Introduction
Heavy metal pollution due to cadmium (Cd), arsenic
and lead is under vital scrutiny as a result of potential
neurotoxicological hazards to the human population (1,2). Cd is a
universal ecological toxicant frequently encountered through metal
industries, in aerosolized form in cigarette smoke and in certain
industrial workplaces, including those where battery manufacture
and smelting take place (3). In
addition to the noxious effect of Cd on vital organs such as the
kidneys, liver and testes, Cd also elicits damaging effects on the
central nervous system (CNS) due to a variety of factors, such as
its toxicity at low concentration, extended half-life (15–30 years
in humans) and low clearance rate (4). Although the blood-brain barrier (BBB)
is impermeable to chronic low levels of Cd exposure, Cd may affect
the permeability of the barrier and reach the brain (5). Preclinical studies have indicated that
Cd induces behavioral disorders, as well as morphological and
biochemical changes in the CNS (6,7). The
brain region displays a high potential for oxidation and a low
antioxidant defense capacity relative to the hepatic system. The
neurotoxic potential of Cd has been attributed to the changes it
induces in the brain enzyme network involved in counteracting
oxidative stress and the disturbance of brain metabolism. Further,
Cd increases the generation of free radicals, in particular
superoxide ions, in the brain and hinders the antioxidant defence
system, thus increasing lipid peroxidation. Moreover, free radicals
generated by Cd interact with mitochondrial sites, leading to the
breakdown of mitochondrial potentials, a consequence of reductions
in intracellular glutathione levels (8). The mechanism by which Cd induces
oxidative stress appears to be based mainly on the disruption of
the prooxidant/antioxidant balance, which may lead to brain injury
via oxidative damage to critical biomolecules, such as thiols,
lipids, proteins and DNA (9).
Dietary polyphenols are proposed to be major
antioxidants that provide health benefits due to their free
radical-scavenging and metal-chelating properties, and the
modulation of enzymatic activity and signal transduction pathways
(10). Chlorogenic acid (CA) is a
potent polyphenolic antioxidant abundantly found in apples, coffee
beans, tomatoes, potatoes and apricots (11,12). CA
is an ester formed between caffeic acid and quinic acid that is
hydrolyzed by intestinal bacteria to various aromatic acid
metabolites (13). The antioxidant
potential of CA is due to the presence of vicinal hydroxyl groups
on an aromatic residue, which enable it to scavenge free radicals
in the aqueous phase (14) and
prevent oxidative DNA damage (15).
Previous studies have reported the antioxidant potential (16,17) and
widespread bioactive effects of CA, such as antimutagenic,
antiviral (18), anticarcinogenic
(19) and tissue protection
(20) effects. The neuroprotective
potential of CA against oxidative insult to the brain has been well
documented (21–23). The present study was carried out to
investigate the neuroprotective efficacy of CA against the changes
to the brain provoked by cadmium.
Materials and methods
Chemicals
CA, cadmium chloride (CdCl2) and other
reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). All
other chemicals were of the highest available commercial grade.
Animals
Male Wistar rats weighing 170–200 g were obtained
from the animal facility at Shandong University (Jinan, China). The
animals were maintained under standard laboratory conditions of
relative humidity (55±5%), temperature (25±2°C) and light (12 h
light/12 h dark). They were fed standard diet pellets and water was
provided ad libitum.
Experimental design
Twenty four rats were divided into four groups (n=6
per group) and subjected to treatment as follows: Group 1: Rats
received distilled water 1 ml/100 g body weight once daily by oral
gavage for 30 days and served as a control group. Group 2: Rats
orally received Cd as CdCl2 in saline (5 mg/kg body
weight) each day for 30 days. Group 3: Rats received CA alone (60
mg/kg body weight) dissolved in distilled water, intragastric
administration once daily for 30 days. Group 4: Rats orally
received Cd (5 mg/kg body weight) followed by intragastric
administration of CA (60 mg/kg body weight) in distilled water once
daily for 30 days.
At the end of the experimental period, rats were
fasted overnight and sacrificed by decapitation. Blood was
collected in a heparinized BD vacutainer (BD Biosciences, Franklin
Lakes, NJ, USA) and plasma samples were collected by centrifugation
at 2,000 × g for 20 min. The whole brain was immediately excised
and rinsed in ice-cold saline to remove the blood. The brain
tissues were homogenized (25%, w/v) in 25 mmol/l phosphate buffer
(pH 7.0) containing 0.25 mol/l sucrose (w/v) and centrifuged at
1,000 × g for 10 min. The clear supernatant was used for various
biochemical assays to assess oxidative stress.
Preparation of mitochondrial
fraction
This was conducted as previously described (24). Brain homogenate (25%, w/v) was
prepared and the supernatant was subject to centrifugation at
12,000 g for 10 min at 4°C (Remi cooling centrifuge, C-24 BL; Remi
Laboratory Instruments, Mumbai, India). The mitochondrial pellets
thus obtained were washed twice with phosphate buffer (pH 7.4) to
remove sucrose and homogenized in phosphate buffer containing 0.5%
Tween-80 (v/v). The supernatant was collected and used for enzyme
analysis, then washed and resuspended in the same buffer.
Biochemical assays
Estimation of acetylcholinesterase (AChE)
activity
The level of AChE in the brain homogenate was
measured according to a previously described method (25) using acetylthiocholine iodide (ATCI)
as a substrate. In this method, AChE in the sample hydrolyzed ATCI
into thiocholine and butyric acid. The thiocholine reacted with
5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) to form
5-thio-2-nitrobenzoic acid. The yellow color that developed was
measured spectrophotometrically at 412 nm. The values are expressed
as µmol of ATCI hydrolyzed/min/mg protein.
Assessment of enzymatic antioxidants
The brain tissue level of superoxide dismutase (SOD)
was estimated by the method of Kakkar et al (26). The reaction was based on the
inhibition of the reduction of the nitro blue tetrazolium chromogen
by NADH and phenazine methosulfate. One unit of SOD activity
corresponds to the amount of enzyme that causes a 50% suppression
of the reduction of nitro blue tetrazolium/min/mg of protein.
The catalase (CAT) activity in the brain homogenate
was analyzed by the method of Sinha et al (27) wherein the breakdown of
H2O2 was evaluated by measuring the UV
absorption of H2O2 at 240 nm. The CAT
activity is expressed as µmol H2O2
consumed/min/mg of protein.
The brain levels of glutathione peroxidase (GPx)
were assayed by the method of Rotruck et al (28). The assay system was based on the
reaction between a measured amount of enzyme preparation
H2O2 and reduced glutathione (GSH) for a
specified time period. The unreacted GSH was then measured by
reaction with DTNB. The GPx activity is expressed as nmol GSH
oxidized/min/mg of protein.
Glutathione S-transferase (GST) was estimated by the
method of Habig et al (29).
The increase in absorbance was measured at 340 nm using
1-chloro-2,4-dinitrochlorobenzene (CDNB) as substrate. GST activity
is expressed in units of nmol of GSH-CDNB conjugate formed/min/mg
of protein.
Estimation of protein content
The protein content of the brain homogenate was
estimated by the method of Lowry et al (30) using bovine serum albumin as a
standard.
Estimation of non-enzymatic antioxidants
The GSH levels in the brain were determined by the
method of Moron et al (31).
The nonprotein sulfhydryl content of cells is in the form of GSH.
DTNB is a disulfide compound that is readily reduced by sulfhydryl
compounds to form a highly colored yellow anion. The optical
density of this yellow substance was measured at 412 nm. Results
are expressed as µg/mg of protein.
The concentration of ascorbic acid (vitamin C) in
the brain was determined by the method of Omaye et al
(32). In this method
dehydroascorbic acid, the oxidized product of ascorbic acid, reacts
with 2,4-dinitrophenylhydrazine to form
bis-2,4-dinitrophenylhydrazone. This undergoes further
rearrangement to form a product with an absorption maximum at 520
nm. Thiourea provides the reducing medium to prevent interference
from non-ascorbic acid chromogens. Results are expressed in µg/mg
of protein.
Vitamin E was analyzed by the method of Desai
(33). Ferric ions were reduced to
ferrous ions in the presence of vitamin E, and a pink-colored
complex was formed. The chelating agent orthophosphoric acid was
added to minimize carotene interference in the assay. Results are
expressed as µg/mg of protein.
Measurement of tissue lipid peroxides (LPOs)
The LPO levels in the brain homogenate were measured
according to the method of Ohkawa et al (34). The final colored end-product was
assayed spectrophotometrically at 532 nm. The LPO level was
expressed as nmol malondialdehyde (MDA)/mg protein.
Determination of membrane-bound ATPase
activities
Na+/K+-ATPase activity in the
brain homogenate was assayed by the method of Bonting (35). In this method, 0.2 ml brain
homogenate was added to a reaction mixture containing Tris HCl
buffer (pH 7.5), 50 mM MgSO4, 50 mM KCl, 600 mM NaCl, 1
mm EDTA, 40 mM ATP and 2.5% ammonium molybdate and incubated for 15
min at 37°C. The reaction was then arrested by the addition of 1 ml
ice cold 10% trichloroacetic acid. Then the amount of inorganic
phosphorus (Pi) liberated in the protein-free supernatant was
estimated. The activity of Mg2+-ATPase in the tissue
homogenate was assayed by the method of Ohinishi (36). Brain levels of Ca2+-ATPase
level were estimated as described by the method of Hjertan and Pan
(37). The activities of these
ATPase enzymes in tissue homogenate were expressed as µmol of Pi
liberated/min/mg protein.
Determination of mitochondrial citric acid cycle
enzymes
The activity of α-ketoglutarate dehydrogenase (KDH)
was estimated from the rate of reduction of NAD+ in the
presence of α-KDH at 340 nm (38).
The activity of isocitrate dehydrogenase (ICDH) was assayed by the
method of King (39). Succinate
dehydrogenase (SDH) was assayed by the method of Nulton-Persson and
Szweda (40) and the activity was
estimated from the rate of reduction of dichloroindophenol (DCIP)
in the presence of sodium succinate at 600 nm. The levels of malate
dehydrogenase (MDH) were estimated by the method of Mehler et
al (41). In this reaction the
rate of oxidation of NADH was measured in the presence of
oxaloacetate at 340 nm.
DNA fragmentation analysis
The DNA fragmentation pattern obtained using agarose
gel electrophoresis was analyzed by the method of Watabe et
al (42). Briefly, the brain
samples were washed twice with phosphate-buffered saline and were
lysed in a solution of 10 mM Tris-HCl (pH 8.0), 10 mM EDTA, 0.5%
(w/v) sodium dodecyl sulfate and 0.1% (w/v) RNase A, with
incubation for 60 min at 50°C. The lysate was then further
incubated for 60 min at 50°C with 1 mg/ml proteinase K and
subjected to electrophoresis on 1% agarose gel for 60 min at 50 V
using 40 mM Tris acetate (pH 7.5) containing 1 mM EDTA. Subsequent
to electrophoresis, DNA was visualized by staining with ethidium
bromide.
Statistical analysis
The results are expressed as mean ± standard
deviation for 6 rats per group. Statistical analysis was performed
by one-way analysis of variance using the SPSS software package for
Windows (version 10.0; SPSS, Inc., Chicago, IL, USA. Post hoc
testing was performed for inter-group comparisons using the least
significance difference (LSD) test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of CA and Cd on brain AChE
status
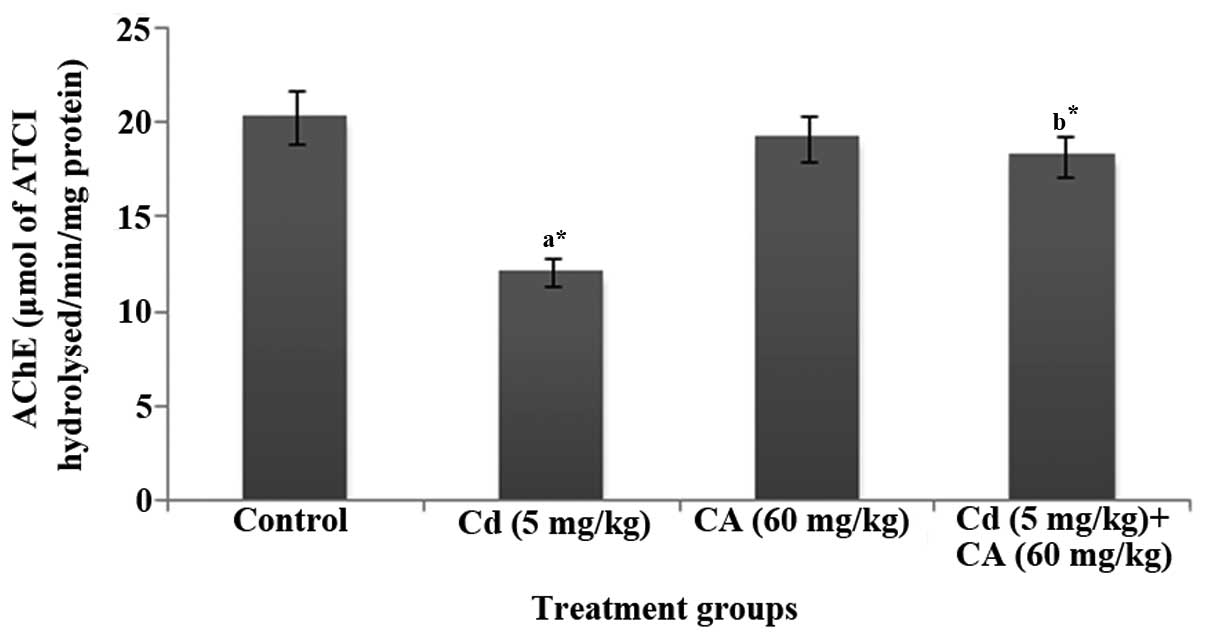
Fig. 1 displays the
activity of AChE in the brains of control and experimental rats. In
the present study, the levels of AChE in the brain were
significantly (P<0.05) decreased in the Cd-treated rats whereas
the administration of CA (60 mg/kg body weight) significantly
(P<0.05) attenuated the Cd-induced reduction in AChE activity,
resulting in normal AChE levels.
Effects of CA and Cd on brain
enzymatic antioxidant levels
The activities of the enzymatic antioxidants SOD,
CAT, GPx and GST in the brains of control and experimental rats are
displayed in Table I. Cd-treated
rats displayed significant (P<0.05) reductions in the activities
of SOD, CAT, GPx and GST when compared with the control rats. The
intragastric administration of CA reversed the noxious effect of Cd
and elicited a significant (P<0.05) restoration of the levels of
antioxidant enzymes to normal values.
 | Table I.Effects of CD and CA on the
activities of enzymatic antioxidants in the brain. |
Table I.
Effects of CD and CA on the
activities of enzymatic antioxidants in the brain.
| Groups | SOD (U/mg
protein) | CAT (U/mg
protein) | GPx (nmol/min/mg
protein) | GST (nmol/min/mg
protein) |
|---|
| Control | 7.22±0.19 | 4.35±0.1 | 2.87±0.13 | 5.59±0.45 |
| Cd (5 mg/kg) |
4.11±0.13a |
1.56±0.07a |
1.38±0.06a |
3.18±0.21a |
| CA (60 mg/kg) |
7.91±0.17a,b |
3.70±0.08a,b |
3.95±0.18a,b |
4.62±0.17b |
| Cd (5 mg/kg) + CA
(60 mg/kg) |
7.10±0.09b |
4.20±0.14b |
4.10±0.15a,b |
5.43±0.10b |
Effects of CA and Cd on brain
non-enzymatic antioxidant levels
Table II displays
the levels of the non-enzymatic antioxidants GSH, vitamin C and
vitamin E in the brains of control and experimental rats. The
levels of GSH and of vitamins C and E were significantly
(P<0.05) diminished in the brain tissues of Cd-treated rats when
compared with control rats. Treatment with CA significantly
(P<0.05) restored the depleted levels of GSH, vitamins C and E
to near normal levels.
 | Table II.Effect of Cd and CA on the levels of
non-enzymatic antioxidants (µg/mg protein). |
Table II.
Effect of Cd and CA on the levels of
non-enzymatic antioxidants (µg/mg protein).
| Groups | GSH | Vitamin C | Vitamin E |
|---|
| Control | 5.82±0.48 | 2.7±0.19 | 2.8±0.24 |
| Cd (5 mg/kg) |
2.16±0.29a |
1.2±0.07a |
1.6±0.18a |
| CA (60 mg/kg) |
5.64±0.35b | 2.6±0.12 | 2.9±0.31 |
| Cd (5 mg/kg) + CA
(60 mg/kg) |
6.12±0.51b |
2.1±0.12b |
2.3±0.21b |
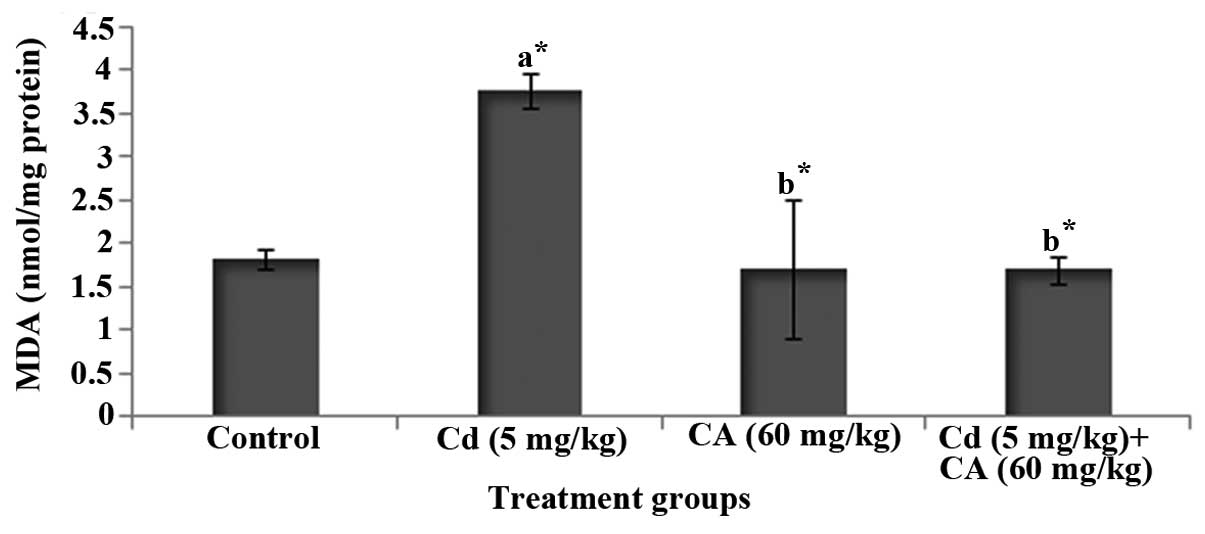
Effects of CA and Cd on brain lipid
peroxidation
Fig. 2 depicts the
level of MDA, an index of lipid peroxidation, in the brains of
control and experimental rats. Cd-treated rats displayed a
significant (P<0.05) increase in the level of MDA in comparison
with control rats. However, treatment with CA significantly
(P<0.05) diminished the degree of elevation of the MDA level in
the brain when compared with that in the rats treated with Cd
alone.
Effect of CA and Cd on membrane-bound
ATPases
The results displayed in Table III are the changes in the
activities of the ATPase enzymes
Na+/K+-ATPase, Ca2+-ATPase and
Mg2+-ATPase in the brains of control and experimental
rats. The Cd-treated rats displayed significant (P<0.05)
reductions in the activities of these ATPase enzymes on comparison
with the control rats. However, treatment with CA significantly
(P<0.05) increased the levels of the three ATPase enzymes in the
brain when compared with those in the rats treated with Cd
alone.
 | Table III.Effect of Cd and CA on the levels of
brain membrane ATPases (µmol phosphorus/min/mg protein). |
Table III.
Effect of Cd and CA on the levels of
brain membrane ATPases (µmol phosphorus/min/mg protein).
| Groups |
Na+/K+-ATPase |
Mg2+-ATPase |
Ca2+-ATPase |
|---|
| Control | 2.27±0.06 | 1.18±0.05 | 1.23±0.07 |
| Cd (5 mg/kg) |
1.18±0.05a |
0.38±0.07a |
0.53±0.07a |
| CA (60 mg/kg) | 2.21±0.04 | 1.07±0.05 | 1.11±0.05 |
| Cd (5 mg/kg) + CA
(60 mg/kg) |
2.15±0.04b |
0.80±0.11b |
0.90±0.07b |
Modulation of mitochondrial membrane
integrity by CA and Cd
Table IV shows the
activities of the citric cycle enzymes ICDH, α-KDH, SDH and MDH in
the brain mitochondria of control and experimental rats.
Significant reductions (P<0.05) in enzyme activities were
brought about by Cd exposure in comparison with the control.
However, treatment with CA restored the levels of mitochondrial
enzymes to normal.
 | Table IV.Effect of Cd and CA on the activities
of brain mitochondrial citric acid cycle enzymes. |
Table IV.
Effect of Cd and CA on the activities
of brain mitochondrial citric acid cycle enzymes.
| Groups | SDH | MDH | α-KDH | ICDH |
|---|
| Control | 43.57±2.35 | 875.85±32.65 | 54.27±3.97 | 155.57±3.88 |
| Cd (5 mg/kg) |
12.65±4.55a |
553.43±26.38a |
23.43±1.83a |
99.41±6.25a |
| CA (60 mg/kg) | 43.94±1.47 | 870.05±31.41 | 52.84±2.73 | 160.64±5.58 |
| Cd (5 mg/kg) + CA
(60 mg/kg) |
26.25±1.35b |
711.59±26.26b |
38.46±1.52b |
134.02±3.57b |
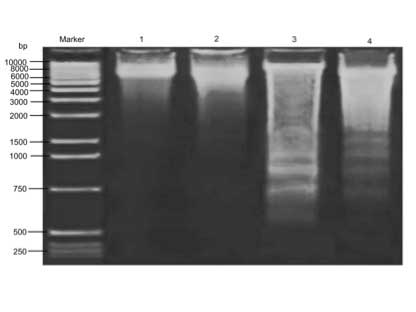
Effects of CA and Cd on brain DNA
fragmentation
Cd provoked oxidative DNA damage as demonstrated by
agarose gel electrophoresis, as shown in Fig. 3. The results indicate that there was
significant DNA fragmentation in the Cd-treated group compared with
the control group. However, CA supplement restored the DNA
integrity in comparison with that in the Cd-treated rats.
Discussion
The brain is a highly complex tissue that regulates
an array of biological metabolic events and utilizes 20% of cardiac
output. However, high concentrations of peroxidizable unsaturated
lipids and minimal antioxidant defence render the brain more prone
to noxious oxidative stress attack than the visceral vital tissues
are (43). Mounting evidence
substantiates the hypothesis that oxidative stress plays a pivotal
role in the etiology of neurodegenerative diseases (44,45). The
neurotoxic effects of Cd are mediated through the depletion of
enzymatic antioxidants and a resultant increase in lipid
peroxidation (5). Further, Cd
exposure elicits modulation of thiol status, alteration of ion
transport and ultimately DNA damage (46).
Research substantiates that alteration of the
cholinergic transmission system is a hallmark of Cd neurotoxicity
(47). AChE is a key biomarker
detected in order to evaluate the neurotoxicity of heavy metals.
There is evidence to suggest that free radicals provoked by heavy
metals are responsible for diminished AChE activity in the brain
(48). In the brain, low levels of
AChE could lead to noxious events such as the accumulation of
acetylcholine, which causes the rapid firing of neurons leading to
convulsions and status epilepticus (49). The modulation of AChE in the brain
displayed by Cd is concentration dependent; at a low concentration
(0.01 mM) activation of AChE occurs whilst at higher concentrations
(>0.1 mM) AChE activity is inhibited (7). Further, Cd induces a conformational
change in AChE that leads to the enzyme becoming unreactive
(50). In the present study toxic
doses (5 mg/kg) of Cd were reflected by diminished levels of AChE
and treatment with CA restored the activity of AChE in the brain.
The neuroprotective effects that CA elicited in the present study
may be due to antioxidant and free radical-scavenging actions.
Furthermore, regulation of the ionic homeostasis imbalance in
neurons and the highly lipophilic nature of CA may be responsible
to its neuroprotective efficacy. Studies suggest that CA has the
ability to cross the BBB so that it may effectively block the
deleterious effects of free radicals within the brains vascular and
cellular compartments (51,52).
In the oxidative stress cascade, lipid peroxidation
is the chief culprit and plays an imperative role in the toxicity
of many xenobiotics (53,54). In the present study, the oxidative
insult of Cd increased the MDA level, an oxidative product of LPO
in brain tissue. Previous studies suggest that Cd-induced lipid
peroxidation is due to the generation of hydroxyl radicals,
superoxide anions, nitric oxide and H2O2
(55–57). Indeed, in the present study,
treatment with CA effectively reduced the MDA level and this may be
due to the ability of CA to transfer electrons, scavenge free
radicals, chelate metals and activate antioxidant enzymes. GSH, the
first line of defense against reactive oxygen species (ROS), is a
readily available source of endogenous sulfhydryl (-SH) groups. It
has been demonstrated that Cd exposure causes a marked decline in
GSH levels, which may be ascribed to direct conjugation with free
or protein-bound -SH groups (58).
Further, GSH depletion may be due to the inhibition of glutathione
reductase, the enzyme responsible for the catalytic conversion of
glutathione disulfide (GSSG) to GSH (59). Cd interferes with the -SH group of
metallothionein, a metal chelating protein (60) and thus reduces the GSH level. Vitamin
C is a vital antioxidant that also acts as an anti-stress factor.
Vitamin E, a lipophilic chain-breaking antioxidant, also plays a
critical role in the detoxification of Cd. The present study
exemplifies the depletion of non-enzymatic antioxidant levels in
the brain, which may be due to increased utilization of these
biomolecules to reduce the Cd-induced oxidative stress. CA directly
acts as a scavenger to inhibit the Cd-mediated lipid peroxidation
and thus reduces the utilization of non-enzymatic antioxidants,
consequently leading to improvements of GSH and vitamin C and E
levels in the brain. The findings observed in the present study are
consistent with previous findings (22).
Cells are equipped with an array of antioxidant
defence mechanisms to counteract the effects of free radicals. The
present study shows that the Cd-induced increase in lipid
peroxidation was accompanied by a concomitant decline in the
activities of the cellular enzymatic antioxidants SOD, CAT, GPx and
GST. The diminished levels of antioxidant enzymes may be due to the
interaction of Cd with the -SH groups of enzymes and the decreased
activity of GPx may be due to competition by Cd-metallothioneins
(61). Further, a number of studies
have indicated that Cd inactivates a wide array of enzymes and
proteins involved in the anti-stress mechanism (62–64). In
one study, the administration of CA significantly elevated the
enzymatic antioxidant status in Cd-intoxicated rats, an effect that
may be due to its anti-lipid peroxidative potential (65).
Membrane-bound ATPase enzymes are highly vulnerable
to peroxidation and the actions of LPOs. They are bound to the
plasma membrane and are involved in the translocation of sodium,
potassium, calcium and magnesium ions. In the brain, the active
transport of sodium and potassium mediated by
Na+/K+-ATPase generates membrane potentials.
In addition, Na+/K+-ATPase regulates the
uptake and release of catecholamines (66,67) and
serotonin (68).
Ca2+-ATPase modulates intracellular calcium levels
(69), and the role of
Mg2+-ATPase is to maintain high brain levels of
intracellular magnesium ions to control the rates of protein
synthesis and cell growth (70).
Thus, ATPases are vital for neuronal functions and to maintain the
resting membrane potential and nerve conduction. Previous studies
indicate that Cd toxicity disrupts the activities of ATPase in CNS
(47,71–73),
reflecting the changes to membrane and neurotransmitter functions.
The reduction of ATPase activity may result from the formation of
Cd-ATPase complexes through -SH groups of the enzyme and/or
increased oxidative stress. Treatment with CA increased the tissue
levels of ATPases, suggesting that it protects the brain by
preserving its structural integrity against Cd challenge (74).
Research into cell death has focused on mitochondria
due to their role as the arbiters of cell fate in response to
stress. Cd is known to cause stress to the mitochondria by
affecting the thiol status and depleting ATP, thereby leading to a
cascade of pathophysiological events that ultimately result in
necrotic cell death (75). The
citric acid cycle is an essential metabolic process that is also an
integral part of the oxidative defense machinery of the cells. When
KDH, ICDH, SDH and MDH are inhibited, the permeability of the inner
mitochondrial membrane is increased, which causes mitochondrial
dysfunction, and subsequently leads to oxidative brain injury.
Notably, in the present study, poisoning with Cd decreased the
levels of these mitochondrial marker enzymes, whilst treatment with
CA significantly attenuated these effects, possibly as a result of
mitochondrial membrane stabilization (76).
Finally, in the present study Cd elevated the extent
of DNA fragmentation, possibly as a result of increased ROS
production during heavy metal exposure. Treatment with the
CD-treated rats with CA profoundly attenuated the toxicity by
diminishing the level of DNA fragmentation, possibly by inhibiting
the oxidative reactive radicals induced by Cd. These results are
consistent with a previous study (77).
In conclusion, the present study provides evidence
for the cytoprotective potential of CA against Cd-provoked
derangement of brain homeostasis. The neuroprotective effects of CA
are mediated through the inhibition of lipid peroxidation and by
supporting the endogenous antioxidant defense systems in brain with
the subsequent restoration of AChE and membrane-bound ATPase enzyme
activities, and prevention of mitochondrial dysfunction and DNA
fragmentation. Recently, it was demonstrated that Cd-induced
neuronal cell death involves the downregulation of phosphatase and
tensin homolog deleted on chromosome 10 (PTEN) and the activation
of phosphoinositide 3′-kinase/protein kinase B (Akt)/mammalian
target of rapamycin (mTOR) (78).
This finding may serve as a basis for future research to explore
the effect of CA on the signaling mechanisms that mediate the
negative effects of Cd.
References
|
1
|
Brender JD, Suarez L, Felkner M, et al:
Maternal exposure to arsenic, cadmium, lead, and mercury and neural
tube defects in offspring. Environ Res. 101:132–139. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jadhav SH, Sarkar SN, Patil RD and
Tripathi HC: Effects of subchronic exposure via drinking water to a
mixture of eight water contaminating metals: a biochemical and
histopathological study in male rats. Arch Environ Contam Toxicol.
53:667–677. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nawrot T, Plusquin M, Hogervorst J, et al:
Environmental exposure to cadmium and risk of cancer: a prospective
population-based study. Lancet Oncol. 7:119–126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fowler BA: Monitoring of human populations
forearly markers of cadmium toxicity; a review. Toxicol Appl
Pharmacol. 238:294–300. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shukla A, Shukla GS and Srimal RC:
Cadmium-induced alterations in blood-brain barrier permeability and
its possible correlation with decreased microvessel antioxidant
potential in rat. Hum Exp Toxicol. 15:400–405. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abu-Taweel GM, Ajarem JS and Ahmad M:
Protective effect of curcumin on anxiety, learning behavior,
neuromuscular activities, brain neurotransmitters and oxidative
stress enzymes in cadmium intoxicated mice. Behav Brain Sci.
3:74–84. 2013. View Article : Google Scholar
|
|
7
|
Carageorgiou H, Tzotzes V, Pantos C,
Mourouzis C, Zarros A and Tsakiris S: In vivo and in-vitro effects
of cadmium on adult rat brain total antioxidant status,
acetylcholinesterase, (Na+, K+)-ATPase and
Mg2+-ATPase activities: protection by L-cysteine. Basic
Clin Pharmacol Toxicol. 94:112–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopez E, Arce C, Oset-Gasque MJ, Cañadas S
and González MP: Cadmium induces reactive oxygen species generation
and lipid peroxidation in cortical neurons in culture. Free Radical
Biol Med. 40:940–951. 2006. View Article : Google Scholar
|
|
9
|
Kasprzak KS: Oxidative DNA and protein
damage in metal-induced toxicity and carcinogenesis. Free Radical
Biol Med. 32:958–967. 2002. View Article : Google Scholar
|
|
10
|
Kasai H, Fukada S, Yamaizumi Z, Sugie S
and Mori H: Action of chlorogenic acid in vegetables and fruits as
an inhibitor of 8-hydroxydeoxyguanosine formation in vitro and in a
rat carcinogenesis model. Food Chem Toxicol. 38:467–471. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suzuki A, Yamamoto N, Jokura H, et al:
Chlorogenic acid attenuates hypertension and improves endothelial
function in spontaneously hypertensive rats. J Hypertens.
24:1065–1073. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shibata H, Sakamoto Y, Oka M and Kono Y:
Natural antioxidant, chlorogenic acid, protects against DNA
breakage caused by monochloramine. Biosci Biotechnol Biochem.
63:1295–1297. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gonthier MP, Verny MA, Besson C, Rémésy C
and Scalbert A: Chlorogenic acid bioavailability largely depends on
its metabolism by the gut microflora in rats. J Nutr.
133:1853–1859. 2003.PubMed/NCBI
|
|
14
|
Rice-Evans CA, Miller NJ and Paganga G:
Structure-antioxidant activity relationships of flavonoids and
phenolic acids. Free Radical Biol Med. 20:933–956. 1996. View Article : Google Scholar
|
|
15
|
Cheng JC, Dai F, Zhou B, Yang L and Liu
ZL: Antioxidant activity of hydroxycinnamic acid derivatives in
human low density lipoprotein: mechanism and structure-activity
relationship. Food Chem. 104:132–139. 2007. View Article : Google Scholar
|
|
16
|
López-Giraldo LJ, Laguerre M, Lecomte J,
et al: Kinetic and stoichiometry of the reaction of chlorogenic
acid and its alkyl esters against the DPPH radical. J Agric Food
Chem. 57:863–870. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato Y, Itagaki S, Kurokawa T, et al: In
vitro and in vivo antioxidant properties of chlorogenic acid and
caffeic acid. Int J Pharm. 403:136–138. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang GF, Shi LP, Ren YD, et al:
Anti-hepatitis B virus activity of chlorogenic acid, quinic acid
and caffeic acid in vivo and in vitro. Antiviral Res. 83:186–190.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gordon MH and Wishart K: Effects of
chlorogenic acid and bovine serum albumin on the oxidative
stability of low density lipoproteins in vitro. J Agric Food Chem.
58:5828–5833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang YZ and Liu ZQ: Chemical kinetic
behavior of chlorogenic acid in protecting erythrocyte and DNA
against radical-induced oxidation. J Agric Food Chem.
56:11025–11029. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakajima Y, Shimazawa M, Mishima S and
Hara H: Water extract of propolis and its main constituents,
caffeoylquinic acid derivatives, exert neuroprotective effects via
antioxidant actions. Life Sci. 80:370–377. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Shi W, Li Y, et al: Neuroprotective
effects of chlorogenic acid against apoptosis of PC12 cells induced
by methylmercury. Environ Toxicol Pharmacol. 26:13–21. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vardi N, Parlakpinar H and Ates B:
Beneficial effects of chlorogenic acid on methotrexate-induced
cerebellar Purkinje cell damage in rats. J Chem Neuroanat.
43:43–47. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson D and Lardy H: Isolation of liver
or kidney mitochondria. Methods Enzymol. 10:94–96. 1967. View Article : Google Scholar
|
|
25
|
Ellman GL, Courtney KD, Andres VJ Jr and
Feather-stone RM: A new and rapid colorimetric determination of
acetylcholinesterase activity. Biochem. Pharmcol. 7:88–95. 1961.
View Article : Google Scholar
|
|
26
|
Kakkar P, Das B and Viswanathan PN: A
modified spectrophotometric assay of superoxide dismutase. Ind J
Biochem Biophys. 21:130–132. 1984.
|
|
27
|
Sinha AK: Colorimetric assay of catalase.
Anal Biochem. 47:389–394. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rotruck JT, Pope AL, Ganther HE, Swanson
AB, Hafeman DG and Hoekstra WG: Selenium: biochemical role as a
component of glutathione peroxidase. Science. 179:588–590. 1973.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Habig WH, Pabst MJ and Jakoby WB:
Glutathione S-transferases. The first enzymatic step in mercapturic
acid formation. J Biol Chem. 249:7130–7139. 1974.PubMed/NCBI
|
|
30
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
31
|
Moron MS, Depierre JW and Mannervik B:
Levels of glutathione, glutathione reductase and glutathione
S-transferase activities in rat lung and liver. Biochim Biophys
Acta. 582:67–78. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Omaye ST, Turnbull JD and Sauberlich HE:
Selected methods for the determination of ascorbic acid in animal
cells, tissues and fluids. Methods Enzymol. 62:3–8. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Desai ID: Vitamin E analysis methods for
animal tissues. Methods Enzymol. 105:138–147. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohkawa H, Ohishi N and Yagi K: Assay for
lipid peroxidation in animal tissues by thiobarbituric acid
reaction. Anal Biochem. 95:351–358. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonting SL: Presence of enzyme systems in
mammalian tissuesMembrane and Ion Transport. Bilter EE: Wiley
Interscience; London: pp. 257–263. 1970
|
|
36
|
Ohinishi T, Suzuki T, Suzuki Y and Ozawa
K: A comparative study of plasma membrane Mg2+ ATPase
activities in normal, regenerating and malignant cells. Biochem
Biophys Acta. 684:67–74. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hjertan S and Pan H: Purification and
characterization of two forms of a low affinity
Ca2+-ATPase from erythrocyte membranes. Biochim Biophys
Acta. 728:281–288. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reed LJ and Mukherjee BB: α-ketoglutarate
dehydrogenase complex from Escherichia coli. Methods Enzymol.
113:55–61. 1969. View Article : Google Scholar
|
|
39
|
King J: The hydrolases - acid and alkaline
phosphatasesPractical Clinical Enzymology. Van D Nostrand Co.;
London: pp. 199–208. 1965
|
|
40
|
Nulton-Persson AC and Szweda LI:
Modulation of mitochondrial function by hydrogen peroxide. J Biol
Chem. 276:23357–23361. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mehler AH, Kornberg A, Grisolia S and
Ochoa S: The enzymatic mechanism of oxidation-reductions between
malate or isocitrate and pyruvate. J Biol Chem. 174:961–977.
1948.PubMed/NCBI
|
|
42
|
Watabe M, Masuda Y, Nakajo S, Yoshida T,
Kuroiwa Y and Nakaya K: The cooperative interaction of two
different signaling pathways in response to bufalin induces
apoptosis in human leukemia U937 cells. J Biol Chem.
271:14067–14072. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bondy SC: Free-radical-mediated toxic
injury to the nervous systemIn: Free Radical Toxicology. Wallace
KB: Taylor and Francis; Oxford: pp. 221–248. 1997
|
|
44
|
Emerit J, Edeas M and Bricaire F:
Neurodegenerative diseases and oxidative stress. Biomed
Pharmacother. 58:39–46. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gandhi S and Abramov AY: Mechanism of
oxidative stress in neurodegeneration. Oxid Med Cell Longev.
2012:4280102012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kumar R, Agarwal AK and Seth PK: Oxidative
stress-mediated neurotoxicity of cadmium. Toxicol Lett. 89:65–69.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Antonio MT, Corredor L and Leret ML: Study
of the activity of several brain enzymes like markers of the
neurotoxicity induced by perinatal exposure to lead and/or cadmium.
Toxicol Lett. 143:331–340. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tsakiris S, Angelogianni P, Schulpis KH
and Starridis JC: Protective effect of L-phenylalanine on rat brain
acetylcholinesterase inhibition induced by free radicals. Clin
Biochem. 33:103–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Olney JW, Collins RC and Sloviter RS:
Excitotoxic mechanisms of epileptic brain damage. Adv Neurol.
44:857–877. 1986.PubMed/NCBI
|
|
50
|
Tomlinson G, Mutus B and McLennan I:
Activation and inactivation of acetylcholinesterase by metal ions.
Can J Biochem. 59:728–735. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chu YF, Brown PH, Lyle BJ, et al: Roasted
coffees high in lipophilic antioxidants and chlorogenic acid
lactones are more neuroprotective than green coffees. J Agric Food
Chem. 57:9801–9808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lapchak PA: The phenylpropanoid
micronutrient chlorogenic acid improves clinical rating scores in
rabbits following multiple infarct ischemic strokes: synergism with
tissue plasminogen activator. Exp Neurol. 205:407–413. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Stohs SJ and Bagchi D: Oxidative
Mechanisms in the toxicity of metal ions. Free Radical Biology and
Medi. 18:321–336. 1995. View Article : Google Scholar
|
|
54
|
Anane R and Creppy EE: Lipid peroxidation
as pathway of aluminium cytotoxicity in human skin fibroblast
cultures: prevention by superoxide dismutase+catalase and vitamins
E and C. Hum Exp Toxicol. 2:477–481. 2001. View Article : Google Scholar
|
|
55
|
O'Brien P and Salasinski HJ: Evidence that
the reactions of cadmium in the presence of metallothionein can
produce hydroxyl radicals. Arch Toxicol. 72:690–700. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Koizumi T, Shirakura G, Kumagai H,
Tatsumoto H and Suzuki KT: Mechanism of cadmium-induced
cytotoxicity in rat hepatocytes: cadmium-induced active
oxygen-related permeability changes of the plasma membrane.
Toxicology. 114:124–134. 1996. View Article : Google Scholar
|
|
57
|
Tandom SK, Singh S, Prasad S, et al:
Reversal of cadmium induced oxidative stress by chelating agent,
antioxidant or their combination in rat. Toxicol Lett. 145:211–217.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rana SV and Verma S: Protective effects of
GSH, vitamin E and selenium on lipid peroxidation in cadmium-fed
rats. Biol Trace Elem Res. 51:161–168. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Dringen R, Gutterer JM and Hirrlinger J:
Glutathione metabolism in brain metabolic interaction between
astrocytes and neurons in the defense against reactive oxygen
species. Eur J Biochem. 267:4912–4916. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Hidalgo J, Aschner M, Zatta P and Vasák M:
Roles of the metallothionein family of proteins in the central
nervous system. Brain Res Bull. 15:133–145. 2001. View Article : Google Scholar
|
|
61
|
Waisberg M, Joseph P, Hale B and
Beyersmann D: Molecular and cellular mechanisms of cadmium
carcinogenesis. Toxicology. 192:95–117. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jamall IS and Sprowls JJ: Effects of
cadmium and dietary selenium on cytoplasmic and mitochondrial
antioxidant defense systems in the heart of rats fed high dietary
copper. Toxicol Appl Pharmacol. 87:102–110. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sarkar S, Yadov P and Bhatnagar D: Lipid
peroxidative damage on cadmium exposure and alterations in
antioxidant system in rat erythrocytes: A study with relation to
time. Biometals. 11:153–157. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Casalino E, Calzaretti G, Sblano C and
Landriscina C: Molecular inhibitory mechanisms of antioxidant
enzymes in rat liver and kidney by cadmium. Toxicology. 179:37–50.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Pari L, Karthikesan K and Menon VP:
Comparative and combined effect of chlorogenic acid and
tetrahydrocurcumin on antioxidant disparities in chemical induced
experimental diabetes. Mol Cell Biochem. 341:109–117. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bogdanski DF, Tissuri A and Brodie BB:
Role of sodium, potassium, ouabain and reserpine in uptake, storage
and metabolism of biogenic amines in synaptosomes. Life Sci.
7:419–428. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mata M, Fink DJ, Gainer H, et al:
Activity-dependent energy metabolism in rat posterior pituitary,
primarily reflects sodium pump activity. J Neurochem. 34:214–215.
1980. View Article : Google Scholar
|
|
68
|
Hernandez R: Brain Na+,
K+-ATPase activity possibly regulated by a specific
serotonin receptor. Brain Res. 408:399–402. 1989. View Article : Google Scholar
|
|
69
|
Skalska J, Bednarczyk P, Piwońska M, et
al: Calcium ions regulate K+ uptake into brain
mitochondria: The evidence for a novel potassium channel. Int J Mol
Sci. 10:1104–1120. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sanui H and Rubin H: The role of magnesium
in cell proliferation and transformationIons, Cell Proliferation
and Cancer. Boynton AL, McKochan WL and Whitfield JP: Academic
Press; New York: pp. 517–537. 1982
|
|
71
|
Rajanna B, Hobson M, Boykin M and Chetty
CS: Effects of chronic treatment with cadmium on ATPases, uptake of
catecholamines and lipid peroxidation in rat brain synaptosomes.
Ecotoxicol Environ Saf. 20:36–41. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Carfagna MA, Ponsler GD and Muhoberac BB:
Inhibition of ATPase activity in rat synaptic plasma membranes by
simultaneous exposure to metals. Chem Biol Interact. 100:53–65.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
El-Missiry MA and Shalaby F: Role of
beta-carotene in ameliorating the cadmium-induced oxidative stress
in rat brain and testis. J Biochem Mol Toxicol. 14:238–243. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lee K, Lee JS, Jang HJ, et al: Chlorogenic
acid ameliorates brain damage and edema by inhibiting matrix
metalloproteinase-2 and 9 in a rat model of focal cerebral
ischemia. Eur J Pharmacol. 689:89–95. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nigam D, Shukla GS and Agarwal AK:
Glutathione depletion and oxidative damage in mitochondria
following exposure to cadmium in rat liver and kidney. Toxicol
Lett. 106:151–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Ho L, Varghese M, Wang J, et al: Dietary
supplementation with decaffeinated green coffee improves
diet-induced insulin resistance and brain energy metabolism in
mice. Nutr Neurosci. 15:37–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xu JG, Hu QP and Liu Y: Antioxidant and
DNA-protective activities of chlorogenic acid isomers. J Agric Food
Chem. 60:11625–11630. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen S, Gu C, Xu C, et al: Celastrol
prevents cadmium-induced neuronal cell death via targeting JNK and
PTEN-Akt/mTOR network. J Neurochem. 128:256–266. 2014. View Article : Google Scholar : PubMed/NCBI
|