Introduction
Diabetes comprises a group of metabolic diseases in
which blood sugar levels are high for a prolonged period of time. A
Chinese epidemiological survey in 2010 revealed that the total
prevalence rate of diabetes was up by 9.7% (1). Erectile dysfunction (ED) is sexual
dysfunction characterized by the inability to develop or maintain
an erection of the penis during sexual activity over the course of
three months (2), and is one of the
chronic complications in males with type 2 diabetes. Numerous
studies have reported that the incidence of ED in diabetic
individuals varies between 30 and 80%, which is six times higher
compared with normal individuals, and augments with an increase in
age and duration of diabetes (3,4). The
pathogenesis of diabetic ED is complex. Diabetic angiopathies
include macrovascular disease, microvascular disease and
endothelial dysfunction, which are all known to be involved in the
pathophysiological process of ED (5–7).
Generally, a diagnosis of ED depends on the illness
history, the international index of erectile function-5 (IIEF-5)
and the hardness scale of penile erection (8,9).
However, ED is often a private matter and considering that patients
are not recognizing ED, there is a doubt that the aforementioned
tests will diagnose ED. Evidently, there are other methods for
diagnosing ED, such as nocturnal penile tumescence, magnetic
resonance angiography, corpus cavernosometry and penile nerve
function. However, these methods are complex and traumatic; thus,
have not been adapted to clinical application (10).
microRNA (miRNA) are small non-coding RNA molecules
(containing ~22 nucleotides) found in animals and plants, which
functions in transcription and post-transcription to negatively
regulate gene expression (11).
Certain miRNA molecules are used as biomarkers since they are
present in the serum. Furthermore, previous studies have indicated
that miRNA plays an important role in the development of diabetes
and diabetic complications (12–15). The
current preliminary trial investigated the relevance of miR-93,
miR-320 and miR-16 in the incidence of diabetic ED. Reverse
transcription quantitative polymerase chain reaction (PCR) was used
to detect the expression levels of the miRNAs in diabetic patients
with ED, and the clinical value of the miRNAs was investigated as
diagnostic evidence for diabetic ED.
Materials and methods
Subjects
In total, 40 diabetic patients with ED (ED group)
were recruited from Nanjing Hospital of Nanjing Medical University
(Nanjing, China) between July 2012 and December 2013. The mean age
was 47.37±5.81 years, and mean diabetes duration was 5.40±2.56
years. The diagnosis criteria of diabetes was in accordance with
the World Health Organization's criteria (16), and IIEF-5 scores were used to assess
whether the diabetic patients suffered from ED. In addition, 40
diabetic patients without ED (NED group) were selected. The mean
age was 46.05±4.88 years, and the duration of diabetes was
5.72±2.33 years. In addition, 40 healthy individuals from the same
community, who did not have any organic lesions, were selected as a
control group; the mean age was 43.9±5.69 years. No statistically
significant differences were observed among the three groups with
regard to age or the duration of diabetes (between the ED and NED
groups). The study was conducted in accordance with the Declaration
of Helsinki and with approval from the Ethics Committee of Nanjing
Medical University. Written informed consent was obtained from all
the participants.
Clinical data
IIEF-5 scores were obtained using a questionnaire.
Body mass index (BMI) was calculated by formula: BMI = Body
weight/height2 (kg/m2). Levels of fasting
plasma glucose (FPG), glycated hemoglobin (HbA1c), total
cholesterol (TC), triglyceride (TG) and total testosterone (TT)
were determined using an exsanguinate assay. The presence of
macroangiopathy (MA), diabetic retinopathy (DR) and diabetic
nephropathy (DN) were evaluated by vascular B ultrasound,
microalbuminuria test and fundus examination.
Blood sample collection
Peripheral blood samples (3 ml) were collected in
the morning and stored in coagulation-promoting tubes (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China). Samples were then
centrifuged at 2,054 × g for 5 min and the supernatant was stored
at −80°C in the centrifuge tube.
Total RNA extraction
A 100-µl sample of serum was treated with phenol and
chloroform (Sigma-Aldrich, St. Louis, MO, USA). RNA was extracted
following treatment with isopropanol and sodium acetate (pH 5.2).
After washing with 75% ethanol, the RNA purity was measured using a
NanoDrop 1000 ultraviolet spectrophotometer (Thermo Fisher
Scientific, Waltham, MA, USA).
Reverse transcription-quantitative
PCR
Total RNA (1 µg) was reverse transcribed into cDNA
in a 20-µl reaction volume using a PrimeScript miRNA qPCR starter
kit (version 2.0; Takara Biotechnology Co. Ltd., Dalian, China),
according to the manufacturer's instructions. LET-7d, LET-7g and
LET-7i were used as internal standards (17). Quantitative PCR was performed with a
PCR 7500 (Applied Biosystems Life Technologies, Foster City, CA,
USA) at the following thermal cycling conditions: One cycle of 30
sec at 95°C, followed by 40 cycles of 5 sec at 95°C and 34 sec at
60°C. The data were presented as the relative expression level of
the gene of interest compared with the internal control gene, as
determined using the 2−ΔΔCt method as follows: ΔCt =
CtmiRNA – 1/3(CtLET-7d + CtLET-7g
+ CtLET-7i). Primers (miR-93, miR-320, miR-16, LEt-7d,
LEt-7g and LEt-7i) were purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA) and the sequences are listed in Table I. Each experiment was repeated three
times.
 | Table I.Primers used for reverse
transcription polymerase chain reaction. |
Table I.
Primers used for reverse
transcription polymerase chain reaction.
| miRNA | Primer |
|---|
| miR-93 |
CAAAGTGCTGTTCGTGCAGGTAG |
| miR-320 |
AAAAGCTGGGTTGAGAGGGCGA |
| miR-16 |
TAGCAGCACGTAAATATTGGCG |
| LET-7d |
AGAGGTAGTAGGTTGCATAGTT |
| LET-7g |
TGAGGTAGTAGTTTGTACAGTT |
| LET-7i |
TGAGGTAGTAGTTTGTGCTGTT |
Statistical analysis
Statistical analysis was performed using SPSS
software, version 17.0 (SPSS, Inc, Chicago, IL, USA). Data are
presented as the mean ± standard deviation. Comparisons between
groups were performed using the t-test or the χ2 test.
Pearson's correlation and linear regression analyses were used to
determine the associations between certain indices in the different
groups. In addition, receiver operating characteristic (ROC) curves
were used to assess the sensitivity and specificity of miR-93,
miR-320 and miR-16 as diagnostic markers for ED. P<0.05 was
considered to indicate a statistically significant difference.
Results
Comparison of the clinical data
As shown in Table
II, there were no statistically significant differences with
regard to age in the three groups (P>0.05), and there was no
statistically significant difference in the duration of diabetes or
the BMI between the ED and NED groups (P>0.05). When compared
with the control group, the BMI was evidently increased in the ED
and NED groups (P<0.001 and P<0.05, respectively). The
differences in FPG, TC and TG levels between the ED and NED groups
were not statistically significant different (P>0.05); however,
the indices in the ED and NED groups were statistically different
when compared with the control group (P<0.05). Furthermore, the
TT level was much lower in the ED group when compared with the NED
and control groups (P<0.05). In addition, the incidence rate of
MA in the ED group was similar to that in the NED group (52.5 vs.
55%, P>0.05); however, the incidence rate of DR (37.5%) and DN
(40%) was higher in the ED group when compared with the NED group
(10 and 17.5%, respectively; P<0.05).
 | Table II.Comparisons of clinical data in three
groups. |
Table II.
Comparisons of clinical data in three
groups.
| Parameter | ED group | NED group | Control group | P1 | P2 | P3 |
|---|
| Age, years | 47.37±5.81 | 46.05±4.88 | 46.9±5.69 | 0.273 | 0.713 | 0.476 |
| Diabetes duration,
years | 5.40±2.56 | 5.72±2.33 | – | 0.554 | – | – |
| BMI,
kg/m2 | 26.2±3.47 | 25.90±3.49 | 23.79±3.47 | 0.721 | <0.001 | 0.002 |
| IIEF-5 score | 16.97±3.95 | 23.82±0.78 | 23.80±0.78 | <0.001 | <0.001 | 0.885 |
| FPG, mmol/l | 10.79±3.22 | 11.07±2.47 | 4.23±1.08 | 0.657 | <0.001 | <0.001 |
| HbA1c, % | 8.22±1.08 | 7.73±1.02 | 4.97±0.83 | 0.039 | <0.001 | <0.001 |
| TC, mmol/l | 5.47±0.70 | 5.27±0.66 | 4.17±0.62 | 0.181 | <0.001 | <0.001 |
| TG, mmol/l | 1.64±1.31 | 1.32±0.35 | 1.07±0.42 | 0.144 | 0.011 | 0.005 |
| TT, ng/ml | 3.69±0.78 | 4.29±1.11 | 4.53±0.97 | 0.006 | <0.001 | 0.305 |
| MA, n (%) | 21 (52.5) | 22 (55) | – | 0.823 | – | – |
| DR, n (%) | 15 (37.5) | 4 (10) | – | 0.004 | – | – |
| DN, n (%) | 16 (40) | 7 (17.5) | – | 0.026 | – | – |
Relative factor and logistic
regression analysis
Pearson's correlation analysis indicated that the
incidence of diabetic ED negatively correlated with the serum TT
levels (r=0.302, P<0.05); however, a positive correlation was
observed with the HbA1c levels (r=0.231, P<0.05; Table III).
 | Table III.Correlation analysis between the
incidence of diabetic erectile dysfunction and other indices. |
Table III.
Correlation analysis between the
incidence of diabetic erectile dysfunction and other indices.
| Correlation | BMI | FPG | HbA1c | TC | TG | TT |
|---|
| R-value | 0.41 | −0.05 | 0.231 | 0.151 | 0.165 | −0.302 |
| P-value | 0.721 | 0.657 | 0.039 | 0.181 | 0.144 | 0.006 |
The predictor-dependent variable was represented by
the presence of ED, while the independent variables were BMI, FPG,
HbA1c, TC, TG and TT. Logistic regression analysis (Table IV) revealed that HbA1c and TT levels
were impacting factors on diabetic ED (P<0.05), and that the
odds ratio values of HbA1c and TT were 1.783 [95% confidence
interval (CI), 1.057–3.007] and 0.437 (95% CI, 0.231–0.827),
respectively, which indicated that HbA1c was a risk factor and TT
was a protective factor for diabetic ED. Specifically, a high HbA1c
level or a low TT level promoted the incidence of diabetic ED.
 | Table IV.Correlations between erectile
dysfunction and biochemical parameters using logistic regression
analysis. |
Table IV.
Correlations between erectile
dysfunction and biochemical parameters using logistic regression
analysis.
| Statistic | BMI | FPG | HbA1c | TC | TG | TT |
|---|
| β-value | 0.04 | 0.066 | 0.578 | 0.298 | 0.351 | −0.828 |
| SE | 0.81 | 0.105 | 0.267 | 0.390 | 0.355 | 0.325 |
| P-value | 0.623 | 0.528 | 0.03 | 0.445 | 0.324 | 0.011 |
| OR | 1.040 | 1.069 | 1.783 | 1.347 | 1.420 | 0.437 |
| 95% CI | 0.888–1.219 | 0.869–1.314 | 1.057–3.007 | 0.627–2.895 | 0.708–2.850 | 0.231–0.827 |
Relative expression levels of miR-93,
miR-320 and miR-16
Expression levels of miR-93, miR-320 and miR-16 were
higher in the ED group when compared with the NED and control
groups (P<0.05; Table V).
 | Table V.Relative expression levels of miR-93,
miR-320 and miR-16 in the three groups. |
Table V.
Relative expression levels of miR-93,
miR-320 and miR-16 in the three groups.
| miRNA | ED group | NED group | Control group | P1 | P2 | P3 |
|---|
| miR-93
(2−ΔΔCt) |
0.98±0.31 |
0.75±0.28 |
0.66±0.19 | <0.05 | <0.05 | 0.88 |
| miR-320
(2−ΔΔCt) |
4.33±0.90 |
3.19±0.86 |
3.09±0.95 | <0.05 | <0.05 | 0.635 |
| miR-16
(2−ΔΔCt) |
1.06±0.30 |
0.71±0.22 |
0.69±0.28 | <0.05 | <0.05 | 0.784 |
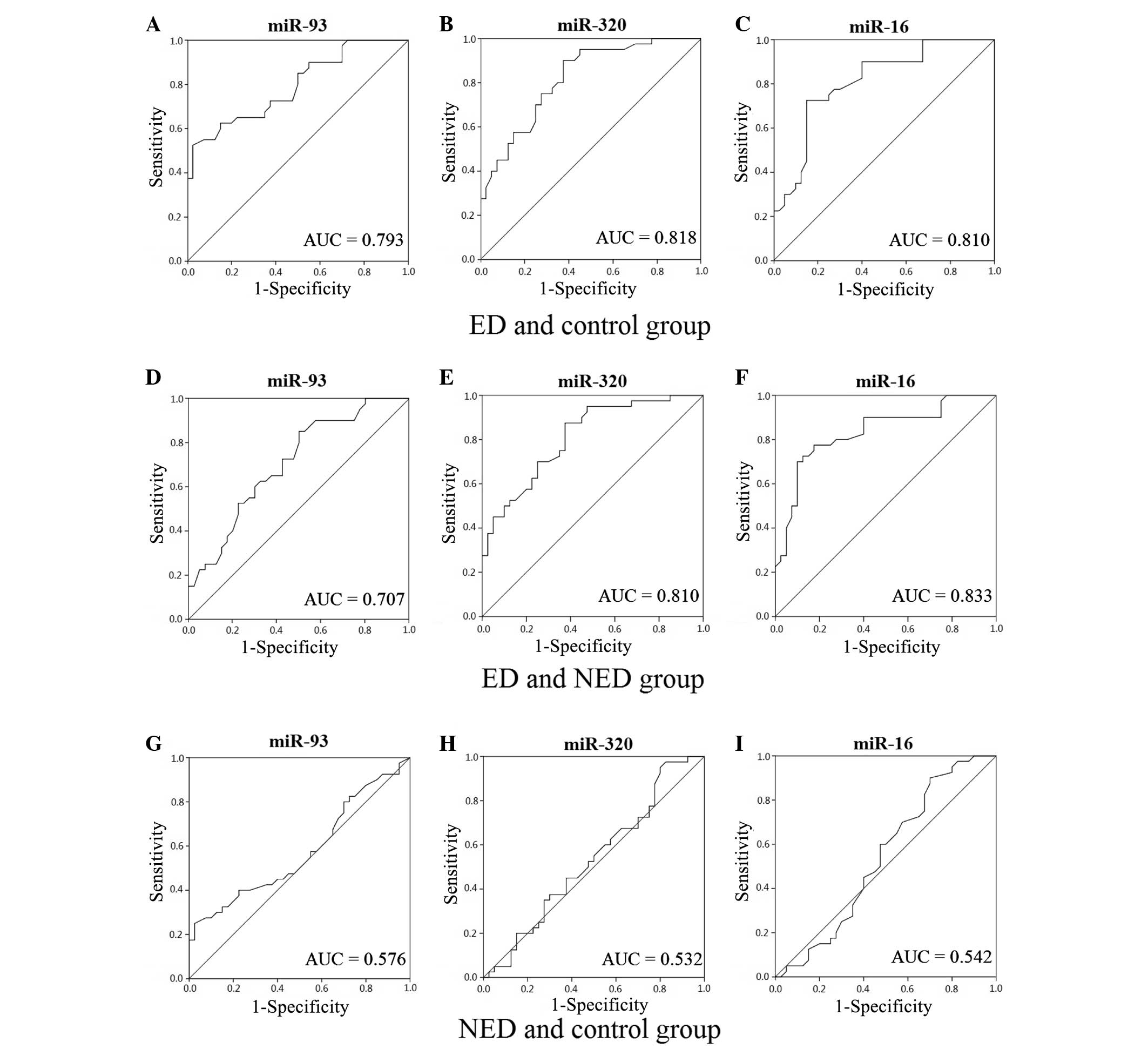
ROC curves for miR-93, miR-320 and
miR-16
When compared with the control group, the area under
the curve (AUC) values for miR-93, miR-320 and miR-16 in the ED
group were 0.793 (95% CI, 0.696–0.890), 0.818 (95% CI, 0.728–0.909)
and 0.810 (95% CI, 0.715–0.905), respectively. Additionally, when
compared with the control group, the AUC values for miR-93, miR-320
and miR-16 in the NED group were 0.576 (95% CI, 0.449–0.702), 0.532
(95% CI, 0.405–0.660) and 0.542 (95% CI, 0.412–0.671),
respectively. When compared with the NED group, the AUC values for
miR-93, miR-320 and miR-16 in the ED group were 0.707 (95% CI,
0.594–0.820), 0.810 (95% CI, 0.718–0.903) and 0.833 (95% CI,
0.743–0.924), respectively (Fig. 1).
The AUC results indicated that miR-93, miR-320 and miR-16 had a
diagnostic value in diabetic ED.
Discussion
Diabetes comprises a number of metabolic diseases in
which the blood sugar levels are high over a prolonged period
(18,19). With a population increasing in age, a
reduction in sporting activity and a high incidence of obesity, the
number of individuals suffering from diabetes is increasing. ED is
characterized by the regular or repeated inability to maintain an
erection and is one of the most common complications of diabetes.
Although ED is a benign disease, the condition impacts the physical
and mental health of the patient, and is closely associated with
the quality of life, the relationship with a sexual partner and
family stability. A retrospective study that analyzed diabetic
complications in the USA over the past 20 years found that since
the prevalence of diabetes continues to increase,
diabetes-associated complications have become a huge burden of the
disease (20).
The risk factors of diabetic ED include high HbA1c
levels, a high BMI, insulin resistance and angiopathy (4,21,22).
Diabetic macroangiopathy, microangiopathy and endothelial
dysfunction are all involved in the pathophysiological progression
of ED. Briefly, diabetic microangiopathy causes atherosclerosis and
thrombosis, which leads to the narrowing of the artery lumen,
subsequently reducing bloodflow to the penis. In addition,
microangiopathy is involved in ED through affecting autonomic
neuropathies (23–27). In the present study, the results
demonstrated that the incidence of DR and DN was significantly
higher in the ED group compared with the NED group (P<0.05),
which indicated that diabetic patients with ED suffered from severe
microangiopathy. Hermans et al (28) reported similar results.
Changes in the androgenic level are also an
important factor in diabetic ED (29,30).
Diabetes affects the secretion of androgen, which plays a key role
in maintaining sexual desire; therefore, low androgenic levels
caused by diabetes reduces sexual desire, which leads to the
incidence of ED. The results of the present study revealed that the
TT level was significantly lower in the ED group when compared with
the NED group (P<0.05). According to the logistic regression
analysis, the TT level was a protective factor in diabetic ED;
thus, the incidence of diabetic ED may increase when the TT level
is lower than the normal level. Kataoka et al (31) found that androgen replacement therapy
for erectile function in rats with type 2 diabetes was able to
increase the intracranial/mean arterial pressure ratio to suppress
inflammation and metabolic disorders and improve the endothelial
and erectile functions. By contrast, the HbA1c level is a risk
factor in diabetic ED; a high HbA1c level promotes the incidence of
diabetic ED (32). In addition, the
HbA1c level is proportional to the average blood glucose
concentration over the previous four weeks to three months. The
long-term dyscontrol of blood sugar levels means that the chronic
complications of patients with diabetes are likely to deteriorate,
including diabetic angiopathy, which may aggravate the conditions
of diabetic ED. In addition, a high HbA1c level is known to
indicate long-term high blood glucose levels, which affect the
function of the hypothalamic-pituitary-gonad axis, causing the
reduction of gonadotropin and follicle-stimulating hormone
secretion (33). Subsequently,
changes are inflicted on Leydig cells, which ultimately impacts
penile erectile function (34).
miRNA are small non-coding RNA molecules of ~22
nucleotides that are found in animals and plants. The molecules
function in transcription and post-transcription to negatively
regulate gene expression. miRNA play a critical role in the
incidence of diabetes and diabetic complications (35). Wang et al (36) reported that the expression of miR-320
was increased in myocardial microvascular endothelial cells
(MMVECs), and that the target genes may be insulin-like growth
factor (IGF)-1 and IGF-1 receptor. It was found that the
upregulation of miR-320 in MMVECs from Goto-Kakizaki rats may be
responsible for the inconsistency between the expression of IGF-1
protein and mRNA; therefore, miR-320 may be associated with
impaired angiogenesis in diabetes (36). Furthermore, Long et al
(37) demonstrated that transfection
of miR-93 prevented the effect of high glucose on vascular
endothelial growth factor (VEGF) downstream targets; thus, tropic
VEGF levels were increased through inhibition of miR-93 gene
expression. Villeneuve et al (38,39)
found that miR-125b may increase the expression of inflammatory
genes in vascular endothelial cells to cause endothelial
dysfunction. Weber et al (40) and Fleissner et al (41) also found that miR-21 was able to
reduce the rate of apoptosis in vascular endothelial cells and
promote the release of nitric oxide to protect the vascular
endothelium. Caporali et al (42) reported that inhibition of miR-15
improved endothelial function in culture conditions mimicking
diabetes mellitus (high D-glucose), and CCNE1 and CDC25A were
direct miR-15 targets. The present study found that miR-93, miR-320
and miR-16 were highly expressed in the diabetic ED group,
indicating that diabetic angiopathy played an important role in the
onset of ED in patients with diabetes.
In conclusion, the results of the current study
demonstrated that the HbA1c level was a risk factor for diabetic
ED, while the TT level was a protective factor, which was the same
as a previous result; however, the BMI, FPG, TC and TG did not show
a correlation with diabetic ED (4,21,22,29–31).
These results may have been caused by the small sample size. In
addition, detecting the levels of miR-93, miR-320 and miR-16
through a quantitative PCR assay and ROC curve analysis may aid the
diagnosis of diabetic ED. However, whether additional miRNAs
exhibit correlations with diabetic ED was unable to be determined
due to the small sample size and certain restrictions. Therefore,
the present study confirmed that detecting the levels of miR-93,
miR-320 and miR-16 may be significant for the diagnosis of diabetic
ED. Due to the complex mechanisms of diabetic ED and the numerous
factors involved, the exact mechanisms underlying diabetic ED
require further study in the future.
References
|
1
|
Yang W, Lu J, Weng J, et al: Prevalence of
diabetes among men and women in China. N Engl J Med. 362:1090–1101.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Montorsi F, Adaikan G, Becher E, et al:
Summary of the recommendations on sexual dysfunctions in men. J Sex
Med. 7:3572–3588. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ma RC, So WY, Yang X, et al: Erectile
dysfunction predicts coronary heart disease in type 2 diabetes. J
Am Coll Cardiol. 51:2045–2050. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cho NH, Ahn CW, Park JY, et al: Prevalence
of erectile dysfunction in Korean men with Type 2 diabetes
mellitus. Diabet Med. 23:198–203. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chew SK, Taouk Y, Xie J, et al: The
relationship of retinal vessel caliber with erectile dysfunction in
patients with type 2 diabetes. Invest Ophthalmol Vis Sci.
54:7234–7239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Canat L, Cicek G, Atis G, Gurbuz C and
Caskurlu T: Is there a relationship between severity of coronary
artery disease and severity of erectile dysfunction? Int Braz J
Urol. 39:465–473. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lin F and Gou X: Panax notoginseng
saponins improve the erectile dysfunction in diabetic rats by
protecting the endothelial function of the penile corpus
cavernosum. Int J Impot Res. 25:206–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen RC, Riley A, Wagner G, Osterloh IH,
Kirkpatrick J and Mishra A: The international index of erectile
function (IIEF): A multidimensional scale for assessment of
erectile dysfunction. Urology. 49:822–830. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatzichristou D, Hatzimouratidis K, Bekas
M, Apostolidis A, Tzortzis V and Yannakoyorgos K: Diagnostic steps
in the evaluation of patients with erectile dysfunction. J Urol.
168:615–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hatzimouratidis K, Amar E, Eardley I, et
al: European Association of Urology: Guidelines on male sexual
dysfunction: Erectile dysfunction and premature ejaculation. Eur
Urol. 57:804–814. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kloosterman WP and Plasterk RH: The
diverse functions of microRNAs in animal development and disease.
Dev Cell. 11:441–450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heneghan HM, Miller N, McAnena OJ, O'Brien
T and Kerin MJ: Differential miRNA expression in omental adipose
tissue and in the circulation of obese patients identifies novel
metabolic biomarkers. J Clin Endocrinol Metab. 96:E846–E850. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Trajkovski M, Hausser J, Soutschek J, et
al: MicroRNAs 103 and 107 regulate insulin sensitivity. Nature.
474:649–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zampetaki A, Kiechl S, Drozdov I, et al:
Plasma microRNA profiling reveals loss of endothelial miR-126 and
other microRNAs in type 2 diabetes. Circ Res. 107:810–817. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong L, Zhu J, Han W, et al: Significance
of serum microRNAs in pre-diabetes and newly diagnosed type 2
diabetes: A clinical study. Acta Diabetol. 48:61–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
World Health Organization, . Definition,
diagnosis and classification of diabetes mellitus and its
complications Report of a WHO consultation. Part 1: diagnosis and
classification of diabetes mellitus. Geneva: World Health
Organization; 1999
|
|
17
|
Chen X, Liang H, Guan D, et al: A
combination of Let-7d, Let-7g and Let-7i serves as a stable
reference for normalization of serum microRNAs. PLoS One.
8:e796522013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McKinlay J and Marceau L: US public health
and the 21st century: Diabetes mellitus. Lancet. 356:757–761. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tricco AC, Ivers NM, Grimshaw JM, et al:
Effectiveness of quality improvement strategies on the management
of diabetes: A systematic review and meta-analysis. Lancet.
379:2252–2261. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gregg EW, Li Y, Wang J, et al: Changes in
diabetes-related complications in the United States, 1990–2010. N
Engl J Med. 370:1514–1523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahmed I, Aamir Au, Anwar E, Ali SS and Ali
A and Ali A: Erectile dysfunction and type 2 diabetes mellitus in
northern Pakistan. J Pak Med Assoc. 63:1486–1490. 2013.PubMed/NCBI
|
|
22
|
Yang G, Pan C and Lu J: Prevalence of
erectile dysfunction among Chinese men with type 2 diabetes
mellitus. Int J Impot Res. 22:310–317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malavige LS and Levy JC: Erectile
dysfunction in diabetes mellitus. J Sex Med. 6:1232–1247. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ponholzer A, Stopfer J, Bayer G, et al: Is
penile atherosclerosis the link between erectile dysfunction and
cardiovascular risk? An autopsy study. Int J Impot Res. 24:137–140.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ellati RT, Dokun AO, Kavoussi PK, Steers
WD, Annex BH and Lysiak JJ: Increased phosphodiesterase type 5
levels in a mouse model of type 2 diabetes mellitus. J Sex Med.
10:362–369. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Angulo J, González-Corrochano R, Cuevas P,
et al: Diabetes exacerbates the functional deficiency of NO/cGMP
pathway associated with erectile dysfunction in human corpus
cavernosum and penile arteries. J Sex Med. 7:758–768. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiu XF, Li XX, Chen Y, et al: Mobilisation
of endothelial progenitor cells: One of the possible mechanisms
involved in the chronic administration of melatonin preventing
erectile dysfunction in diabetic rats. Asian J Androl. 14:481–486.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hermans MP, Ahn SA and Rousseau MF:
Erectile dysfunction, microangiopathy and UKPDS risk in type 2
diabetes. Diabetes Metab. 35:484–489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corona G and Maggi M: Patients with
testosterone deficiency syndrome and type 2 diabetes. Arch Esp
Urol. 66:711–722. 2013.PubMed/NCBI
|
|
30
|
Hackett G, Cole N, Bhartia M, Kennedy D,
Raju J and Wilkinson P: Testosterone replacement therapy with
long-acting testosterone undecanoate improves sexual function and
quality-of-life parameters vs. placebo in a population of men with
type 2 diabetes. J Sex Med. 10:1612–1627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kataoka T, Hotta Y, Maeda Y and Kimura K:
Assessment of androgen replacement therapy for erectile function in
rats with type 2 diabetes mellitus by examining nitric
oxide-related and inflammatory factors. J Sex Med. 11:920–929.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cheng D, Fei Y, Liu Y, Li J, Xue Q, Wang X
and Wang N: HbA1C variability and the risk of renal status
progression in Diabetes Mellitus: A meta-analysis. PLoS One.
9:e1155092014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Juarez DT, Demaris KM, Goo R, Mnatzaganian
CL and Wong Smith H: Significance of HbA1c and its measurement in
the diagnosis of diabetes mellitus: US experience. Diabetes Metab
Syndr Obes. 7:487–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sabanayagam C, Khoo EY, Lye WK, et al:
Diagnosis of diabetes mellitus using HbA1c in Asians: Relationship
between HbA1c and retinopathy in a multiethnic Asian population. J
Clin Endocrinol Metab. 100:689–696. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Natarajan R, Putta S and Kato M: MicroRNAs
and diabetic complications. J Cardiovasc Transl Res. 5:413–422.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM
and Hu RM: MicroRNA-320 expression in myocardial microvascular
endothelial cells and its relationship with insulin-like growth
factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol.
36:181–188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Long J, Wang Y, Wang W, Chang BH and
Danesh FR: Identification of microRNA-93 as a novel regulator of
vascular endothelial growth factor in hyperglycemic conditions. J
Biol Chem. 285:23457–23465. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Villeneuve LM, Reddy MA, Lanting LL, Wang
M, Meng L and Natarajan R: Epigenetic histone H3 lysine 9
methylation in metabolic memory and inflammatory phenotype of
vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA.
105:9047–9052. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Villeneuve LM, Kato M, Reddy MA, Wang M,
Lanting L and Natarajan R: Enhanced levels of microRNA-125b in
vascular smooth muscle cells of diabetic db/db mice lead to
increased inflammatory gene expression by targeting the histone
methyltransferase Suv39h1. Diabetes. 59:2904–2915. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Weber M, Baker MB, Moore JP and Searles
CD: MiR-21 is induced in endothelial cells by shear stress and
modulates apoptosis and eNOS activity. Biochem Biophys Res Commun.
393:643–648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fleissner F, Jazbutyte V, Fiedler J, et
al: Short communication: Asymmetric dimethylarginine impairs
angiogenic progenitor cell function in patients with coronary
artery disease through a microRNA-21-dependent mechanism. Circ Res.
107:138–143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Caporali A, Meloni M, Völlenkle C, et al:
Deregulation of microRNA-503 contributes to diabetes
mellitus-induced impairment of endothelial function and reparative
angiogenesis after limb ischemia. Circulation. 123:282–291. 2011.
View Article : Google Scholar : PubMed/NCBI
|