's instructions were followed. Brain coronal
cryosections were incubated in a reaction buffer, which contained
40 µg/ml fluorescein isothiocyanate-labeled dye-quenched
(DQ)-gelatin, at 37°C for 2 h. Gelatinases cleaved the DQ-gelatin
to yield the peptides and form fluorescence, which was
representative of the net gelatinolytic activity. A fluorescence
microscope (Carl Zeiss AG, Oberkochen, Germany) was used to examine
the fluorescent imaging.
Western blot analysis
Denatured protein samples were resolved using
SDS-PAGE and transferred to polyvinylidene difluoride membrane
(Millipore, Billerica, MA, USA). Subsequent to blocking, the
membrane was incubated overnight at 4°C, with rabbit polyclonal
anti-MMP-9 (#3852; 1:500; Cell Signaling Technology, Inc., Boston,
MA, USA) and anti-occludin (#71-1500; 1:1,000; Invitrogen Life
Technologies), and mouse monoclonal anti-claudin-5 (#4C3C2; 1:500;
Invitrogen Life Technologies) and anti-β-actin (#sc-47778; 1:2,000;
Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) primary
antibodies. The membrane was then incubated with goat anti-mouse or
anti-rabbit horseradish peroxidase-conjugated secondary antibodies
(1:2,000; Santa Cruz Biotechnology Inc.). ECL Advance™ western
blotting detection reagents (GE Healthcare, Little Chalfont, UK)
were used to perform immunodetection.
Statistical analysis
Data are expressed as the mean ± standard deviation.
For two-group experiments, comparisons were made using an unpaired
Student's t-test. SPSS 16.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for the statistical analysis. P<0.05
was considered to indicate a statistically significant
difference.
Results
Reduced infarct volume, neurological
score and brain edema in a rat model of cerebral
ischemia/reperfusion injury following TMP treatment
The first experiment was performed to investigate
the neuroprotective effect of TMP during cerebral ischemia and
reperfusion. As shown in Fig. 3A and
B, the brains subjected to ischemia/reperfusion injury
exhibited a maximal infarct volume of 31.2±4.14%, whereas following
treatment with TMP that volume dropped to 18.9±3.75% (P<0.01).
TMP was also found to reduce the neurological score and brain edema
in brains with ischemia/reperfusion injury (P<0.05, Fig. 3C and D). These results reveal that
TMP plays a neuroprotective role against cerebral
ischemia/reperfusion injury.
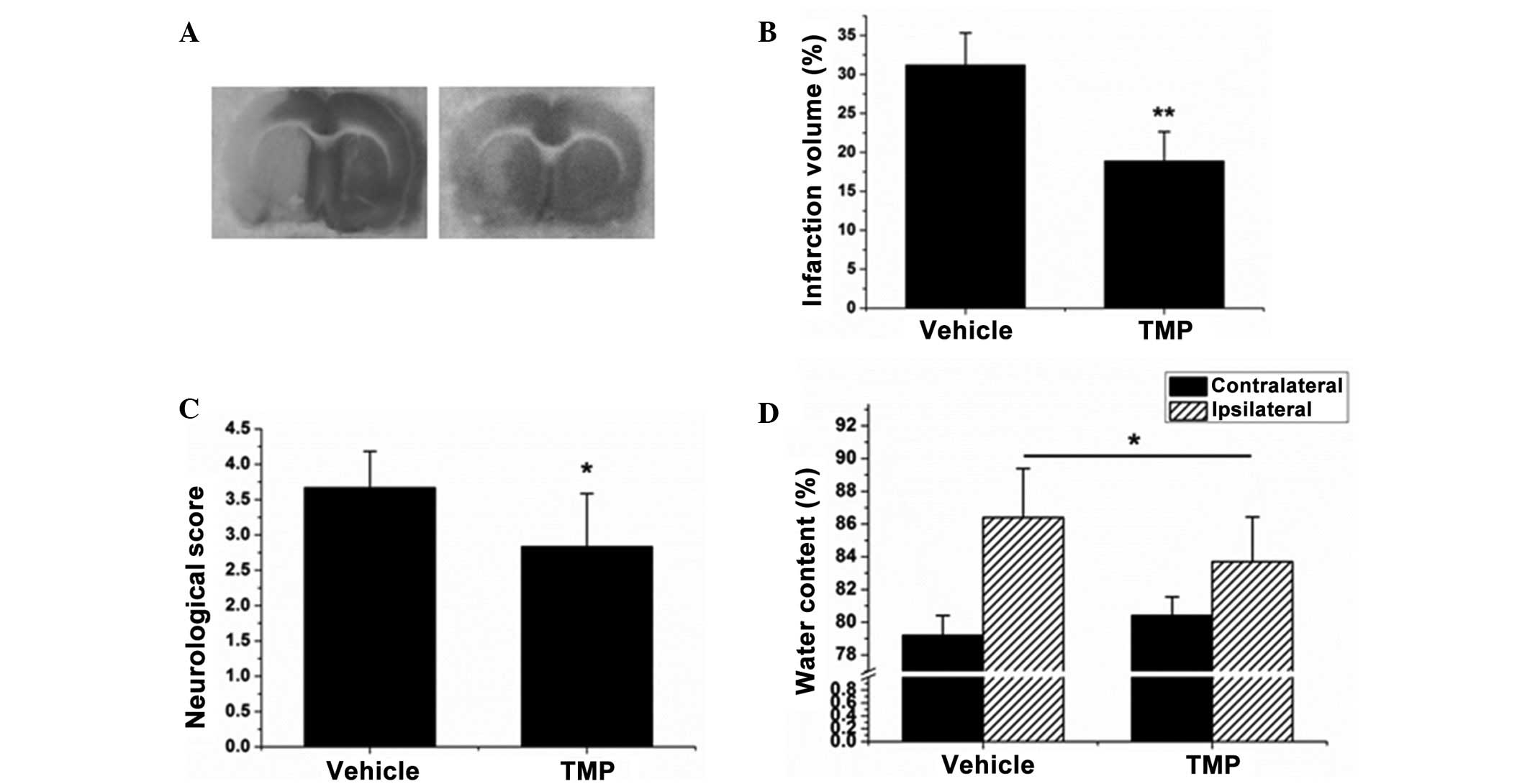 | Figure 3.TMP reduces the infarct volume,
neurological score and brain edema during cerebral ischemia and
reperfusion in rats. (A) The infarct volume of each treatment group
was assayed by 2,3,5-triphenyltetrazolium chloride staining. (B)
The relative infarct volume percentage was calculated with
ImageJ-1.38x software (National Institutes of Health, Bethesda, MD,
USA) and presented as a bar graph. (C) The neurological score of
each rat was determined and recorded. (D) The water content was
calculated according to the difference between the wet and dry
weight, and presented as a bar graph. Data are expressed as the
mean ± standard deviation, n=8. **P<0.01, *P<0.05. TMP,
2,3,5,6-tetramethylpyrazine. |
Decreased BBB permeability during rat
cerebral ischemia and reperfusion following TMP treatment
BBB leakage is the characteristic feature of
cerebral ischemia/reperfusion injury, contributing to the formation
of brain edema and hemorrhage. Based on the effect of the
TMP-induced reduction in brain edema in rats with cerebral
ischemia/reperfusion injuries, the next experiment was performed to
examine the effect of TMP on BBB integrity. The results
demonstrated that TMP significantly reduced the BBB permeability
(from 9.49±1.97 to 4.82±1.14 µg/g brain) (P<0.01, Fig. 4A). The impairment of tight junctions
plays the key role in BBB opening, and it was observed that the
expression of occludin and claudin-5, two tight junction proteins,
was significantly elevated in the ischemia/reperfusion-injured
brains in the TMP treatment group compared with that in the vehicle
group (P<0.05, Fig. 4B and C).
These data indicate that TMP can significantly reduce BBB
permeability during cerebral ischemia/reperfusion injury.
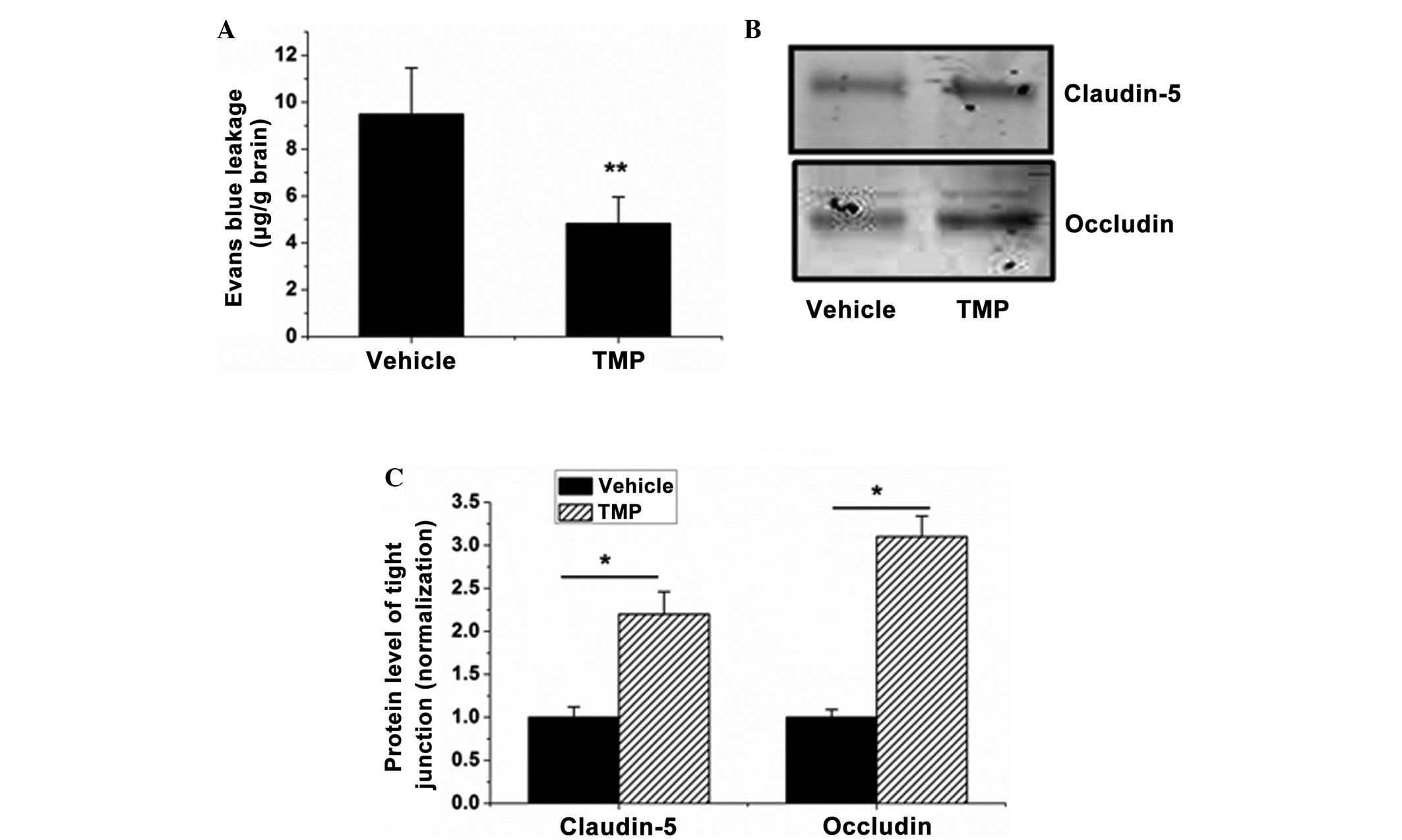 | Figure 4.TMP decreases BBB permeability and
tight junction impairment in brains with cerebral
ischemia/reperfusion injury. (A) BBB permeability in each group was
evaluated by measuring the Evans blue extravasation. (B) Western
blot analysis was used to determine the expression of occludin and
claudin-5 in the protein samples of the brains isolated from the
TMP- or saline-treated rats. (C) The blots of occludin and
claudin-5 were quantified with ImageJ-1.38x software (National
Institutes of Health, Bethesda, MD, USA) and presented in the bar
graph. Data are expressed as the mean ± standard deviation, n=6.
**P<0.01, *P<0.05. TMP, 2,3,5,6-tetramethylpyrazine; BBB,
blood-brain barrier. |
Decreased MMP activity during rat
cerebral ischemia and reperfusion following TMP treatment
MMPs are a group of zinc-dependent proteinases
responsible for the matrix and tight junction degradation (22). MMP activation and the secondary
impairment of tight junction proteins are the main causes of BBB
leakage. In order to study the relevant mechanisms involved in BBB
protection, the MMP activities in brain sections and the MMP-9
expression were observed. Fig. 5A and
B show that TMP treatment reduced the protein level of MMP-9.
Correspondingly, TMP markedly reduced the elevated MMP activity in
the brains of rats subjected to MCAO, as shown by in situ
zymography with a fluorescence-conjugated substrate (Fig. 5C). These results suggest that TMP
decreased the MMP activity during rat cerebral ischemia and
reperfusion.
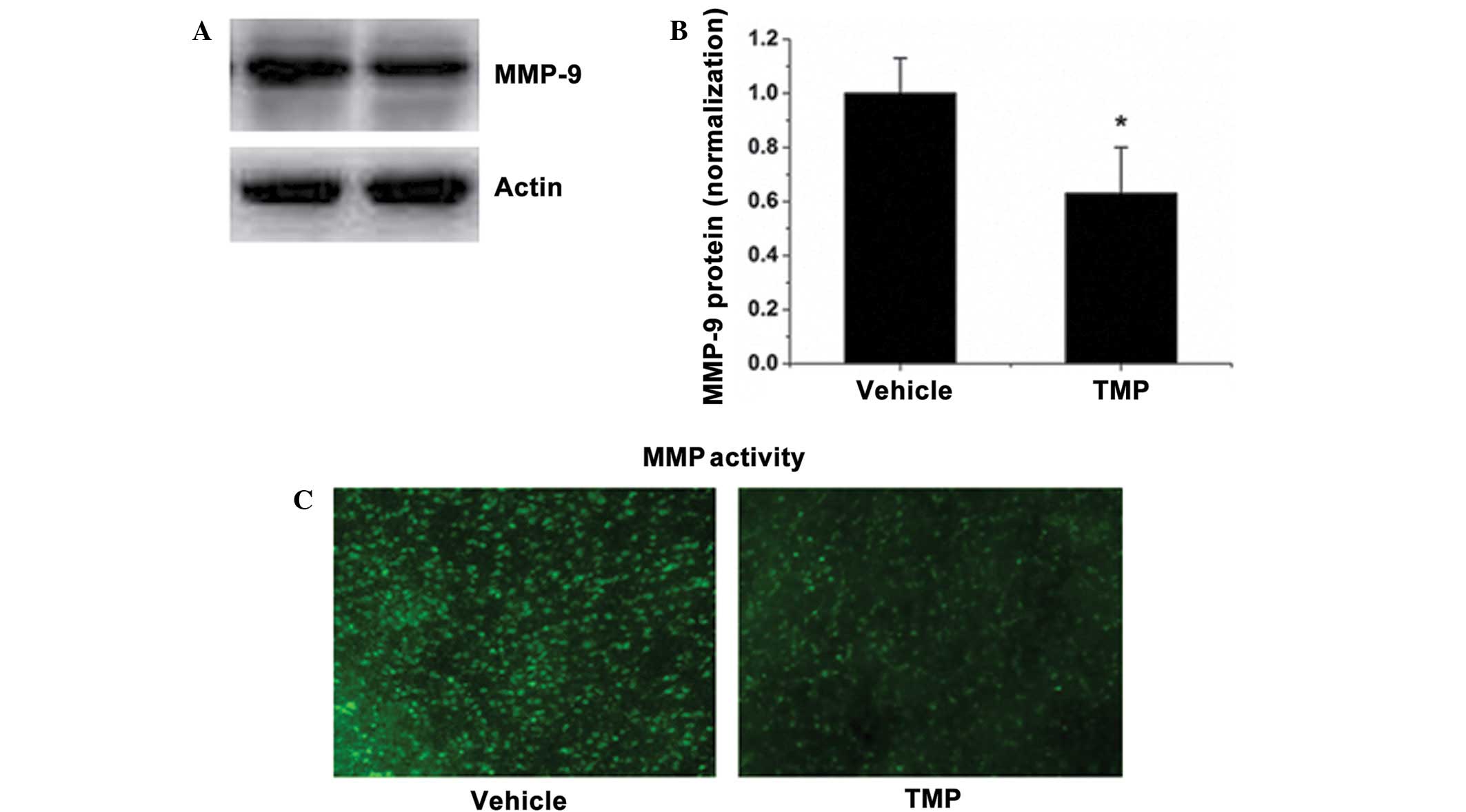 | Figure 5.TMP reduces MMP-9 expression and MMP
activity in the brain. (A) The MMP-9 expression level in the brains
of TMP- or saline-treated rats was determined by western blotting.
(B) Quantitative data of the western blot bands of MMP-9
expression. The average band intensity of MMP-9 was quantified with
ImageJ-1.38x software (National Institutes of Health, Bethesda, MD,
USA) and presented as a bar graph. (C) MMP activity in cryosections
in each group was determined in situ, using a
fluorescence-conjugated substrate. Data are expressed as the mean ±
standard deviation, n=6. *P<0.05. TMP,
2,3,5,6-tetramethylpyrazine; MMP, matrix metalloproteinase. |
Discussion
The present study investigated the effects of TMP at
a neurovascular level and found that i) TMP simultaneously reduced
the infarct volume, neurological score, brain edema and BBB
breakdown in a rat model of cerebral ischemia and reperfusion, a
finding that is consistent with previously reported findings; ii)
TMP reduced BBB permeability in the brain of rats with
ischemia/reperfusion damage; iii) activated MMPs and impaired tight
junctions in ischemic brains were significantly attenuated by TMP
treatment.
During cerebral ischemia and reperfusion, toxic
substances can readily pass from the blood to the brain due to the
increased permeability of the BBB induced by reperfusion insults;
this results in an enlarged infarct volume. Thus, maintaining BBB
integrity is a critical therapeutic strategy for the prevention of
cerebral ischemia/reperfusion injury. The most notable finding of
the present study is that TMP can protect the BBB integrity during
cerebral ischemia and reperfusion.
The BBB is composed of extracellular matrix,
microvascular endothelial cells and astrocytic endfeet. The primary
responsibility of the BBB is the strict regulation of trans-BBB
permeability. In this regard, the endothelial tight junctions of
the capillary are the main mediators, restricting the transfer of
vascular-derived substances (23).
The most important membrane-associated tight junction proteins
include occludins, claudins and junctional adhesion molecules,
which are connected to the cytoskeleton by the zonula occludens
(24,25). Elevated MMP activity and tight
junctional impairment are typical events associated with the BBB
dysfunction during cerebral ischemia/reperfusion. The in
situ zymography assay and the western blot analysis found that
TMP treatment reduced MMP activity, decreased MMP-9 expression and
attenuated the loss of the tight junction proteins occludin and
claudin-5. These results further confirmed that TMP was capable of
protecting the BBB from increased permeability during cerebral
ischemia and reperfusion.
In conclusion, this study is the first, to the best
of our knowledge, to demonstrate that TMP can preserve the BBB
integrity in this model of ischemic stroke in rats. The mechanisms
underlying this protection could be associated with the regulation
of MMPs and tight junctions. The findings of the present study
could be applied in a therapeutic or preventive strategy for
patients that have or are at high risk of suffering a stroke.
Acknowledgements
This study was supported by the National Natural
Science of Foundation of China (no. 81072947), the Guangdong
Natural Science Foundation (no. 8152800007000001) and the
Collaborative Innovation Center for Key Technology of the Wudang
Genuine Medicines Industry in Hubei Province (no.
2011JH-2103CXTT08).
References
|
1
|
Gu Y, Dee CM and Shen J: Interaction of
free radicals, matrix metalloproteinases and caveolin-1 impacts
blood-brain barrier permeability. Front Biosci (Schol Ed).
3:1216–1231. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion - from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gu Y, Chen J and Shen J: Herbal medicines
for ischemic stroke: Combating inflammation as therapeutic targets.
J Neuroimmune Pharmacol. 9:313–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Obermeier B, Daneman R and Ransohoff RM:
Development, maintenance and disruption of the blood-brain barrier.
Nat Med. 19:1584–1596. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han JZ, Sun J, Zhu QG, Liu JY, Hu JH and
Chen F: A modified LC-MS/MS method for determination of
tetramethylpyrazine in microdialysis samples and calibration of
home-made linear probes. Biomed Chromatogr. 26:1276–1281. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chun-sheng L, Hsiao-meng Y, Yun-hsiang H,
Chun P and Chi-fen S: Radix Salviae Miltiorrhizae and Rhizoma
Ligustici Wallichii in coronary heart disease. Chin Med J (Engl).
4:43–46. 1978.PubMed/NCBI
|
|
7
|
Zhu XL, Xiong LZ, Wang Q, Liu ZG, Ma X,
Zhu ZH, Hu S, Gong G and Chen SY: Therapeutic time window and
mechanism of tetramethylpyrazine on transient focal cerebral
ischemia/reperfusion injury in rats. Neurosci Lett. 449:24–27.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kao TK, Ou YC, Kuo JS, Chen WY, Liao SL,
Wu CW, Chen CJ, Ling NN, Zhang YH and Peng WH: Neuroprotection by
tetramethylpyrazine against ischemic brain injury in rats.
Neurochem Int. 48:166–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao X, Liu Y, Qi C, Qiu F, Chen X, Zhang
J and Yang P: Neuroprotection and enhanced neurogenesis by
tetramethylpyrazine in adult rat brain after focal ischemia. Neurol
Res. 32:547–555. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao SL, Kao TK, Chen WY, Lin YS, Chen SY,
Raung SL, Wu CW, Lu HC and Chen CJ: Tetramethylpyrazine reduces
ischemic brain injury in rats. Neurosci Lett. 372:40–45. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kao TK, Chang CY, Ou YC, Chen WY, Kuan YH,
Pan HC, Liao SL, Li GZ and Chen CJ: Tetramethylpyrazine reduces
cellular inflammatory response following permanent focal cerebral
ischemia in rats. Exp Neurol. 247:188–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shih YH, Wu SL, Chiou WF, Ku HH, Ko TL and
Fu YS: Protective effects of tetramethylpyrazine on kainate-induced
excitotoxicity in hippocampal culture. Neuroreport. 13:515–519.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li SY, Jia YH, Sun WG, Tang Y, An GS, Ni
JH and Jia HT: Stabilization of mitochondrial function by
tetramethylpyrazine protects against kainate-induced oxidative
lesions in the rat hippocampus. Free Radic Biol Med. 48:597–608.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lu C, Zhang J, Shi X, Miao S, Bi L, Zhang
S, Yang Q, Zhou X, Zhang M, Xie Y, et al: Neuroprotective effects
of tetramethylpyrazine against dopaminergic neuron injury in a rat
model of Parkinson's disease induced by MPTP. Int J Biol Sci.
10:350–357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leng YF, Gao XM, Wang SX and Xing YH:
Effects of tetramethylpyrazine on neuronal apoptosis in the
superficial dorsal horn in a rat model of neuropathic pain. Am J
Chin Med. 40:1229–1239. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fan LH, Wang KZ, Cheng B, Wang CS and Dang
XQ: Anti-apoptotic and neuroprotective effects of
Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC
Neurosci. 7:482006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng L, Ke N, Cheng F, Guo Y, Li S, Li Q
and Li Y: The protective mechanism of ligustrazine against renal
ischemia/reperfusion injury. J Surg Res. 166:298–305. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang Y, Hsiao G, Chen SH, Chen YC, Lin
JH, Lin KH, Chou DS and Sheu JR: Tetramethylpyrazine suppresses
HIF-1alpha, TNF-alpha, and activated caspase-3 expression in middle
cerebral artery occlusion-induced brain ischemia in rats. Acta
Pharmacol Sin. 28:327–333. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li XY, He JL, Liu HT, Li WM and Yu C:
Tetramethylpyrazine suppresses interleukin-8 expression in
LPS-stimulated human umbilical vein endothelial cell by blocking
ERK, p38 and nulear factor-kappaB signaling pathways. J
Ethnopharmacol. 125:83–89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu Y, Zheng G, Xu M, et al: Caveolin-1
regulates nitric oxide-mediated matrix metalloproteinases activity
and blood-brain barrier permeability in focal cerebral ischemia and
reperfusion injury. J Neurochem. 120:147–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yu F, Kamada H, Niizuma K, Endo H and Chan
PH: Induction of mmp-9 expression and endothelial injury by
oxidative stress after spinal cord injury. J Neurotrauma.
25:184–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Candelario-Jalil E, Thompson J, Taheri S,
Grossetete M, Adair JC, Edmonds E, Prestopnik J, Wills J and
Rosenberg GA: Matrix metalloproteinases are associated with
increased blood-brain barrier opening in vascular cognitive
impairment. Stroke. 42:1345–1350. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vorbrodt AW and Dobrogowska DH: Molecular
anatomy of intercellular junctions in brain endothelial and
epithelial barriers: Electron microscopist's view. Brain Res Brain
Res Rev. 42:221–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sandoval KE and Witt KA: Blood-brain
barrier tight junction permeability and ischemic stroke. Neurobiol
Dis. 32:200–219. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Krizbai IA and Deli MA: Signalling
pathways regulating the tight junction permeability in the
blood-brain barrier. Cell Mol Biol (Noisy-le-grand). 49:23–31.
2003.PubMed/NCBI
|