Introduction
Skeletal strength and bone mineral homeostasis are
strictly regulated by bone remodeling (1). The process of bone remodeling consists
primarily of two functional events: Osteoblastic bone formation and
osteoclastic bone resorption (2).
The disruption of the bone remodeling process is considered to
cause metabolic bone diseases, such as osteoporosis. Numerous
humoral factors, such as cytokines and prostaglandins, have been
shown to participate in the bone remodeling process (3).
Transforming growth factor-β (TGF-β), which is a
member of the TGF-β superfamily that consists of >40 members,
such as bone morphogenetic proteins (BMPs) and activin, is well
recognized as a stimulator of osteoblastic bone formation (4,5). TGF-β
stimulates the deposit of bone matrix and the proliferation of
osteoblasts (5). TGF-β, which is
produced by osteoblasts and subsequently embedded into the bone
matrix, is released and activated via osteoclastic bone resorption,
the first stage of bone remodeling (5,6). Thus,
TGF-β is fundamental for the regulation of bone remodeling, acting
as a coupling factor between bone resorption and bone formation
(7).
Vascular endothelial growth factor (VEGF) plays an
essential role in angiogenesis (8).
The osteoblast lineage is currently considered to be an important
source of VEGF among bone cells (8).
It has been demonstrated that VEGF receptors are expressed by
osteoblasts and osteoclasts (8). A
variety of physiological stimuli, including hormonal, mechanical
and environmental factors, reportedly modulate the production of
VEGF by osteoblasts, suggesting that osteoblast-synthesized VEGF is
important for the autocoid-mediated control of angiogenesis in bone
(8). We have previously demonstrated
that VEGF synthesis in osteoblast-like MC3T3-E1 cells is stimulated
by TGF-β and positively regulated by p38 mitogen-activated protein
(MAP) kinase, p44/p42 MAP kinase and stress-activated protein
kinase/c-Jun N-terminal kinase (SAPK/JNK) (9,10);
however, the details underlying VEGF synthesis in osteoblasts are
yet to be clarified.
Resveratrol is a polyphenolic compound found in
grape and berry skins and red wine. It is currently believed that
the various biological abilities of resveratrol mainly stem from
its antioxidant and anti-inflammatory effects, which are mediated
via the activation of sirtuin 1 (SIRT1) (11,12). Low
mortality rates from coronary heart disease have been reported in
France, where there is frequent consumption of red wine (13). Since red wine is an abundant source
of resveratrol, the so-called French paradox is considered to be
associated with the uptake of resveratrol (13). In the matter of bone health, it has
recently been reported that the risk of hip fracture in women who
have a preference for wine consumption is lower than that in female
former drinkers, non-drinkers or drinkers with different alcoholic
beverage preferences (14). It has
also recently been observed that resveratrol inhibits the
BMP-4-induced VEGF synthesis via the suppression of the p70 S6
kinase in osteoblast-like MC3T3-E1 cells (15). In addition, we previously
demonstrated that resveratrol attenuates the osteoprotegerin
synthesis stimulated by prostaglandin F2α
(PGF2α) or PGD2 in MC3T3-E1 cells (16,17);
however, the exact mechanism by which bone metabolism is affected
by resveratrol remains to be elucidated. The aim of the present
study, therefore, was to investigate the effect of resveratrol on
the TGF-β-induced VEGF synthesis and the mechanism in
osteoblast-like MC3T3-E1 cells.
Materials and methods
Materials
Resveratrol and SRT1720 were obtained from
Calbiochem-Novabiochem Corp. (La Jolla, CA, USA). TGF-β and mouse
VEGF enzyme-linked immunosorbent assay (ELISA) kits were obtained
from R&D Systems, Inc. (Minneapolis, MN, USA). Rabbit
polyclonal phospho-specific Smad2 (#3101), Smad2/3 (#3102), p38 MAP
kinase (#9212), phospho-specific p44/p42 MAP kinase (#9101),
p44/p42 MAP kinase (#9102), SAPK/JNK (#9252), and rabbit monoclonal
phospho-specific p38 MAP kinase (#4511) and phospho-specific
SAPK/JNK (#4671) antibodies were obtained from Cell Signaling
Technology, Inc. (Beverly, MA, USA). An enhanced chemiluminescence
(ECL) western blotting detection system was obtained from GE
Healthcare (Little Chalfont, UK). The rest of the chemicals and
materials were purchased from commercial sources. Resveratrol and
SRT1720 were dissolved in dimethyl sulfoxide, the maximum
concentration of which did not exceed 0.1% and affected neither the
assay for VEGF nor the detection of the protein level using western
blotting.
Cell culture
The cloned osteoblast-like MC3T3-E1 cells were
derived from a newborn mouse calvaria (18) and were maintained as previously
described (19). Briefly, the cell
culture was performed using α-minimum essential medium (α-MEM),
which contained 10% fetal bovine serum (FBS), at 37°C in a
humidified atmosphere of 5% CO2/95% air. The cells were
seeded into 35- or 90-mm diameter dishes (5×104 and
2×105 cells/dish, respectively) in α-MEM containing 10%
FBS. Five days later, the medium was exchanged for α-MEM containing
0.3% FBS. Experiments with the cells were performed after 48 h.
Assay for VEGF
The cultured cells were pretreated with a variety of
resveratrol doses (0–50 µM) or SRT1720 for 60 min and then
stimulated by 5 ng/ml TGF-β or vehicle in 1 ml α-MEM containing
0.3% FBS for the indicated periods. Following incubation, the
conditioned medium was collected and the VEGF concentration was
measured using the VEGF ELISA kit, according to the manufacturer's
instructions (R&D Systems, Inc.).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The cultured cells were pretreated with 50 µM
resveratrol, 10 µM SRT1720 or vehicle for 60 min, and were
subsequently stimulated by 5 ng/ml TGF-β or vehicle in α-MEM
containing 0.3% FBS for 12 h. TRIzol® reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) and the Omniscript® RT kit (Qiagen
Inc., Valencia, CA, USA) were used for the isolation of the total
RNA and its transcription into complementary DNA, respectively. The
RT-qPCR was performed in capillaries using a LightCycler® system
and the FastStart DNA Master SYBR Green I provided with the kit
(Roche Diagnostics, Basel, Switzerland). The mouse VEGF and GAPDH
mRNA sense and antisense primers were synthesized based on the
method described in the study by Simpson et al (20). A melting curve analysis and agarose
electrophoresis were used to evaluate the amplified products. GAPDH
mRNA levels were used to normalize the levels of VEGF mRNA.
Western blot analysis
The cultured cells were pretreated with a variety of
doses of resveratrol or 20 µM SRT1720 for 60 min, and were then
stimulated by 5 ng/ml TGF-β in α-MEM containing 0.3% FBS for the
indicated periods. The cells were washed twice with
phosphate-buffered saline and then lysed, homogenized and sonicated
in a lysis buffer that contained 2% sodium dodecyl sulfate (SDS),
50 mM dithiothreitol, 62.5 mM Tris/HCl (pH 6.8) and 10% glycerol.
SDS-polyacrylamide gel electrophoresis was performed using the
method of Laemmli (21) in 10%
polyacrylamide gels. The protein was separated and transferred onto
an Immun-Blot® polyvinylidene difluoride (PVDF) membrane (Bio-Rad,
Hercules, CA, USA). The membrane was blocked using 5% skimmed,
dried milk in Tris-buffered saline-Tween 20 [TBS-T; 20 mM Tris-HCl
(pH 7.6), 137 mM NaCl and 0.1% Tween 20] for 1 h prior to
incubation with the primary antibody. Western blotting was
performed as previously described (22), using primary antibodies against
phospho-specific Smad2, Smad2/3, phospho-specific p38 MAP kinase,
p38 MAP kinase, phospho-specific p44/p42 MAP kinase, p44/p42 MAP
kinase, phospho-specific SAPK/JNK or SAPK/JNK, with
peroxidase-labeled goat-anti rabbit immunoglobulin G secondary
antibodies (Kirkegaard & Perry Laboratories, Inc.,
Gaithersburg, MD, USA). The primary and secondary antibodies were
diluted at 1:1,000 with 5% skimmed, dried milk in TBS-T.
Visualization of the peroxidase activity on the membrane was
performed using X-ray film and the ECL western blotting detection
system.
Densitometric analysis
A scanner and image analysis software (ImageJ
version 1.47; National Institutes of Health, Bethesda, MD, USA)
were used to perform densitometric analysis. The phosphorylated
protein levels were presented as the fold increase relative to the
unstimulated control cell values and calculated by normalizing the
background-subtracted signal intensity of each phosphorylation
signal to the respective total protein signal.
Statistical analysis
Data analysis was performed using analysis of
variance, followed by the Bonferroni method for multiple
comparisons between pairs. P<0.05 was considered to indicate a
statistically significant difference. All data are presented as the
mean ± standard error of the mean of triplicate determinations from
three independent cell preparations.
Results
Effect of resveratrol on the
TGF-β-stimulated VEGF release in MC3T3-E1 cells
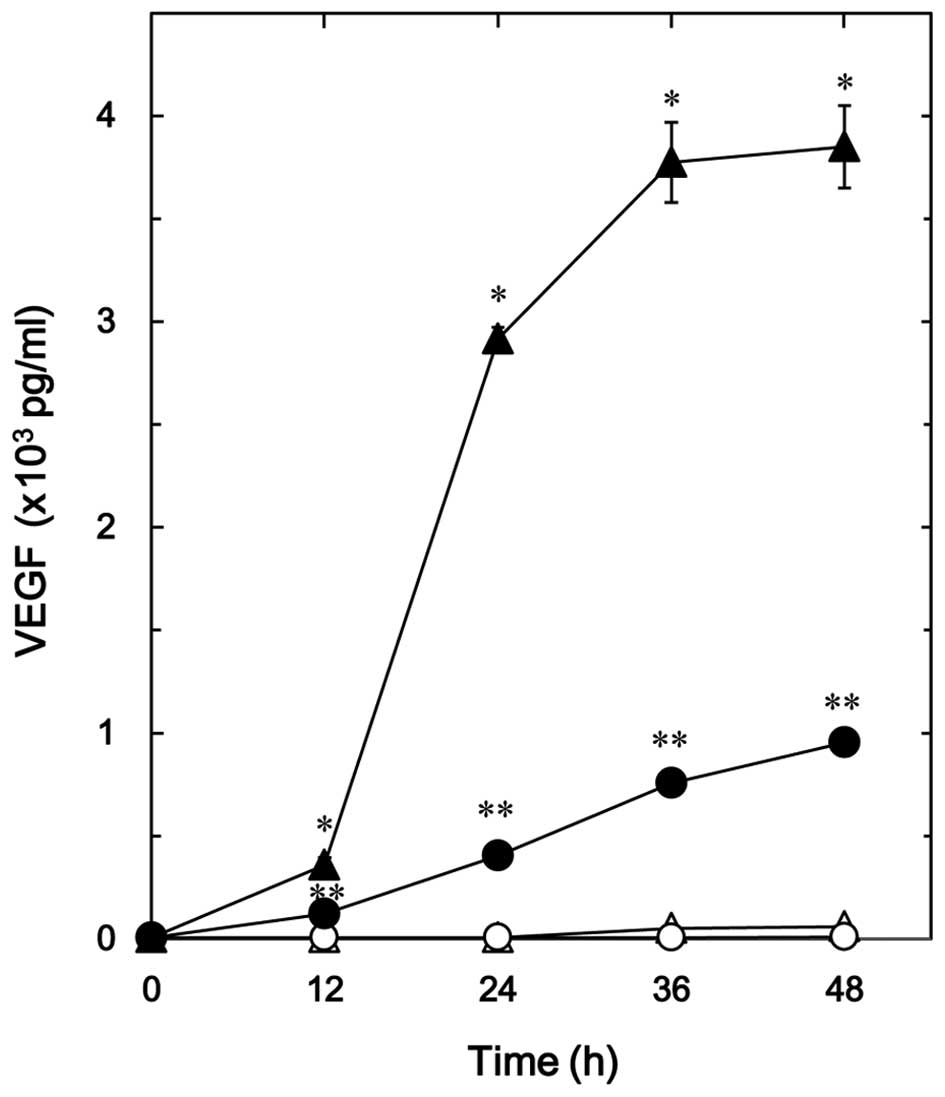
The effect of resveratrol on the TGF-β-stimulated
VEGF release in osteoblast-like MC3T3-E1 cells was firstly
examined. Resveratrol, which alone had little effect on the VEGF
release, significantly reduced the TGF-β-stimulated VEGF release in
a time-dependent manner up to 48 h (Fig.
1). The suppressive effect of resveratrol on the
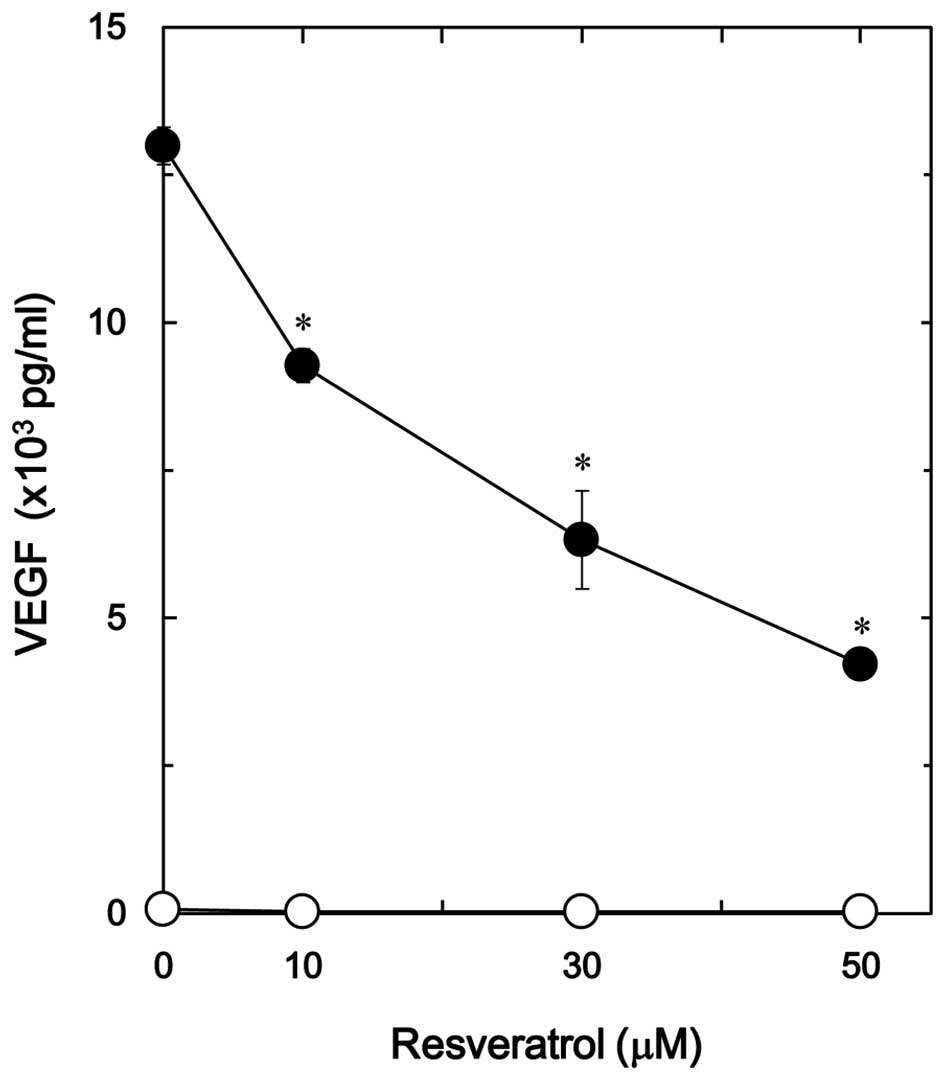
TGF-β-stimulated VEGF release was dose-dependent in the range
between 10 and 50 µM (Fig. 2). The
maximum inhibitory effect of resveratrol was observed at 50 µM,
which caused an ∼70% decrease in the TGF-β-effect.
Effect of SRT1720 on the
TGF-β-stimulated VEGF release in MC3T3-E1 cells
It has been demonstrated that resveratrol activates
SIRT1, resulting in the exertion of the biological effects
(11,12). Thus, the effect of SRT1720, which is
a synthetic SIRT1 activator (23),
was then examined on the TGF-β-stimulated VEGF release in MC3T3-E1
cells. SRT1720 reduced the TGF-β-stimulated VEGF release in a
time-dependent manner (Fig. 3A). In
addition, SRT1720 decreased the TGF-β-induced VEGF release in a
dose-dependent manner (Fig. 3B). The
inhibitory effect of SRT1720 that was observed at 3 µM on the VEGF
release caused an ∼60% decrease in the TGF-β-effect.
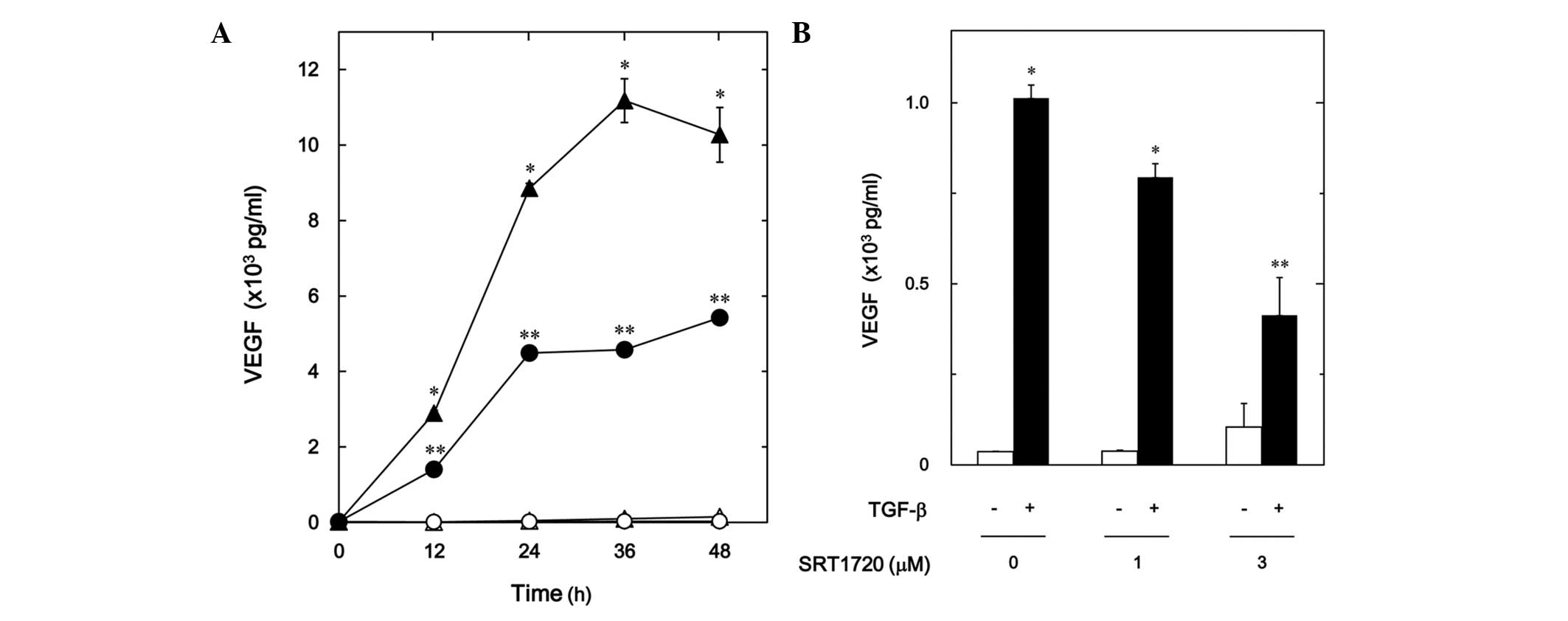 | Figure 3.Effect of SRT1720 on the
TGF-β-stimulated VEGF release in MC3T3-E1 cells. (A) The cultured
cells were pretreated with 10 µM SRT1720 (●,○) or vehicle (▲,△) for
60 min, and then stimulated by 5 ng/ml TGF-β (●,▲) or vehicle (○,△)
for the indicated periods. (B) The cultured cells were pretreated
with various doses of SRT1720 for 60 min, and then stimulated by 5
ng/ml TGF-β or vehicle for 48 h. VEGF concentrations of the
conditioned media were determined by enzyme-linked immunosorbent
assay. Each value represents the mean ± standard error of the mean
of triplicate determinations from three independent cell
preparations. *P<0.05, compared with the value of the control.
**P<0.05, compared with the value of TGF-β alone. TGF-β,
transforming growth factor-β; VEGF, vascular endothelial growth
factor. |
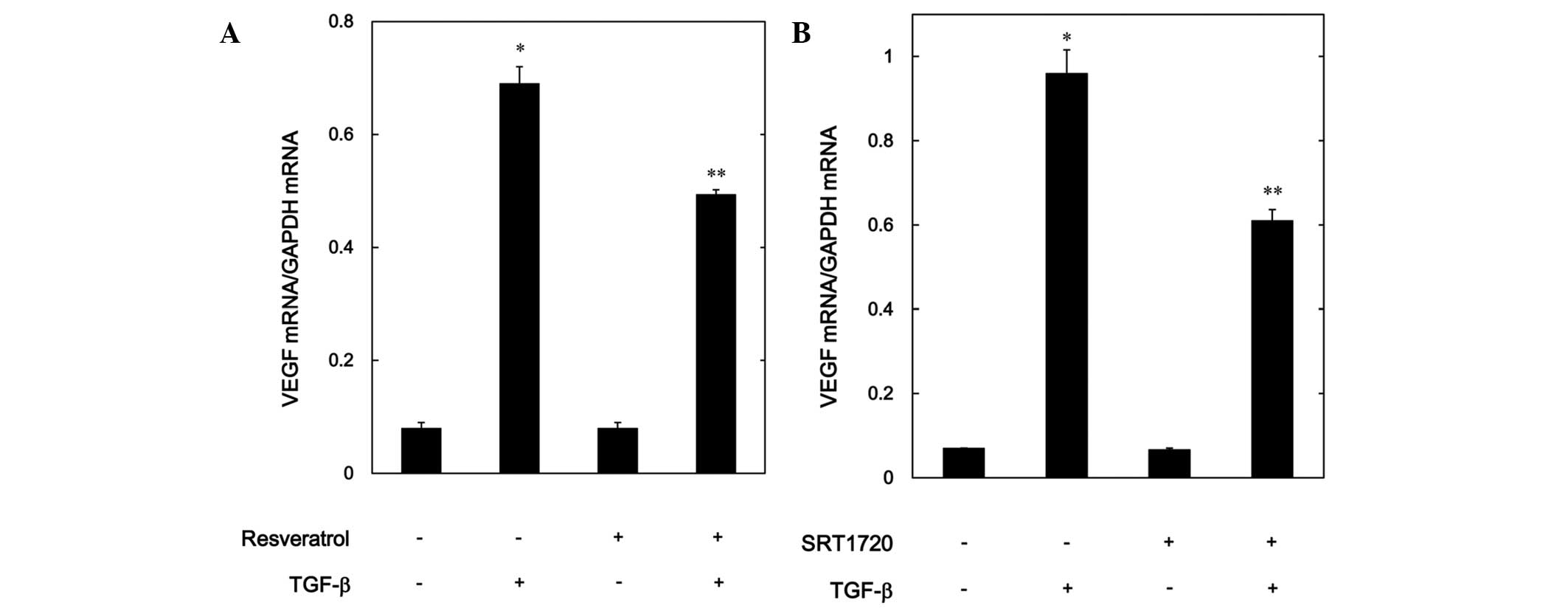
Effects of resveratrol or SRT1720 on
the TGF-β-induced expression levels of VEGF mRNA in MC3T3-E1
cells
In order to investigate whether or not the
suppressive effect of resveratrol or SRT1720 on the
TGF-β-stimulated VEGF release was mediated through transcriptional
events, the effect of resveratrol or SRT1720 on the TGF-β-induced
VEGF mRNA expression levels was further examined using RT-qPCR.
Although resveratrol by itself had little effect on the mRNA levels
of VEGF, it significantly suppressed the mRNA expression levels
induced by TGF-β (Fig. 4A).
Additionally, SRT1720 markedly reduced the VEGF mRNA expression
levels stimulated by TGF-β in a similar manner to resveratrol
(Fig. 4B).
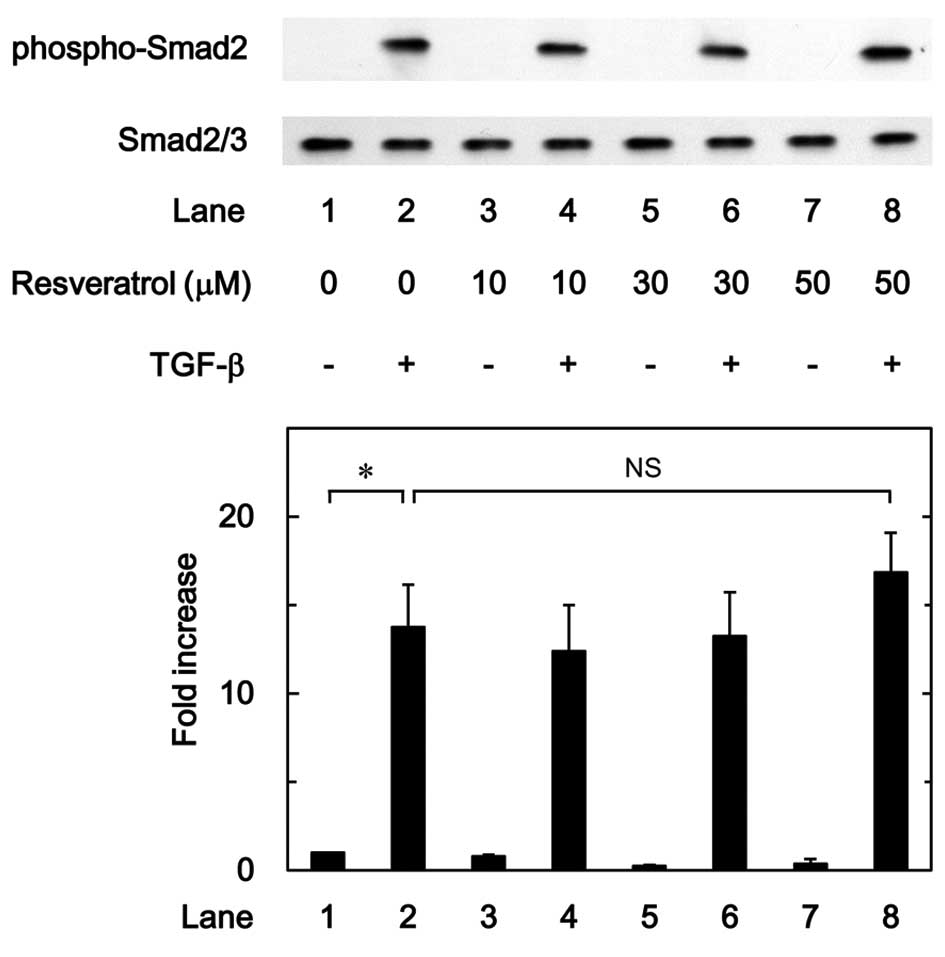
Effect of resveratrol on the
TGF-β-induced phosphorylation of Smad in MC3T3-E1 cells
It is generally recognized that the effect of TGF-β
is mainly mediated through the Smad-dependent pathway (24). It was found that Smad is involved in
the TGF-β-stimulated VEGF synthesis in osteoblasts. In order to
clarify whether the suppressive effect of resveratrol on the
TGF-β-stimulated VEGF synthesis was mediated by the modulation of
Smad activation in MC3T3-E1 cells, the effect of resveratrol on the
TGF-β-induced phosphorylation of Smad2 was examined; however, it
was demonstrated that resveratrol had little effect on the
TGF-β-induced phosphorylation of Smad2 in the range between 10 and
50 µM (Fig. 5).
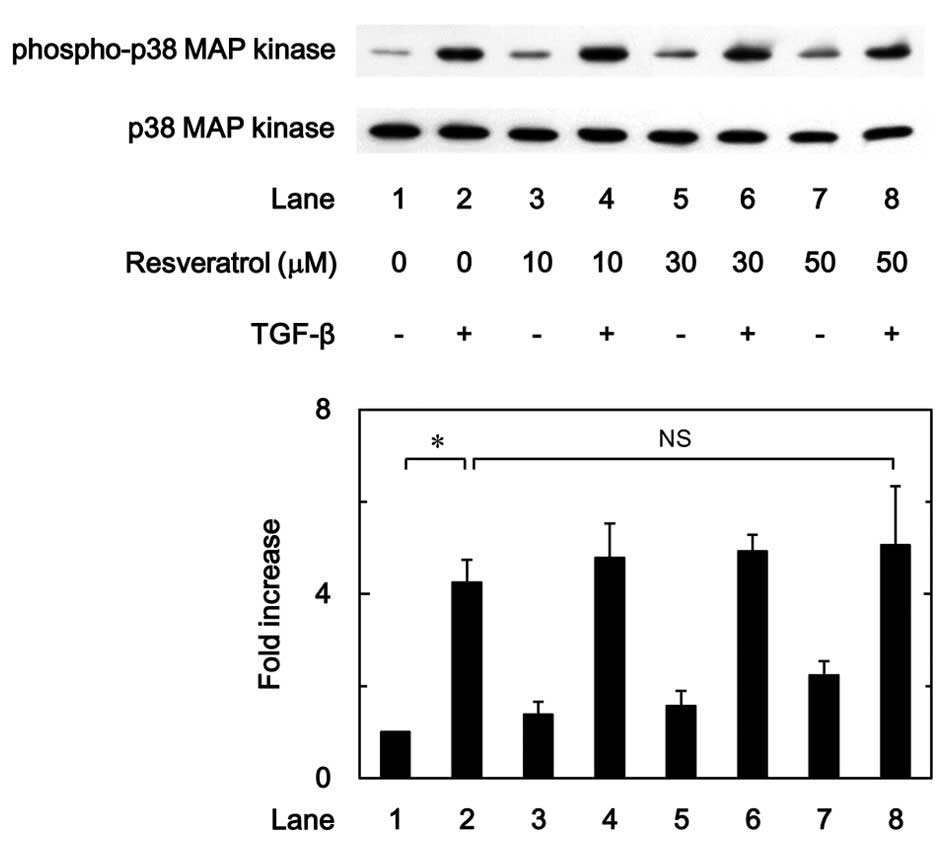
Effect of resveratrol on the
phosphorylation of p38 MAP kinase induced by TGF-β in MC3T3-E1
cells
In our previous study it was reported that the
TGF-β-induced VEGF synthesis in osteoblast-like MC3T3-E1 cells was
positively regulated by p38 MAP kinase, p44/p42 MAP kinase and
SAPK/JNK (9,10). The effect of resveratrol on the
TGF-β-induced phosphorylation of p38 MAP kinase was therefore
examined in the present study. The results showed that the
phosphorylation of p38 MAP kinase stimulated by TGF-β was not
affected by resveratrol in the range between 10 and 50 µM (Fig. 6).
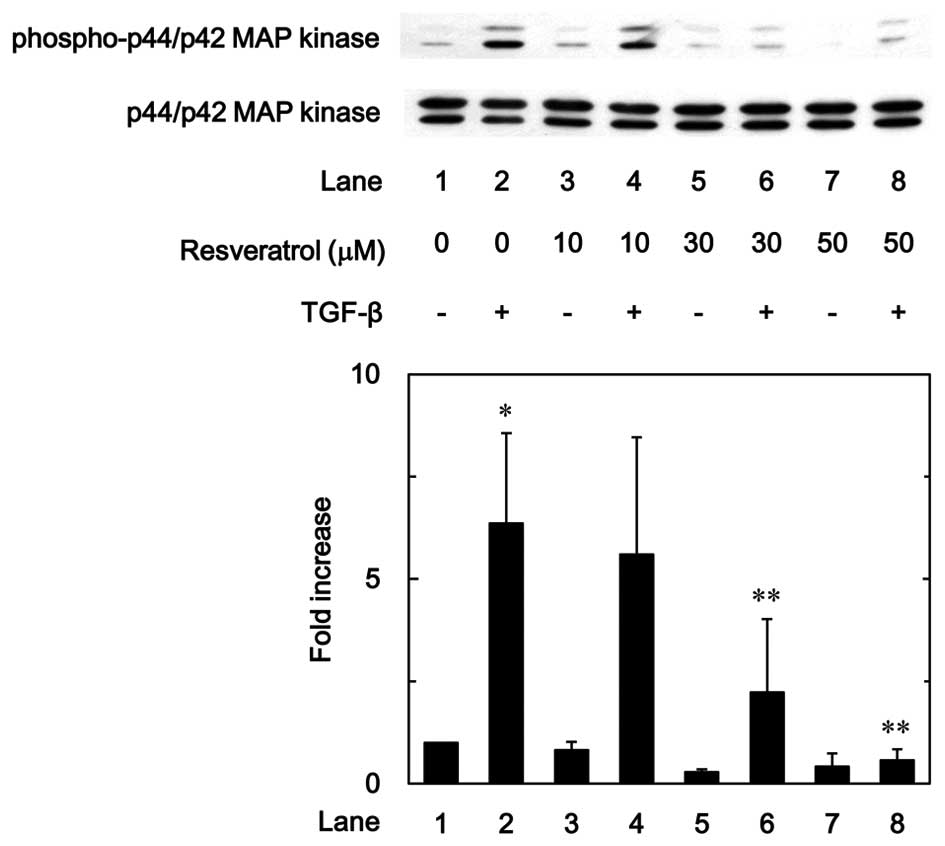
Effect of resveratrol on the
TGF-β-induced phosphorylation of p44/p42 MAP kinase and SAPK/JNK in
MC3T3-E1 cells
To investigate whether the inhibitory effect of
resveratrol on the TGF-β-stimulated VEGF synthesis was mediated
through the modulation of p44/p42 MAP kinase and/or SAPK/JNK
activation in MC3T3-E1 cells, the effect of resveratrol on the
TGF-β-induced phosphorylation of p44/p42 MAP kinase or SAPK/JNK was
examined. Resveratrol markedly suppressed the TGF-β-induced
phosphorylation of p44/p42 MAP kinase in a dose-dependent manner in
the range between 10 and 50 µM (Fig.
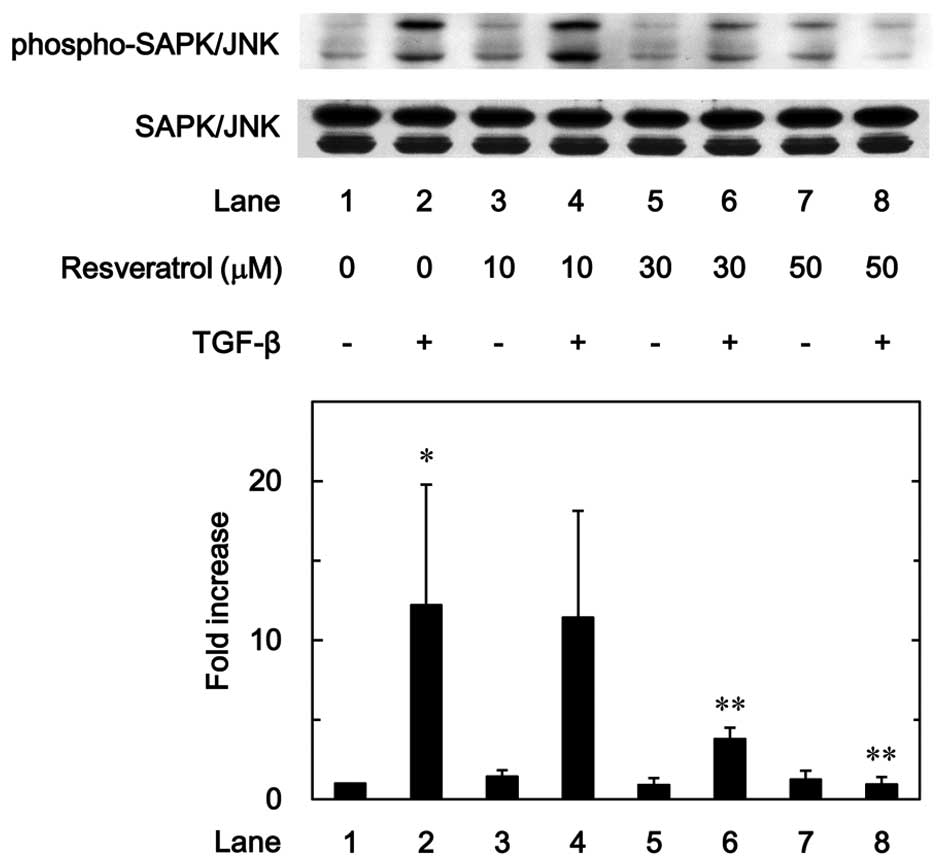
7). In addition, as shown in Fig.
8, the phosphorylation of SAPK/JNK stimulated by TGF-β was
dose-dependently attenuated by resveratrol in the range between 10
and 50 µM.
Effect of SRT1720 on the TGF-β-induced
phosphorylation of Smad, p38 MAP kinase, p44/p42 MAP kinase or
SAPK/JNK in MC3T3-E1 cells
The effects of SRT1720 on the TGF-β-induced
phosphorylation of Smad2, p38 MAP kinase, p44/p42 MAP kinase or
SAPK/JNK in osteoblast-like MC3T3-E1 cells were further
investigated. SRT1720 hardly affected the TGF-β-induced
phosphorylation of Smad2 or p38 MAP kinase (Fig. 9A and B); however, SRT1720
significantly suppressed the TGF-β-induced phosphorylation of
p44/p42 MAP kinase or SAPK/JNK (Fig. 9C
and D). Thus, the present findings suggest that SRT1720 mimics
the effects of resveratrol on the TGF-β-induced phosphorylation of
Smad2, p38 MAP kinase, p44/p42 MAP kinase and SAPK/JNK.
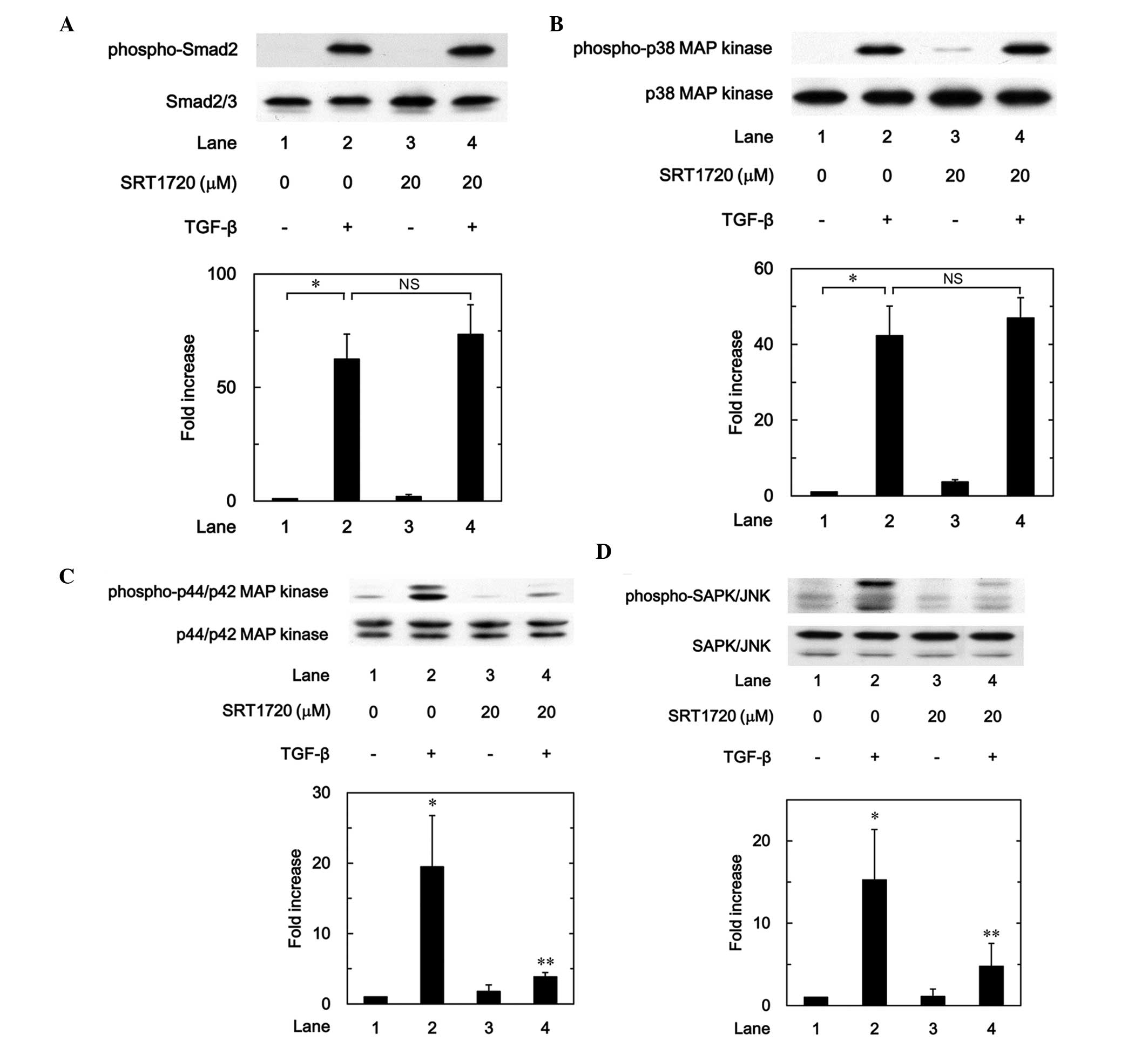 | Figure 9.Effects of SRT1720 on the
TGF-β-induced phosphorylation of (A) Smad, (B) p38 MAP kinase, (C)
p44/p42 MAP kinase or (D) SAPK/JNK in MC3T3-E1 cells. The cultured
cells were pretreated with 20 µM SRT1720 for 60 min, and then
stimulated by 5 ng/ml TGF-β or vehicle for 120 min. The cell
extracts were subsequently subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and western blot
analysis with antibodies against phospho-specific Smad2, Smad2/3,
phospho-specific p38 MAP kinase, p38 MAP kinase, phospho-specific
p44/p42 MAP kinase, p44/p42 MAP kinase, phospho-specific SAPK/JNK
or SAPK/JNK. The histograms show a quantitative representation of
the levels of TGF-β-induced phosphorylation obtained from a laser
densitometric analysis of three independent experiments. Each value
represents the mean ± standard error of the mean of triplicate
determinations. **P<0.05, compared with the value of the
control. **P<0.05, compared with the value of TGF-β alone.
TGF-β, transforming growth factor-β; SAPK/JNK, stress-activated
protein kinase/c-Jun N-terminal kinase; MAP, mitogen-activated
protein; NS, not significant. |
Discussion
In the present study, it was demonstrated that
resveratrol, a polyphenolic compound found in grape and berry skins
and red wine, significantly suppressed the TGF-β-stimulated release
of VEGF in osteoblast-like MC3T3-E1 cells. It was additionally
found that SRT1720, an activator of SIRT1 with a potency 1,000
times greater than that of resveratrol (23), markedly reduced the VEGF release
induced by TGF-β in these cells. Resveratrol has been shown to
induce the activation of SIRT1 and extend the life span of yeast
and mammalian mouse models (25,26);
therefore, it is possible that the inhibitory effect of resveratrol
on the TGF-β-induced VEGF release is mediated, at least to a
certain extent, by the activation of SIRT1 in MC3T3-E1 cells. In
addition, it was observed that resveratrol and SRT1720 reduced the
expression levels of VEGF mRNA following their upregulated by TGF-β
in those cells. Based on these findings, it is most likely that the
suppressive effect of resveratrol on the TGF-β-stimulated VEGF
synthesis is exerted at a point upstream of the transcriptional
level in osteoblast-like MC3T3-E1 cells.
The TGF-β super family includes TGF-β, BMPs and
activin (4). TGF-β signaling is a
simple linear cascade involving the TGF-β ligands, the Smad signal
transducers and two types of receptors (type I and II). The Smad
complex directly binds to particular elements of the DNA and
regulates the expression of the target gene (4). The present study showed that the
TGF-β-induced phosphorylation of Smad2 was not affected by
resveratrol in MC3T3-E1 cells; therefore, it is unlikely that the
effect of resveratrol observed was mediated through the
Smad-dependent pathway in osteoblast-like MC3T3-E1 cells. It has
been previously recognized, however, that the effects of TGF-β are
also exerted through the Smad-independent pathways, such as the MAP
kinase pathway (27). We have
previously shown that TGF-β upregulates the synthesis of VEGF
through the activation of p38 MAP kinase, p44/p42 MAP kinase and
SAPK/JNK in osteoblast-like MC3T3-E1 cells (9,10). Thus,
in the present study it was examined whether resveratrol could
affect the phosphorylation of p38 MAP kinase, p44/p42 MAP kinase or
SAPK/JNK induced by TGF-β in these cells. Although resveratrol
failed to affect the TGF-β-induced phosphorylation of p38 MAP
kinase, the phosphorylation of both p44/p42 MAP kinase and SAPK/JNK
stimulated by TGF-β was significantly reduced by resveratrol in the
MC3T3-E1 cells. Based on these findings, it is most likely that the
suppressive effect of resveratrol on the TGF-β-stimulated VEGF
synthesis is mediated by the inhibition of both p44/p42 MAP kinase
and SAPK/JNK in osteoblast-like MC3T3-E1 cells. In addition, it was
observed that SRT1720 reduced the phosphorylation of p44/p42 MAP
kinase and SAPK/JNK without affecting the phosphorylation of Smad2
or p38 MAP kinase, suggesting that the described inhibitory effect
of resveratrol is mediated via the activation of SIRT1 in
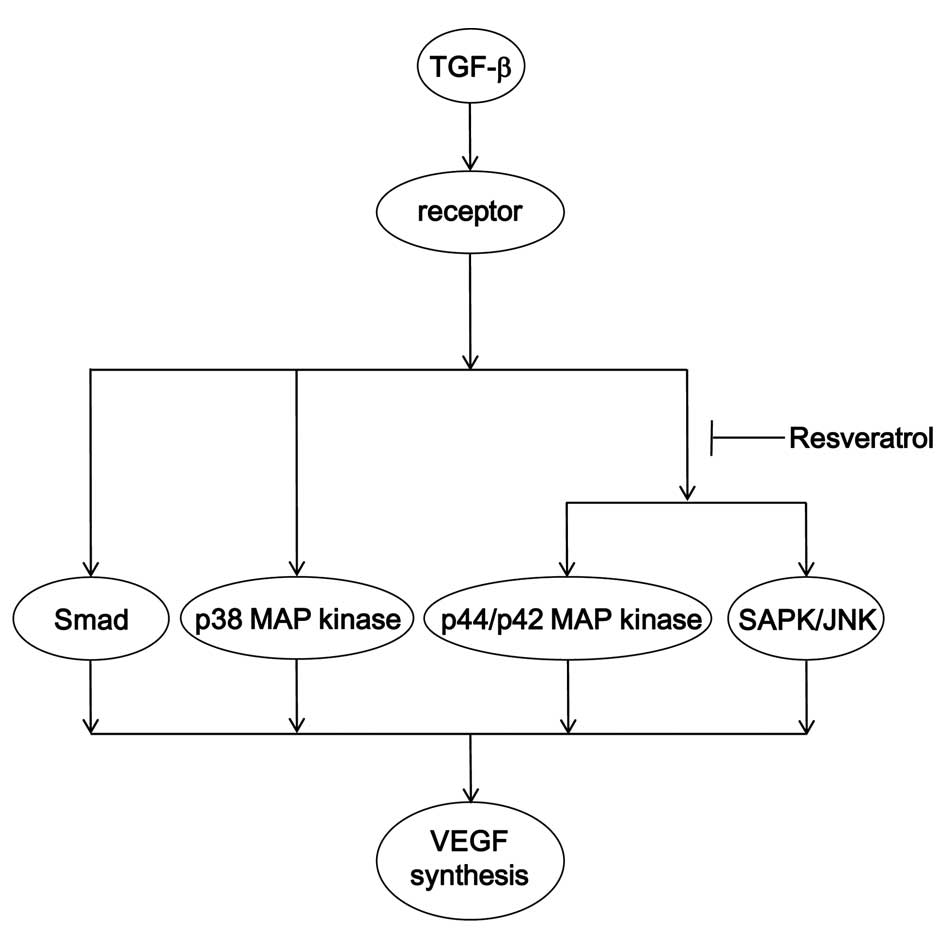
osteoblast-like MC3T3-E1 cells. A potential mechanism underlying
the action of resveratrol on the TGF-β-stimulated VEGF synthesis in
osteoblasts is summarized in Fig.
10.
TGF-β has a stimulatory effect on osteoblastic bone
formation (5). TGF-β reportedly
functions as an autacoid in osteoblasts, and modulates cellular
functions, including VEGF production (8). VEGF is an essential mediator of
angiogenesis, and plays a pivotal role in the process of bone
formation and fracture healing (8).
During bone fracture repair, osteoclasts produce heparinase, an
enzyme promoting the release of VEGF from heparin in an active
form, and contribute to local angiogenesis, osteoclast formation
and callus resorptive activities (5). Callus resorption is one of the
essential steps initiating the process of bone formation. With
regard to the favorable effect of resveratrol on the human health,
particularly bone health (14), it
is possible that the suppressive effect of resveratrol on the
TGF-β-induced VEGF synthesis in osteoblasts provides adequate
vascularization for the process of bone remodeling. Appropriate
vascularization is required in the regulation of bone turnover, and
adequate VEGF synthesis is considered to be essential for
maintaining both the quality and the quantity of bone mass. These
findings concerning the inhibitory effect of resveratrol on the
TGF-β-stimulated VEGF synthesis in osteoblasts may provide new
information about the role of VEGF as a ‘gate keeper’ of bone
tissue quality via the regulation of bone remodeling. Further
investigation is necessary to determine the exact mechanism through
which resveratrol affects VEGF synthesis in osteoblasts. In
conclusion, the present findings strongly suggest that the
TGF-β-stimulated VEGF synthesis is suppressed by resveratrol
through the inhibition of p44/p42 MAP kinase and SAPK/JNK in
osteoblasts, and that the inhibitory effect is exerted, at least in
part, via SIRT1 activation.
Acknowledgements
This investigation was supported in part by the
Grant-in-Aid for Scientific Research (no. 19591042) from the
Ministry of Education, Science, Sports and Culture of Japan and
Research Funding for Longevity Sciences (no. 23-9, 25-4) from the
National Center for Geriatrics and Gerontology, Japan. The authors
would like to thank Yumiko Kurokawa for her skillful technical
assistance.
References
|
1
|
Kular J, Tickner J, Chim SM and Xu J: An
overview of the regulation of bone remodelling at the cellular
level. Clin Biochem. 45:863–873. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chim SM, Tickner J, Chow ST, Kuek V, Guo
B, Zhang G, Rosen V, Erber W and Xu J: Angiogenic factors in bone
local environment. Cytokine Growth Factor Rev. 24:297–310. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parfitt AM: Targeted and nontargeted bone
remodeling: Relationship to basic multicellular unit origination
and progression. Bone. 30:5–7. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moustakas A and Heldin CH: The regulation
of TGFbeta signal transduction. Development. 136:3699–3714. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tang Y, Wu X, Lei W, Pang L, Wan C, Shi Z,
Zhao L, Nagy TR, Peng X, Hu J, et al: TGF-beta1-induced migration
of bone mesenchymal stem cells couples bone resorption with
formation. Nat Med. 15:757–765. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karsenty G and Wagner EF: Reaching a
genetic and molecular understanding of skeletal development. Dev
Cell. 2:389–406. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhen G and Cao X: Targeting TGFβ signaling
in subchondral bone and articular cartilage homeostasis. Trends
Pharmacol Sci. 35:227–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Clarkin CE and Gerstenfeld LC: VEGF and
bone cell signalling: An essential vessel for communication? Cell
Biochem Funct. 31:1–11. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tokuda H, Hatakeyama D, Akamatsu S, Tanabe
K, Yoshida M, Shibata T and Kozawa O: Involvement of MAP kinases in
TGF-beta-stimulated vascular endothelial growth factor synthesis in
osteoblasts. Arch Biochem Biophys. 415:117–125. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kanno Y, Ishisaki A, Yoshida M, Tokuda H,
Numata O and Kozawa O: SAPK/JNK plays a role in transforming growth
factor-beta-induced VEGF synthesis in osteoblasts. Horm Metab Res.
37:140–145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Blander G and Guarente L: The Sir2 family
of protein deacetylases. Annu Rev Biochem. 73:417–435. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koo SH and Montminy M: In vino veritas: A
tale of two sirt1s? Cell. 127:1091–1093. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Renaud S and de Lorgeril M: Wine, alcohol,
platelets, and the French paradox for coronary heart disease.
Lancet. 339:1523–1526. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kubo JT, Stefanick ML, Robbins J,
Wactawski-Wende J, Cullen MR, Freiberg M and Desai M: Preference
for wine is associated with lower hip fracture incidence in
post-menopausal women. BMC Womens Health. 13:362013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kondo A, Otsuka T, Kuroyanagi G, Yamamoto
N, Matsushima-Nishiwaki R, Mizutani J, Kozawa O and Tokuda H:
Resveratrol inhibits BMP-4-stimulated VEGF synthesis in
osteoblasts: Suppression of S6 kinase. Int J Mol Med. 33:1013–1018.
2014.PubMed/NCBI
|
|
16
|
Kuroyanagi G, Tokuda H,
Matsushima-Nishiwaki R, Kondo A, Mizutani J, Kozawa O and Otsuka T:
Resveratrol suppresses prostaglandin F(2α)-induced osteoprotegerin
synthesis in osteoblasts: Inhibition of the MAP kinase signaling.
Arch Biochem Biophys. 542:39–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kuroyanagi G, Mizutani J, Kondo A,
Yamamoto N, Matsushima-Nishiwaki R, Otsuka T, Kozawa O and Tokuda
H: Suppression by resveratrol of prostaglandin D2-stimulated
osteoprotegerin synthesis in osteoblasts. Prostaglandins Leukot
Essent Fatty Acids. 91:73–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sudo H, Kodama HA, Amagai Y, Yamamoto S
and Kasai S: In vitro differentiation and calcification in a new
clonal osteogenic cell line derived from newborn mouse calvaria. J
Cell Biol. 96:191–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kozawa O, Tokuda H, Miwa M, Kotoyori J and
Oiso Y: Cross-talk regulation between cyclic AMP production and
phosphoinositide hydrolysis induced by prostaglandin E2
in osteoblast-like cells. Exp Cell Res. 198:130–134. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Simpson DA, Feeney S, Boyle C and Stitt
AW: Retinal VEGF mRNA measured by SYBR green I fluorescence: A
versatile approach to quantitative PCR. Mol Vis. 6:178–183.
2000.PubMed/NCBI
|
|
21
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kato K, Ito H, Hasegawa K, Inaguma Y,
Kozawa O and Asano T: Modulation of the stress-induced synthesis of
hsp27 and alpha B-crystallin by cyclic AMP in C6 rat glioma cells.
J Neurochem. 66:946–950. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Milne JC, Lambert PD, Schenk S, et al:
Small molecule activators of SIRT1 as therapeutics for the
treatment of type 2 diabetes. Nature. 450:712–716. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo X and Wang XF: Signaling cross-talk
between TGF-beta/BMP and other pathways. Cell Res. 19:71–88. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Howitz KT, Bitterman KJ, Cohen HY, et al:
Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baur JA, Pearson KJ, Price NL, et al:
Resveratrol improves health and survival of mice on a high-calorie
diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moustakas A and Heldin CH: Non-Smad
TGF-beta signals. J Cell Sci. 118:3573–3584. 2005. View Article : Google Scholar : PubMed/NCBI
|