Introduction
Myopia is a global health problem that has social,
educational and economic consequences, and significantly affects
the quality of life of sufferers (1). There is growing evidence to suggest
that the prevalence of myopia is increasing; it is one of the five
ocular conditions that are considered an immediate priority by the
World Health Organization's Global Initiative for the Elimination
of Avoidable Blindness (2). There is
considerable variation in the prevalence rate of myopia worldwide.
Currently, ~1/3 of the world's population is affected (3,4), and in
certain populations in East Asia, the incidence rate of myopia is
>80% (5–8). Although there are numerous methods of
improving the blurred vision associated with myopia, including
wearing corrective lenses or refractive surgery, possible
interventions for the pathogenesis of myopia have been intensively
studied. Several studies have revealed that a parental history of
myopia may be linked to the prevalence of the disorder; however, it
is not yet understood whether parental myopia denotes a common
family environment or a genetic susceptibility (9–11).
A number of epidemiological studies have
demonstrated a positive association between the prevalence of
myopia in parents and a child's risk of developing myopia (12–14).
However, there are large inconsistencies in the odds ratios (ORs)
among these studies (ranging from 1.48 to 7.90). Furthermore, other
studies have identified no statistically significant association
between parental myopia and a child's risk of developing myopia
(15–17). These contrasting conclusions may be
due to differences in the study designs. The association between
parental myopia and a child's risk of developing myopia has not
yet, to the best of our knowledge, been investigated through
meta-analysis. Therefore, the present study conducted a
meta-analysis, by extracting data from observational studies with
various designs, to quantitatively investigate the association
between parental myopia and a child's risk of developing
myopia.
Materials and methods
Study outline
The current study systematically reviewed
potentially eligible literature for a meta-analysis of prospective
cohort, cross-sectional and case-control studies in accordance with
the Meta-analysis of Observational Studies in Epidemiology (MOOSE)
guidelines and the Preferred Reporting Items for Systematic Reviews
and Meta-analyses (PRISMA) statement (18,19).
Search strategy
The MEDLINE (articles from 1966 to June 1, 2013),
Embase (articles from 1980 to June 1, 2013), and Ovid (articles
from 1950 to June 1, 2013) databases were searched for prospective
cohort, cross-sectional and case-control studies that did not have
access restrictions. All relevant studies using Medical Subject
Headings (MeSH) or free text words were selected. The MeSH search
strategies were followed and the search terms for exposure (parent,
parental, family, history), and outcomes (myopia, myopic,
short-sight, short sight, near-sight, near sight, refractive
errors) were combined. Furthermore, a number of potential studies
were identified electronically by searching the reference lists of
the relevant publications. These publications were scrutinized in
an effort to identify additional relevant studies.
Selection criteria
The reviewers independently evaluated potential
published studies that quantitatively estimated the association
between parental myopia and a child's risk of developing myopia.
The titles of the studies were first evaluated to ascertain the
possibility of the study fitting the selection criteria of the
meta-analysis. The abstracts, as well as the methods and results,
of studies that were deemed potentially relevant were subsequently
reviewed. Those studies over which there was uncertainty as to
whether they fulfilled the selection criteria were also reviewed.
Any discrepancies between the reviewers were resolved through
arbitration, and any differences were settled by consensus. Studies
were included in the meta-analysis if they fulfilled the following
criteria: i) children, adolescents or youth were included as
participants; ii) the exposure of interest was parents with myopia;
iii) the outcome of interest was myopia amongst children (prevalent
or incident) and; iv) risk estimates, including relative risks
(RRs), ORs, hazard ratios (HRs), or other measures that it was
possible to transform into ORs with 95% confidence intervals (CIs),
were reported. Studies that did not meet the inclusion criteria
were excluded during the initial review phase.
Data extraction and quality
assessment
The reviewers independently extracted all data using
a standardized data collection form. Any inconsistencies were
resolved through discussion and by consulting the original
articles. The following data were collected from each study: first
author's surname, publication year, country, recruitment date, size
of the study population, gender and age of the participants, number
of cases, measure and range of exposure, and risk estimates with
corresponding CIs of a child's risk of developing myopia. ORs and
95% CIs that reflected the degree of control for potential
confounders were extracted for use in the main analyses. A third
reviewer resolved any disagreement in the abstracted data.
Statistical analysis
The OR was used to assess associations across
studies. RRs and HRs were transformed into ORs using a previously
described method (20). The OR was
pooled to summarize the associations between one or two parents
with myopia and a child's risk of developing the condition.
Pooled estimations and complete analyses across
studies were obtained using random-effects models throughout the
meta-analysis (21). The
heterogeneity of the studies was assessed using Cochran's Q test
and the I2 statistic (22). As suggested by Higgins and Thompson,
I2 values of 25, 50 and 75% were considered to indicate
low, moderate and high heterogeneity, respectively (23). Subgroup analyses were conducted to
assess the potential association between a child's risk of
developing myopia and relevant study characteristics (including
participants' age, geographical location, follow-up time,
recruitment date and the study design) as possible sources of
heterogeneity. A funnel plot of the overall ORs was generated and
this produced a standard error (ER) which was used to assess
publication bias using Egger's and Begg's regression tests. The
‘trim and fill’ procedure was also performed to ascertain the
possible effect of publication bias in the meta-analysis. This
method considered the possibility of hypothetical ‘missing’ studies
and imputed their RRs, thus obtaining a pooled RR that combined the
hypothetical missing studies with the actual studies used (24,25).
Stata 10 (StataCorp, College Station, TX, USA) was used to carry
out all the analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Final study inclusions
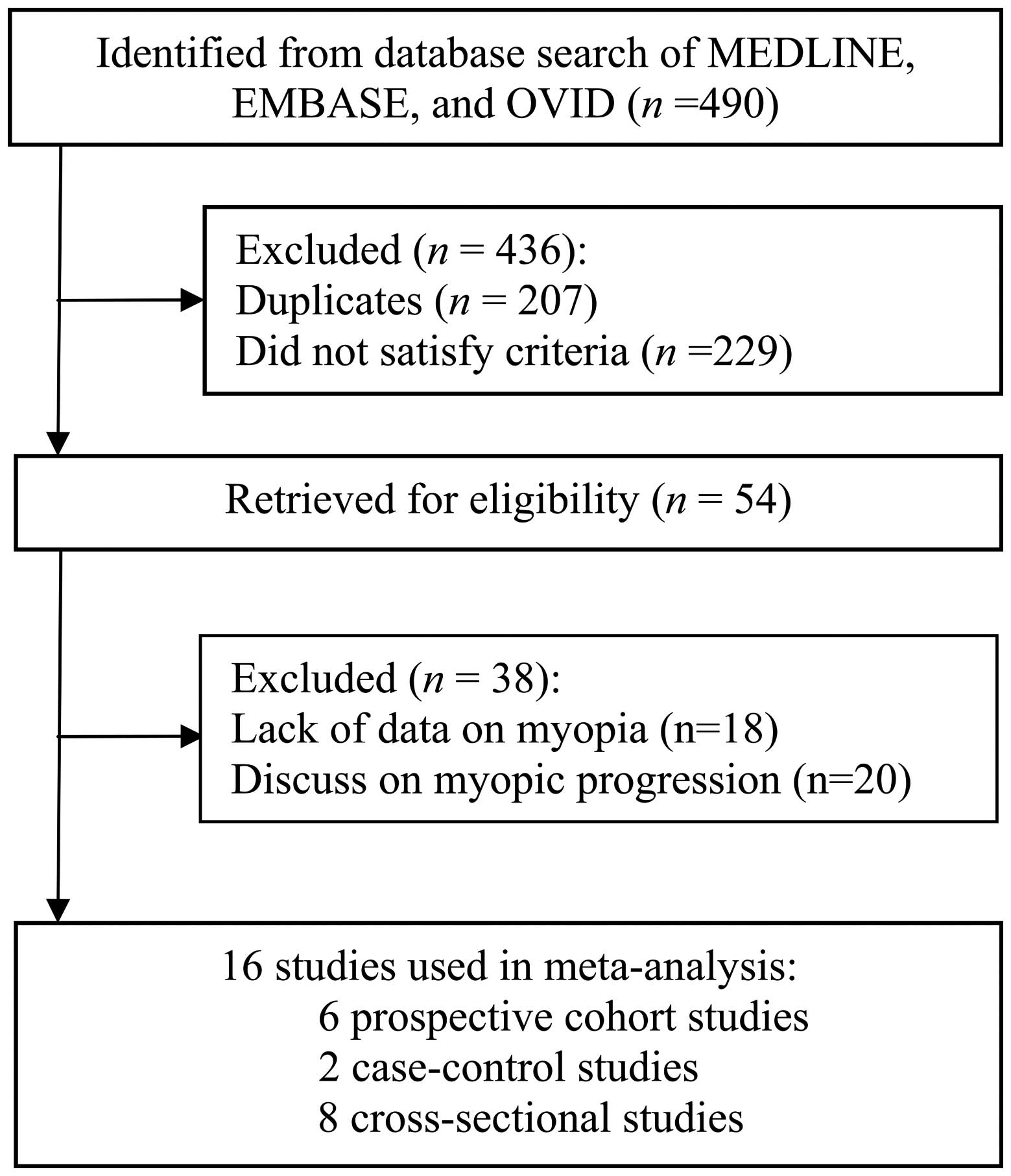
Fig. 1 shows the
detailed procedure that was employed to search for the literature
included in the current meta-analysis. Following the evaluation of
titles and abstracts, 54 manuscripts were identified that fulfilled
the selection criteria of the present study. The full texts of
these studies were reviewed for eligibility. Following this review,
a number of manuscripts were excluded due to information, including
outcome, the exposure of interest or essential data, being omitted.
A total of 16 studies were eligible for final inclusion (12–17,26–34). Of
these, six were prospective cohort (12,13,15,17,31,32),
eight were cross-sectional (14,16,26–30,35) and
two were case-control studies (33,34).
Characteristics and quality of the
study cohorts
Table I shows the
characteristics of the included studies. The present meta-analysis
included 16 studies with 8,393 cases of myopia. The selected
studies were from four continents (seven from Asia (14,16,26,28,32,34,35),
four from Europe (17,29,31,33),
three from the United States (12,13,30) and
two from Australia (15,27). All of the studies recruited male and
female participants aged ≤31 years. The majority of studies used
questionnaires to ascertain the parents' history of myopia and to
assess whether parents wore spectacles or contact lenses at the
time of the study. Seven of the studies identified the
participants' myopia as a spherical equivalent refraction (SER)
≤-0.5 diopters (D) (14–16,27,28,33,35) and
two studies identified the participants' myopia as a SER ≤-0.75 D
(26,32). Three of the studies reported the
participants' myopia as a SER ≤-0.75 D in the horizontal and
vertical meridians following cycloplegic autorefraction (12,13,30). The
participants' myopia in another four studies was identified as SER
≤-1.5 D (17), a visual acuity ≥6/9
following correction with a concave lens >0.5 D (34), a SER between −0.75 and −2.99 D
(31), and self-reported wearing of
‘minus’ glasses (29).
 | Table I.Characteristics of the studies
included in the meta-analysis of published studies on a child's
risk of developing myopia with myopic parents. |
Table I.
Characteristics of the studies
included in the meta-analysis of published studies on a child's
risk of developing myopia with myopic parents.
| Study (Ref.) | Country | Design | Study name | Gender and age
(years) | Recruitment date
(years of follow-up) | No. of cases (study
size) | Definition of
myopia | Adjustments |
|---|
| French et al
2013 (15) | Australia | Cohort | Sydney Adolescent
Vascular and Eye Study (SAVES) | Male/female
(9.5) | 2003–2005
(6.1) | 335 (2,059) | SER ≤-0.5 D | Age and gender |
| Xiang et al
2012 (14) | China |
Cross-sectional | Guangzhou
refractive error study in children (RESC) | Male/female
(5–15) | 2002 (−) | 3,421 (4,364) | SER ≤-0.5 D | – |
| Wu et al
2010 (26) | Taiwan |
Cross-sectional | – | Male/female
(7–12) | 2007 (−) | 45 (145) | SER ≤-0.75 D | – |
| Low et al
2010 (16) | Singapore |
Cross-sectional | STrabismus,
Amblyopia and Refractive error Singaporean children (STARS)
study | Male/female
(0.5–6) | 2006 and 2008
(−) | 301 (3,009) | SER ≤-0.5 D | Family clusters,
age gender, height, time spent outdoors, and words or pictures
read |
| Jones-Jordan et
al 2010 (13) | United States | Cohort Evaluation
of Ethnicity and Refractive Error (CLEERE) | Collaborative
Longitudinal | Male/female
(6) | 1989–2005
(5.32) | 334 (1,854) | SER ≤-0.75 D in
both the horizontal and vertical meridians. | Gender, site and
ethnicity |
| Konstantopoulos
et al 2008 (33) | Greece | Case-control | – | Male/female
(17–31) | 2002–2003 (−) | 99 (200) | SER ≤-0.5 D | – |
| Williams et
al 2008 (17) | UK | Cohort | Avon Longitudinal
Study of Parents and Children (ALSPAC) | Male/female
(7–10) | 1991 (7) | 102 (6,871) | SER ≤-1.5 D | Gender, mother's
partner's education, and ethnicity |
| Ip et al
2007 (27) | Australia |
Cross-sectional | Sydney Myopia Study
(SMS) | Male/female
(11.1–14.4) | – | 220 (2,353) | SER ≤-0.5 D | Age, gender, near
work, time spent outdoors, and ethnicity |
| Jones et al
2007 (12) | United States | Cohort | Orinda Longitudinal
Study of Myopia | Male/female
(8–9) | 1989 (12) | 111 (514) | SER ≤-0.75 D in the
horizontal and vertical meridians. | Hours spent doing
sports/being outdoors and reading |
| Onal et al
2007 (31) | Turkey | Cohort | – | Male/female
(18–26) | 2003 (1) | 68 (207) | SER between −0.75
and −2.99 D | – |
| Saw et al
2006 (32) | Singapore | Cohort | Singapore Cohort
Study Of the Risk Factors for Myopia (SCORM) | Male/female
(7–9) | 1999 (3) | 454 (994) | SER ≤-0.75 D | Age, gender,
school, parental income, books read per week, and IQ |
| Khader et al
2006 (28) | Jordan |
Cross-sectional | | Male/female
(12–17) | – | 313 (1,777) | SER ≤-0.5 D | – |
| Khandekar et
al 2005 (34) | Oman | Case-control | – | Male/female
(16–17) | 2002–2003 (−) | 1440 (2,853) | Vision 6/9 or more
following correction with concave lens of >0.5 D | – |
| Vannas et al
2003 (29) | Finland |
Cross-sectional | – | Male/female
(19.2) | 1999–2000 (−) | 782 (3,524) | Self-reported
wearing of ‘minus’ glasses | Conscript's
education, eye color, BMI, sunglasses wearing, region, myopic
siblings, parents' education, unweighted near-work and weighted
near-work |
| Saw et al
2002 (35) | Singapore and
China |
Cross-sectional | – | Male/female
(7–9) | 1999–2000 (−) | 299 (957) | SER ≤-0.5D | Books read per
week, age, night-light use prior to 2 years of age and country |
| Mutti et al
2002 (30) | United States |
Cross-sectional | Orinda Longitudinal
Study of Myopia | Male/female
(13–14) | 1991 (5) | 67 (366) | SER ≤-0.75D in the
horizontal and vertical meridians | Diopter-hours per
week, sports, reading, local ITBS percentile score and ITBS total
language local percentile score |
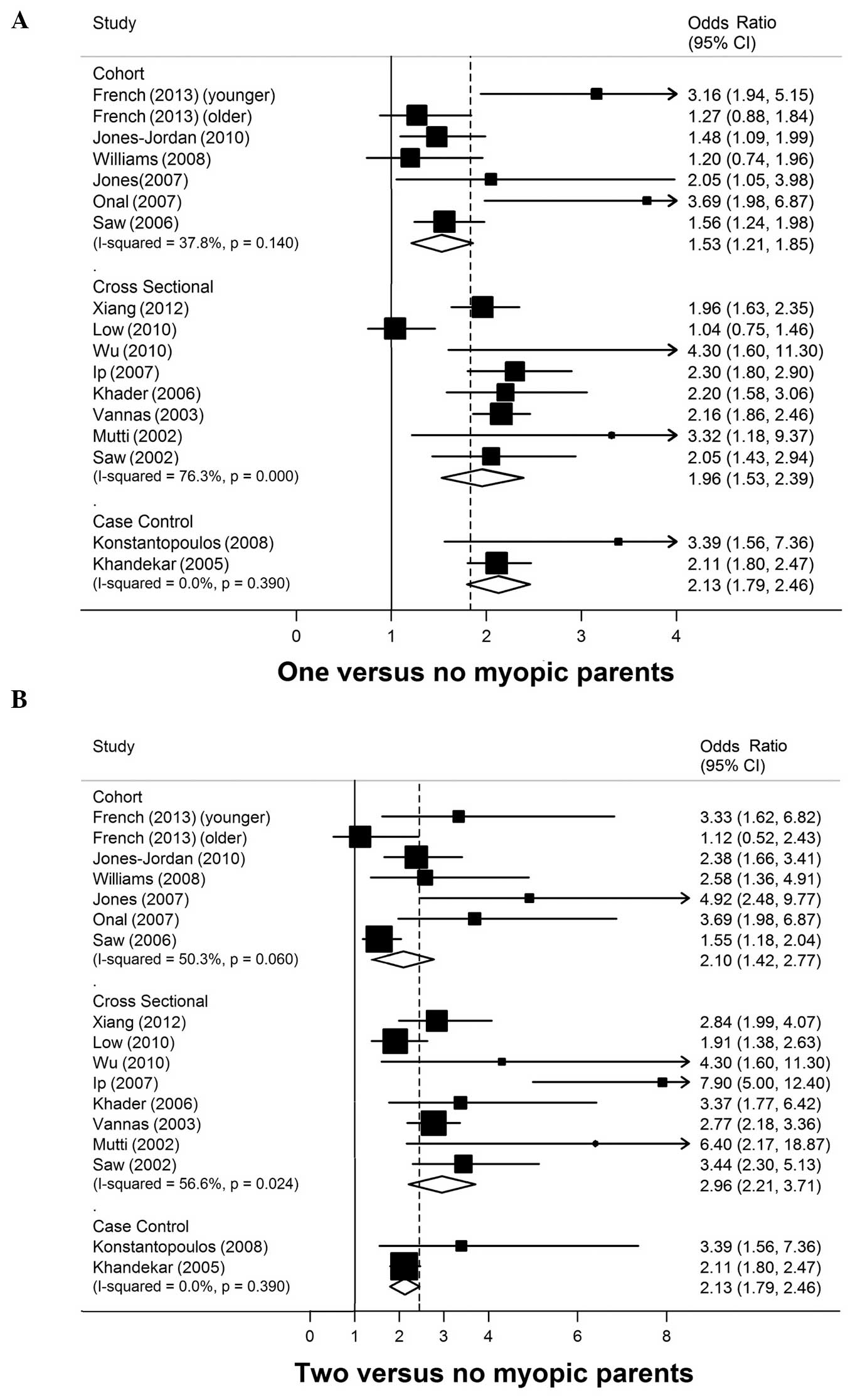
Main analyses
A total of 16 studies with 31,677 participants and
8,393 cases of myopia were included in the present analysis. The
pooled ORs for each study and for the studies that combined data
for ‘having parents with myopia’ vs. ‘no parents with myopia’ are
shown in Fig. 2. There was a
statistically significant positive association between myopia in
one or two parents and a child's risk of developing myopia. The ORs
and 95% CIs in the prospective cohort, cross-sectional and
case-control studies were 1.53 (95% CI, 1.21–1.85), 1.96 (95% CI,
1.53–2.39), and 2.13 (95% CI, 1.79–2.46), respectively, for myopia
in one parent and 2.10 (95% CI, 1.42–2.77), 2.96 (95% CI,
2.21–3.71), and 2.13 (95% CI, 1.79–2.46), respectively, for myopia
in two parents. No heterogeneity was observed in the case-control
studies with myopia in one or two parents (P=0.39,
I2=0%).
Publication bias
Visual inspection of the funnel plot revealed a
certain level of asymmetry. The Begg's test (P=0.18 and P=1.00 for
included cohort and cross-sectional studies, respectively) and
Egger's test (P=0.05 and P=0.46 for included cohort and
cross-sectional studies, respectively) did not suggest any evidence
for publication bias in the analysis of participants with one
parent with myopia. However, Egger's test implied a certain level
of publication bias in studies that investigated the association
between a child's risk of myopia and myopia in two parents (P=0.03
and P=0.04 for included cohort and cross-sectional studies,
respectively) whereas Begg's test did not (P=0.05 and P=0.22 for
included cohort and cross-sectional studies, respectively). The
‘trim and fill’ method identified the existence of possible missing
studies in the analysis of participants with myopia in one parent
(two and three missing cohort and cross-sectional studies,
respectively) and with myopia in two parents (three and four
missing cohort and cross-sectional studies, respectively). However,
the filled studies did not influence the results (for myopia in one
parent OR, 1.44; 95% CI, 1.03–1.84 in cohort studies; OR, 1.85; 95%
CI, 1.47–2.23 in cross-sectional studies; for myopia in two
parents: OR, 1.79; 95% CI, 1.09–2.48 in cohort studies; OR, 2.56;
95% CI, 1.78–3.35 in cross-sectional studies).
Subgroup and sensitivity analyses
With respect to the sensitivity analysis, Table II shows the results of subgroup
analyses stratified according to the study characteristics. For the
prospective cohort studies, age, geographical location, recruitment
date or years of follow-up did not significantly influence the
association between myopia in one parent and a child's risk of
developing myopia. Neither age nor years of follow-up influenced
the association between myopia in two parents and a child's risk of
developing the condition. Geographical location and recruitment
date were identified to be possible sources of heterogeneity
(P<0.01 and P=0.03, respectively) in the studies of myopia in
two parents.
 | Table II.Subgroup analysis for myopia (one or
two parents with myopia vs. no parents with myopia). |
Table II.
Subgroup analysis for myopia (one or
two parents with myopia vs. no parents with myopia).
|
|
| Heterogeneity
within subgroup in participants with one myopic parent | P-value for
heterogeneity between subgroups | Heterogeneity
within subgroup in participants with two myopic parents | P-value for
heterogeneity between subgroups |
|---|
|
|
|---|
| Subgroups | No. of studies | OR (95% CI) | I2
(%) | P-value for
heterogeneity | OR (95% CI) | I2
(%) | P-value for
heterogeneity |
|---|
| Cohort |
|
|
|
|
|
|
|
|
|
| Age
(years) |
|
|
|
|
|
|
|
|
|
|
<12 | 5 | 1.55
(1.21–1.89) | 29.1 | 0.23 | 0.54 | 52.24
(1.44–3.04) | 49.1 | 0.1 | 0.47 |
|
≥12 | 2 | 1.53
(1.21–1.86) | 72.4 | 0.06 |
| 22.15
(−0.32–4.62) | 72.8 | 0.06 |
|
|
Geographical location |
|
|
|
|
|
|
|
|
|
|
Asia and
Australia | 2 | 1.60
(1.04–2.16) | 60.7 | 0.08 | 0.86 | 21.52
(0.96–2.07) | 21.7 | 0.28 | <0.01 |
|
US and Europe | 4 | 1.53
(1.02–2.05) | 33.7 | 0.21 |
| 42.63
(1.90–3.37) | 0 | 0.47 |
|
|
Recruitment date (years) |
|
|
|
|
|
|
|
|
|
|
<1999 | 3 | 1.42
(1.07–1.77) | 63.0 | 0.04 | 0.61 | 32.53
(1.76–3.30) | 0 | 0.41 | 0.03 |
|
≥1999 | 3 | 1.77
(1.13–2.41) | 0 | 0.53 |
| 31.75
(0.95–2.55) | 45.5 | 0.14 |
|
|
Follow-up (years) |
|
|
|
|
|
|
|
|
|
|
<6 | 3 | 1.58
(1.18–1.99) | 34.1 | 0.22 | 0.46 | 32.09
(1.20–2.99) | 61.8 | 0.07 | 0.97 |
|
≥6 | 3 | 1.56
(0.95–2.17) | 50.5 | 0.11 |
| 32.43
(0.95–3.92) | 56.1 | 0.08 |
|
|
Cross-sectional |
|
|
|
|
|
|
|
|
|
| Age
(years) |
|
|
|
|
|
|
|
|
|
|
<2 | 4 | 1.70
(1.00–2.40) | 81.0 | <0.01 | <0.01 | 42.61
(1.76–3.46) | 47.0 | 0.13 | 0.12 |
|
≥12 | 4 | 2.20
(1.95–2.45) | 0 | 0.92 |
| 44.16
(2.02–6.30) | 62.6 | 0.05 |
|
|
Geographical location |
|
|
|
|
|
|
|
|
|
|
Asia | 5 | 1.80
(1.20–2.40) | 78.3 | <0.01 | <0.01 | 52.65
(1.89–3.41) | 37.3 | 0.17 | 0.17 |
|
Others | 3 | 2.20
(1.93–2.46) | 0 | 0.79 |
| 35.16
(1.05–9.28) | 74.6 | 0.02 |
|
The present study examined age and geographical
location as possible sources of heterogeneity in the
cross-sectional studies. Age and geographical location were
observed as sources of heterogeneity (both P<0.01) in studies
with myopia in one parent; however there was no evidence that age
or geographical location were a source of heterogeneity in studies
with myopia in two parents.
Discussion
The current study, which included 31,677
participants and 8,393 cases of myopia, revealed that parental
myopia has a significant positive association with a child's risk
of developing myopia. Children of two parents with myopia have a
higher risk of developing myopia than those who have one parent
with myopia.
The underlying mechanisms responsible for this
association may be consistent with genetic and environmental
factors, or with gene-environment interactions (27,36–41).
Zadnik et al reported that the eyes of children who had two
parents with myopia had longer axial lengths and a smaller
hyperopic refractive error than the eyes of children who had one or
no parents with myopia (11). A
cross-sectional sample from the Orinda Longitudinal Study of Myopia
in 716 children, aged from 6 to 14 years, confirmed these results
(30). These studies suggest that
the size of pre-myopic eyes may be influenced by parental myopia
(42), and that a higher number of
myopic parents is associated with an increase in the axial length
of eyes in childhood (11,43).
A number of previous studies have extensively
examined the impact of genetic effects on myopia in humans
(44–46). The analysis of genes involved in the
scleral extracellular matrix (ECM) is a common feature of studies
on syndromic high myopia (47). A
meta-analysis that investigated the genetic variants of the high
myopia present in the Han Chinese population confirmed that four
single nucleotide polymorphisms (SNPs) have genome-wide
significance. Of these SNPs, rs2730260 is located on the VIPR2
gene, which is positioned in the MYP4 locus, whilst the other three
SNPs (rs7839488, rs4395927, and rs4455882) are in the same linkage
disequilibrium block, which is located on the SNTB1 gene. The VIPR2
and SNTB1 genes are expressed in the retina and in the retinal
pigment epithelium. The authors of the study therefore suggested
that variants of the VIPR2 and SNTB1 genes increase susceptibility
to high myopia in the Han Chinese population (48).
A large number of chromosomal localizations have
been reported (MYP1-MYP17) for cases of non-syndromic high myopia;
however, only a few specific genes have been identified. MYP16 is
an exception since mutations in the cadherin-associated protein,
situated on this gene, have been identified and replicated
(47,49). Although there are several issues with
replication, Wojciechowski (44)
demonstrated that a number of the reported mutations form a
coherent nexus of linked structural and metabolic constituents in
the scleral ECM. A recent genome-wide meta-analysis, which included
27 studies with participants of European ancestry and five Asian
cohorts, identified 16 new loci for refractive error in individuals
with European ancestry. Eight of these loci are shared with
individuals of Asian descent. The new loci include candidate genes
with functions in neurotransmission (GRIA4), ion transport (KCNQ5),
retinoic acid metabolism (RDH5), ECM remodeling (LAMA2 and BMP2),
and eye development (SIX6 and PRSS56) (50). Genes associated with a hereditary
susceptibility for myopia may explain the results obtained in the
present meta-analysis.
Myopia with a positive parental history is
frequently assumed to have a hereditary origin, although families
are known to share lifestyle behaviors as well as genes.
Alternatively, there is a theory that parents with myopia, who are
generally more educated, create environments that may lead to the
development of myopia in their children. For instance, these
parents may place higher educational demands on their children, who
may therefore spent less time outdoors (14). Furthermore, parents who read
extensively may also encourage their children to read more
frequently (16). Since a limited
number of influential factors were studied in the current analysis,
further literature evidence should be quoted in order to confirm
the important role of environmental factors in the development of
myopia in children.
To the best of our knowledge, the current study is
the first meta-analysis to estimate the association between myopia
in one or two parents and a child's risk of developing the
condition. A major strength of the present study is the large
number of participants (n=31,677) and cases of myopia (n=8,393)
from different ethnic groups that were included in the analyses,
which significantly increased the statistical power of the study.
Another advantage was the comprehensive and elaborate literature
search that was conducted. Three comprehensive databases were
searched using a wide-range of search terms.
The present meta-analysis had certain limitations.
Firstly, as it is based on the results of observational studies,
the possibility that other factors may explain the observed
associations between parental myopia and a child's risk of
developing myopia cannot be excluded. Therefore, the possibility of
residual confounders remains. It is also difficult to completely
rule out that either genetic factors or a shared parent-child
environment was responsible for the observed associations.
Secondly, data deficits, data restriction and data
of variable quality were used with varying definitions for myopia,
and this may have weakened the strength of the associations
observed. As myopia in parents was mainly identified using
questionnaires, the possibility of recall bias and error were
inevitable. The definition of childhood myopia also varied between
studies and this may have resulted in an over or under estimation
of the risk.
Publication bias existed in our analysis, as shown
by the funnel plot and the Egger's and Begg's tests. Furthermore,
the ‘trim and fill’ method identified possible missing studies.
Nevertheless, the meta-analysis revealed that ‘filled’ studies did
not influence the results. The OR of the pooled estimate was
modified from 1.53 (95% CI, 1.21–1.85) to 1.44 (95% CI, 1.03–1.84)
in cohort studies and from 1.96 (95% CI, 1.53–2.39) to 1.85 (95%
CI, 1.47–2.23) in cross-sectional studies with myopia in one
parent, and from 2.10 (95% CI, 1.42–2.77) to 1.79 (95% CI,
1.09–2.48) in cohort studies, and from 2.96 (95% CI, 2.21–3.71) to
2.56 (95% CI, 1.78–3.35) in cross-sectional studies with myopia in
two parents.
Finally, methodological differences in the designs
of the studies may have introduced heterogeneity. Following
subgroup analysis, the present study revealed that geographical
location and recruitment year may be possible sources of
heterogeneity in the current analysis, which included cohort
studies with myopia in two parents. With respect to the
cross-sectional studies, age and geographical location were
identified as possible sources of heterogeneity in the current
analysis with myopia in one parent. Further well-designed cohort
studies with adequate controls for confounding factors are
required, particularly studies that allow for long-term follow-up
of children as well as studies amongst populations in East
Asia.
The present meta-analysis included cross-sectional
and case-control studies that had small or inadequate sample sizes.
This may have resulted in large effect estimates and the
heterogeneous entry criteria may have limited the study results.
These issues may have reduced the strength of the results obtained
in the current study.
In conclusion, the present meta-analysis revealed a
significant positive association between parental myopia and a
child's risk of developing myopia. Furthermore, the study
demonstrated that children with two myopic parents have a higher
risk of developing myopia than those with one myopic parent.
References
|
1
|
Saw SM, Katz J, Schein OD, Chew SJ and
Chan TK: Epidemiology of myopia. Epidemiol Rev. 18:175–187. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pararajasegaram R: VISION 2020-the right
to sight: from strategies to action. Am J Ophthalmol. 128:359–360.
1999.PubMed/NCBI
|
|
3
|
He M, Zeng J, Liu Y, Xu J, Pokharel GP and
Ellwein LB: Refractive error and visual impairment in urban
children in southern China. Invest Ophthalmol Vis Sci. 45:793–799.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu HM, Seet B, Yap EP, Saw SM, Lim TH and
Chia KS: Does education explain ethnic differences in myopia
prevalence? A population-based study of young adult males in
Singapore. Optom Vis Sci. 78:234–239. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morgan I and Rose K: How genetic is school
myopia? Prog Retin Eye Res. 24:1–38. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kempen JH, Mitchell P, Lee KE, et al: The
prevalence of refractive errors among adults in the United States,
Western Europe, and Australia. Arch Ophthalmol. 122:495–505. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vitale S, Ellwein L, Cotch MF, Ferris FL
III and Sperduto R: Prevalence of refractive error in the United
States, 1999–2004. Arch Ophthalmol. 126:1111–1119. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin LL, Shih YF, Hsiao CK and Chen CJ:
Prevalence of myopia in Taiwanese schoolchildren: 1983 to 2000. Ann
Acad Med Singapore. 33:27–33. 2004.PubMed/NCBI
|
|
9
|
Young TL, Metlapally R and Shay AE:
Complex trait genetics of refractive error. Arch Ophthalmol.
125:38–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rose KA, Morgan IG, Smith W and Mitchell
P: High heritability of myopia does not preclude rapid changes in
prevalence. Clin Experiment Ophthalmol. 30:168–172. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zadnik K, Satariano WA, Mutti DO, Sholtz
RI and Adams AJ: The effect of parental history of myopia on
children's eye size. JAMA. 271:1323–1327. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jones LA, Sinnott LT, Mutti DO, Mitchell
GL, Moeschberger ML and Zadnik K: Parental history of myopia,
sports and outdoor activities, and future myopia. Invest Ophthalmol
Vis Sci. 48:3524–3532. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jones-Jordan LA, Sinnott LT, Manny RE, et
al: Early childhood refractive error and parental history of myopia
as predictors of myopia. Invest Ophthalmol Vis Sci. 51:115–121.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiang F, He M and Morgan IG: The impact of
parental myopia on myopia in Chinese children: population-based
evidence. Optom Vis Sci. 89:1487–1496. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
French AN, Morgan IG, Mitchell P and Rose
KA: Risk factors for incident myopia in Australian schoolchildren:
the Sydney adolescent vascular and eye study. Ophthalmology.
120:2100–2108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Low W, Dirani M, Gazzard G, et al: Family
history, near work, outdoor activity, and myopia in Singapore
Chinese preschool children. Br J Ophthalmol. 94:1012–1016. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Williams C, Miller LL, Gazzard G and Saw
SM: A comparison of measures of reading and intelligence as risk
factors for the development of myopia in a UK cohort of children.
Br J Ophthalmol. 92:1117–1121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stroup DF, Berlin JA, Morton SC, et al:
Meta-analysis Of Observational Studies in Epidemiology (MOOSE)
group: Meta-analysis of observational studies in epidemiology: a
proposal for reporting. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: PRISMA Group: Preferred reporting items for systematic reviews
and meta-analyses: the PRISMA statement. Ann Intern Med.
151:264–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ronksley PE, Brien SE, Turner BJ, Mukamal
KJ and Ghali WA: Association of alcohol consumption with selected
cardiovascular disease outcomes: a systematic review and
meta-analysis. BMJ. 342:d6712011. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Der Simonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Higgins JP and Thompson SG: Quantifying
heterogeneity in a meta-analysis. Stat Med. 21:1539–1558. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Egger M, Davey Smith G, Schneider M and
Minder C: Bias in meta-analysis detected by a simple, graphical
test. BMJ. 315:629–634. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sutton AJ, Duval SJ, Tweedie RL, Abrams KR
and Jones DR: Empirical assessment of effect of publication bias on
meta-analyses. BMJ. 320:1574–1577. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu PC, Tsai CL, Hu CH and Yang YH: Effects
of outdoor activities on myopia among rural school children in
Taiwan. Ophthalmic Epidemiol. 17:338–342. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ip JM, Huynh SC, Robaei D, et al: Ethnic
differences in the impact of parental myopia: findings from a
population-based study of 12-year-old Australian children. Invest
Ophthalmol Vis Sci. 48:2520–2528. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khader YS, Batayha WQ, Abdul-Aziz SM and
Al-Shiekh-Khalil MI: Prevalence and risk indicators of myopia among
schoolchildren in Amman, Jordan. East Mediterr Health J.
12:434–439. 2006.PubMed/NCBI
|
|
29
|
Vannas AE, Ying GS, Stone RA, Maguire MG,
Jormanainen V and Tervo T: Myopia and natural lighting extremes:
risk factors in Finnish army conscripts. Acta Ophthalmol Scand.
81:588–595. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mutti DO, Mitchell GL, Moeschberger ML,
Jones LA and Zadnik K: Parental myopia, near work, school
achievement, and children's refractive error. Invest Ophthalmol Vis
Sci. 43:3633–3640. 2002.PubMed/NCBI
|
|
31
|
Onal S, Toker E, Akingol Z, et al:
Refractive errors of medical students in Turkey: one year follow-up
of refraction and biometry. Optom Vis Sci. 84:175–180. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saw SM, Shankar A, Tan SB, et al: A cohort
study of incident myopia in Singaporean children. Invest Ophthalmol
Vis Sci. 47:1839–1844. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Konstantopoulos A, Yadegarfar G and
Elgohary M: Near work, education, family history, and myopia in
Greek conscripts. Eye (Lond). 22:542–546. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khandekar R, Al Harby S and Mohammed AJ:
Determinants of myopia among Omani school children: a case-control
study. Ophthalmic Epidemiol. 12:207–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saw SM, Zhang MZ, Hong RZ, Fu ZF, Pang MH
and Tan DT: Near-work activity, night-lights, and myopia in the
Singapore-China study. Arch Ophthalmol. 120:620–627. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang CL, Yen E, Su JY, et al: Impact of
family history of high myopia on level and onset of myopia. Invest
Ophthalmol Vis Sci. 45:3446–3452. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kurtz D, Hyman L, Gwiazda JE, et al: COMET
Group: Role of parental myopia in the progression of myopia and its
interaction with treatment in COMET children. Invest Ophthalmol Vis
Sci. 48:562–570. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feldkamper M and Schaeffel F: Interactions
of genes and environment in myopia. Dev Ophthalmol. 37:34–49. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu MM and Edwards MH: The effect of having
myopic parents: an analysis of myopia in three generations. Optom
Vis Sci. 76:387–392. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Saw SM, Nieto FJ, Katz J, Schein OD, Levy
B and Chew SJ: Familial clustering and myopia progression in
Singapore school children. Ophthalmic Epidemiol. 8:227–236. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pacella R, McLellan J, Grice K, Del Bono
EA, Wiggs JL and Gwiazda JE: Role of genetic factors in the
etiology of juvenile-onset myopia based on a longitudinal study of
refractive error. Optom Vis Sci. 76:381–386. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pan CW, Ramamurthy D and Saw SM: Worldwide
prevalence and risk factors for myopia. Ophthalmic Physiol Opt.
32:3–16. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Saw SM, Chua WH, Hong CY, et al: Nearwork
in early-onset myopia. Invest Ophthalmol Vis Sci. 43:332–339.
2002.PubMed/NCBI
|
|
44
|
Wojciechowski R: Nature and nurture: the
complex genetics of myopia and refractive error. Clin Genet.
79:301–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Baird PN, Schache M and Dirani M: The
GEnes in Myopia (GEM) study in understanding the aetiology of
refractive errors. Prog Retin Eye Res. 29:520–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hornbeak DM and Young TL: Myopia genetics:
a review of current research and emerging trends. Curr Opin
Ophthalmol. 20:356–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Morgan IG, Ohno-Matsui K and Saw SM:
Myopia. Lancet. 379:1739–1748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shi Y, Gong B, Chen L, et al: A
genome-wide meta-analysis identifies two novel loci associated with
high myopia in the Han Chinese population. Hum Mol Genet.
22:2325–2333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Li YJ, Goh L, Khor CC, et al: Genome-wide
association studies reveal genetic variants in CTNND2 for high
myopia in Singapore Chinese. Ophthalmology. 118:368–375. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Verhoeven VJ, Hysi PG, Wojciechowski R, et
al: Genome-wide meta-analyses of multiancestry cohorts identify
multiple new susceptibility loci for refractive error and myopia.
Nat Genet. 45:314–318. 2013. View Article : Google Scholar : PubMed/NCBI
|