Introduction
Atherosclerosis is a complex disease involving
numerous cells, including macrophages, lymphocytes, neutrophils,
endothelial cells and vascular smooth muscle cells (VSMCs)
(1). During the last decades, a
large number of studies have suggested that VSMCs may play a key
role in the genesis and development of atherosclerosis (1,2). At an
early stage, macrophages derived from peripheral blood mononuclear
cells secrete various cytokines, some of which promote VSMC
migration from the tunica media to the subendothelial region, where
they proliferate and take up highly oxidized low-density
lipoprotein, forming foam cells. Over time, the proliferation and
migration of VSMCs result in the expansion of the lesion. Then,
with the death of the foam cells and further accumulation of
lipids, the advanced fibro-fatty lesion develops, which is mainly
composed of accumulated VSMCs and smooth muscle cell (SMC)-derived
extracellular matrix (ECM) (2). In
addition, a number of cytokines, chemokines and growth factors,
including monocyte chemotactic protein-1 (MCP-1) and angiotensin II
(Ang II), are involved in the regulation of VSMCs in
atherosclerosis (3,4).
Mimecan, also known as osteoglycin or osteoinductive
factor, was originally isolated from bovine bone and belongs to the
family of small leucine-rich proteoglycans (SLRPs) (5,6). The
structural hallmarks of SLRPs are leucine-rich repeats (LRRs) and a
cysteine-rich cluster in the N-terminal region, which have highly
conserved spacing and are involved in the regulation of cell
proliferation and differentiation (7,8). Mimecan
demonstrates a unique sequence of cysteine-rich regions and six
LRRs. There is increasing evidence demonstrating that mimecan is
involved in arteriogenesis and atherosclerosis. Shanahan et
al (9) demonstrated that mimecan
is a novel marker of differentiated VSMCs and may be an essential
component of the normal vascular matrix. Using quantitative
polymerase chain reaction (qPCR), mimecan was observed to be
decreased in the media of vessel walls and increased in the
activated endothelium and thickened neointima (9). Immunohistochemistry experiments
revealed that mimecan accumulated in the leading edge of migrating
SMCs in rabbit atherosclerotic lesions (10). These results indicated that mimecan
played a role in atherosclerosis (10). A previous study reported that mimecan
is distributed in the adventitia of collateral arteries, expressed
mostly in SMCs and perivascular fibroblasts in the rabbit femoral
artery ligation model. The observation also confirmed that mimecan
is downregulated in arteriogenesis (11). Another study demonstrated that
mimecan may play a significant role in the regulation of cellular
growth and differentiation (12). It
has been reported that mimecan gene expression is downregulated by
certain growth factors and cytokines associated with vascular
injury, and activated by the tumor suppressor protein p53 (13,14).
Although the existing literature indicates that
mimecan plays a role in arteriogenesis and cellular growth, there
is no direct evidence of the effect of mimecan on cell
proliferation, apoptosis and migration in VSMCs. The purpose of the
present study was to observe the effect of mimecan on cultured
human aortic smooth muscle cells (HASMCs) in vitro.
Materials and methods
HASMC cultures
HASMCs were grown in culture flasks in medium 231
with smooth muscle growth supplement (SMGS). Cells, medium 231 and
SMGS were all purchased from Cascade Biologics (Portland, OR, USA).
The cells were incubated at 37°C in a humidified 5% CO2
atmosphere. HASMCs were subcultured when the cells reached
approximately 70–80% confluence. Cells from passages 4 to 10 were
used for experiments.
Stable transfection
Full-length human mimecan cDNA was generated by PCR
amplification, incorporating XhoI and BglII (Takara,
Kyoto, Japan) restriction sites and adding three FLAG tags. The
primers for the full-length human mimecan were
5′-ACTGAGATCTATGAAGACTCTGCAGTCTACACTTCTCCTGTTACTGCTTGTGCCTCTGATAAAGCCAGCACCACCAACCCAGCAGGACTCACGC-3′
and
5′-CTGGCTCGAGTTACTTGTCGTCATCGTCTTTGTAGTCGATGTCATGGTCTTTGTAGTCTCCGTCATGGTCTTTGTAGTCAAAGTATGACCCTAT-3′.
The PCR product was ligated into the pMSCV-puromycin-IRES-GFP (PIG;
Clontech, Mountain View, CA, USA) vector, resulting in a PIG
lentiviral construct. Recombinant lentiviruses were produced by
transient co-transfection of three plasmids in HEK293T (ATCC,
Manassas, VA, USA) cells using Lipofectamine 2000 (Invitrogen,
Carlsbad, CA, USA) according to the manufacturer's instructions.
The three plasmids were h-mimecan-3FLAG-PIG or empty vector PIG,
the envelope protein VSV-G and the packaging protein gag-pol
(Clontech). Infectious lentivirus was harvested at 48 h
post-transfection and filtered through 0.45 µm-pore cellulose
acetate filters (Millipore, Billerica, MA, USA). HASMCs were plated
on a six-well plate, and the medium was changed to perfect medium
(medium 231 with SMGS) without antibiotics prior to transduction.
When the cells reached 60% confluence, the lentiviruses and 8 µg/ml
polybrene (Invitrogen) were added to each well. Following
incubation for 6–8 h at 37°C, the medium containing lentiviruses
was removed and replaced with perfect medium. When the cells
reached 90% confluence, 1 µg/ml puromycin (Gibco, New York, NY,
USA) was added for 4 days. Human mimecan expression was verified by
reverse transcription (RT)-qPCR and western blot analysis.
RT-qPCR
Total RNA was extracted from transduced cells using
TRIzol (Invitrogen). The cDNA was synthesized from 1 µg RNA using a
Takara reverse transcription kit according to the manufacturer's
instructions. RT-qPCR was performed using SYBR-Green PCR Master mix
(Takara) according to the manufacturer's instructions. The sense
primer for GAPDH was 5′-GATCATCAGCAATGCCTCCTGC-3′, and the
antisense primer was 5′-GGT CAT GAG TCC TTC CAC GAT ACC-3′. The
sense primer for mimecan was 5′-TAC TTG GAC CAT AAT GCC CTGG-3′,
and the antisense primer was 5′-AAC TGG TGT CAT TAG CCT TGC-3′. The
resultant 127-bp fragment was confirmed by sequencing and comparing
with the sequence of the human mimecan gene expression region in
GeneBank.
Western blot analysis
When the cells reached 90% confluence, they were
harvested to extract total cell protein. The proteins were
separated by 10% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (Roche, Basel, Switzerland), after which the
membranes were probed with the following antibodies: The primary
antibody, anti-FLAG (1:1,000; Sigma-Aldrich, St. Louis, MO, USA);
the horseradish peroxidase-coupled secondary antibody (1:2,000;
DAKO, Glostrup, Denmark); and anti-GAPDH (1:10,000; Kangchen
Raybiotech, Shanghai, China). The results were analyzed using the
LAS-4000 system (Fujifilm, Tokyo, Japan).
Cell proliferation assay
Cell proliferation was measured by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. HASMCs transduced with h-mimecan-3FLAG-PIG or empty vector
were seeded at a density of 1×103 cells/well on 96-well
plates. The cells were incubated at 37°C for 24 h, following which
20 µl methyl thiazolyl tetrazolium (5 mg/ml MTT, Sangon Biotech,
Shanghai, China) was added into each well and incubated for 3–4 h
at 37°C. The medium was then carefully removed, and 200 µl dimethyl
sulphoxide (DMSO, Invitrogen) was added to each well.
Spectrophotometric readings were normalized using cell-free media
as a blank. The plate was read spectrophotometrically by measuring
the absorbance of the dye at a wavelength of 490 nm. Four wells per
treatment were used for the assay, and each experiment was
performed in triplicate.
Cell apoptosis assay
To measure the effect of overexpression of mimecan
on HASMC apoptosis, the cells were seeded in six-well plates at a
density of 1×104 per well and incubated for 36 h at
37°C. The cells were then harvested. Cells from each well were
suspended in 195 µl binding buffer, and 5 µl Annexin
Ⅴ-phosphatidylethanolamine (PE) was added. The mixture was
incubated for 10 min at room temperature in the dark and then
harvested and suspended in 200 µl binding buffer according to the
manufacturer's instructions (Beyotime Institute of Biotechnology,
Shanghai, China). The cells were subjected to flow cytometry to
measure the apoptosis rate with a cytometer (Beckman Coulter, Brea,
CA, USA). Experiments were repeated three times.
Cell migration assay
The migration activity of cells was assayed in
Transwell cell culture chambers (BD Biosciences, Franklin Lakes,
NJ, USA) and sterile cloning cylinders (Bel-Art Products, Wayne,
NJ, USA). HASMCs (2×103) were resuspended in 200 µl
condition medium (medium 231 with 0.1% bovine serum albumin). The
cell suspension was added to the upper compartment of the chamber,
and incubated with 700 µl perfect medium (medium 231 with SMGS) in
the lower compartment for 24 h at 37°C. Following incubation, the
inserts were removed from the wells, and the cells on the upper
surface of the filters were removed by cotton swabs. The filters
were fixed with 4% paraformaldehyde (Sangon Biotech) and stained
with crystal violet staining reagent (Beyotime Institute of
Biotechnology). Migrated cells were manually counted using a light
microscope. Cells in nine random high-power fields were counted for
each migration well to determine the number of migrated cells.
HASMCs (1×103) were resuspended in 50 µl condition
medium. The suspension was added to the inner wall of the sterile
cloning cylinders, and the edge of the sterile cloning cylinders
were marked using a syringe needle. After the cells adhered, the
sterile cloning cylinders were removed and 500 µl perfect medium
was added to the 12-well plate. The cells were incubated for 24 h
at 37°C to evaluate their migration ability. The migrated distance
(mm) was measured at ten points along the edge of the sterile
cloning cylinder using the Image Pro Plus, version 6.0 (Media
Cybernetics, Inc., Bethesda, MA, USA) image analysis system. Each
assay was performed in triplicate.
Statistical analysis
Data are presented as the means ± standard
deviation. Statistical analyses were performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 and P<0.01
were considered to indicate a statistically significant
difference.
Results
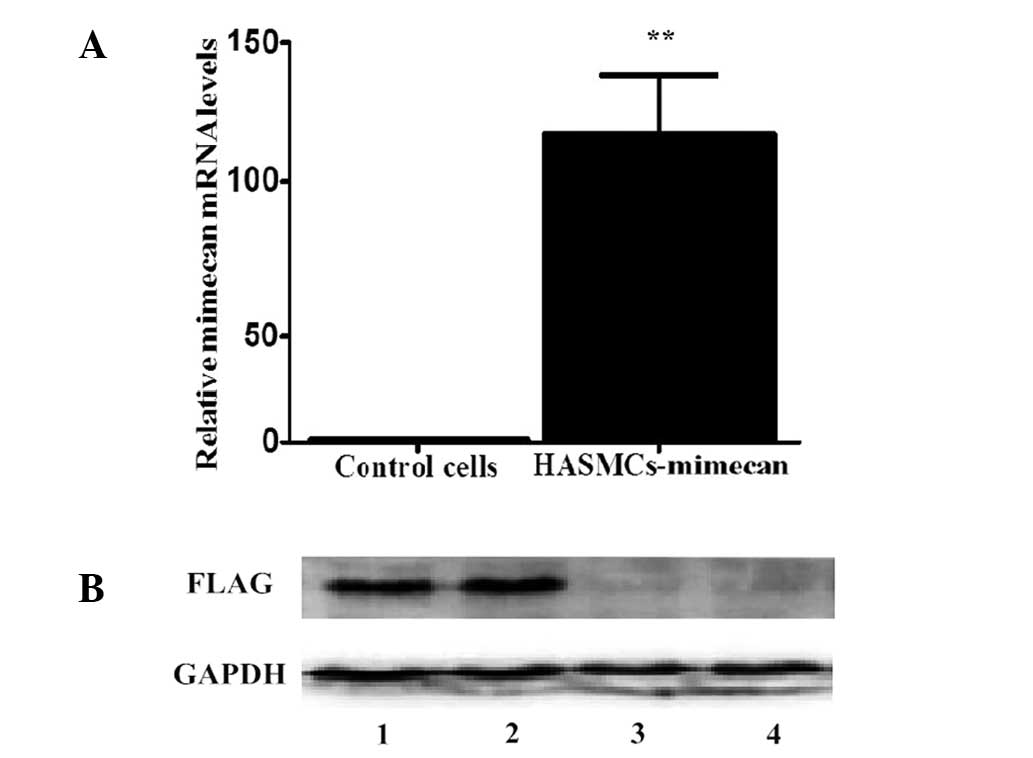
Transduction of h-mimecan-3FLAG-PIG
results in target-specific overexpression
To determine the effect of mimecan on HASMCs, a
lentiviral system was used to stably overexpress mimecan in
vitro. The HASMCs transduced with h-mimecan-3FLAG-PIG
(HASMC-mimecan) demonstrated a 100-fold increase in mimecan mRNA
levels compared with control cells as determined by RT-qPCR
(Fig. 1A; P<0.01). HASMCs
transduced with empty vectors were used as control cells. The
transduction of HASMCs with mimecan resulted in specific
overexpression of mimecan protein, as determined by western blot
analysis (Fig. 1B). The transduction
efficiency was demonstrated by the expression of GFP (data not
shown).
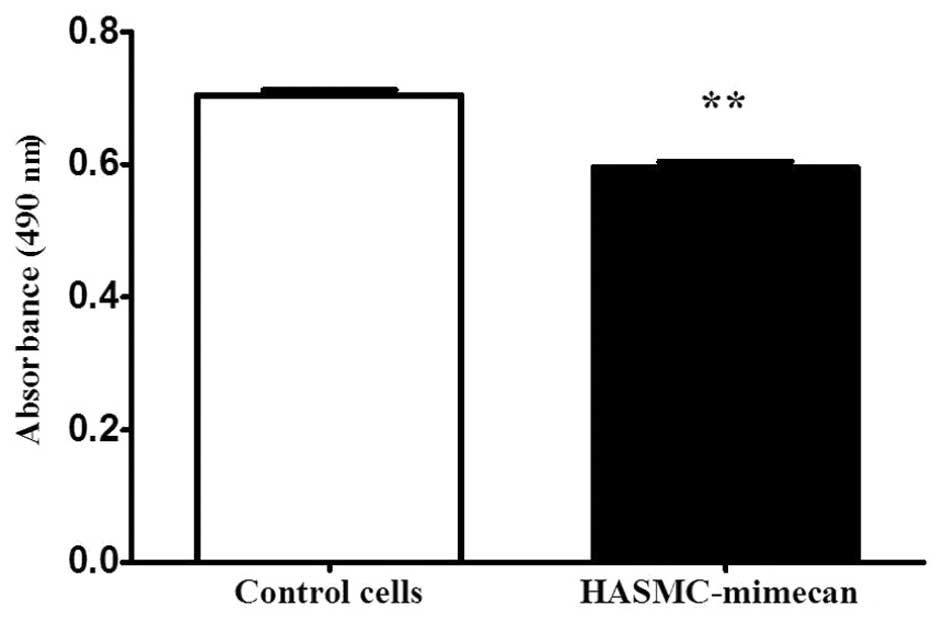
Mimecan inhibits proliferation of
HASMCs
The effect of mimecan overexpression on the
proliferation of HASMCs was measured by MTT assay. The
proliferation rate was quantified by measuring the absorbance at
490 nm. The results demonstrated that overexpression of mimecan
decreased the proliferation rate by 16% (P<0.01; Fig. 2).
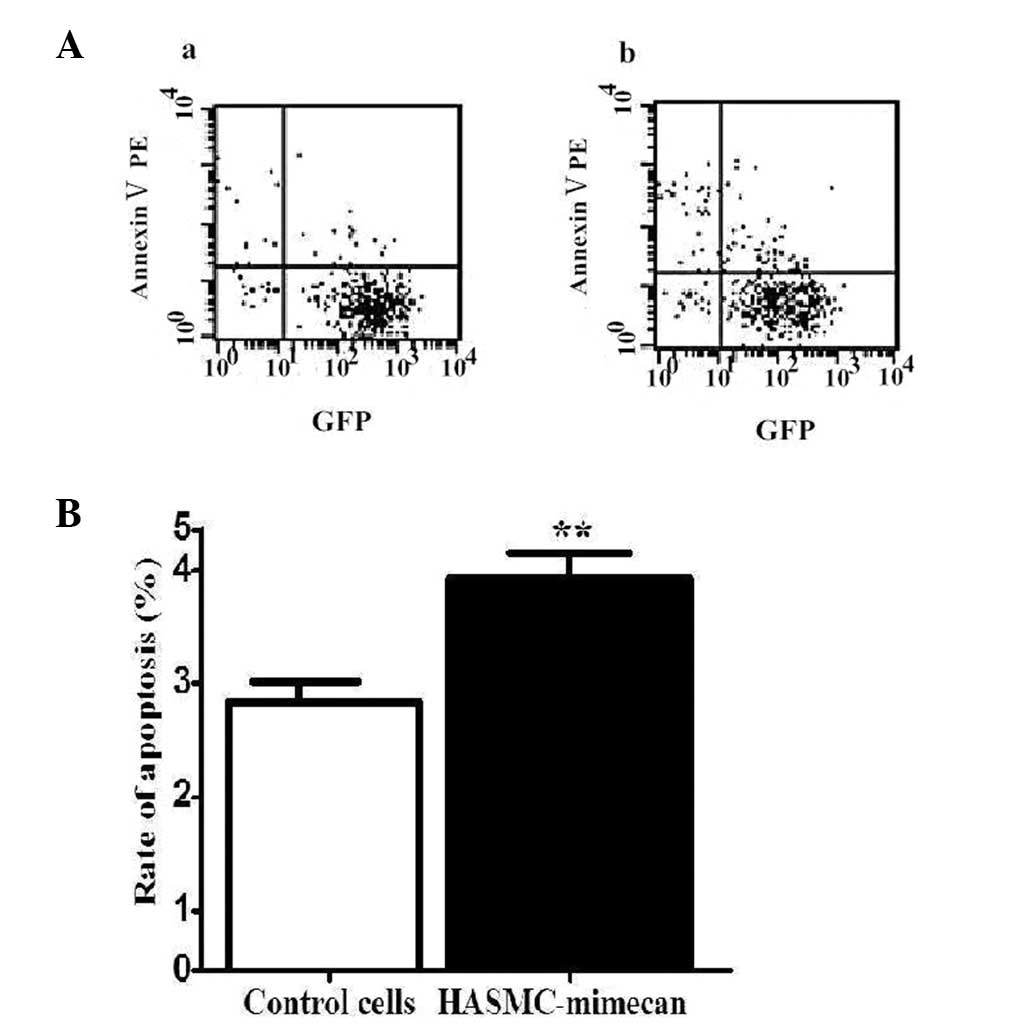
Mimecan induces apoptosis in
HASMCs
Cells undergoing apoptosis revealed translocation of
phosphatidylserine (PS) to the outer leaflets of the plasma
membrane. Apoptosis levels were measured by staining of Annexin
Ⅴ-PE targeted to PS. The rate of apoptosis was assessed by flow
cytometry. The results reveal that overexpression of mimecan
increased the cell apoptosis rate compared with that of control
cells (Fig. 3; P<0.05).
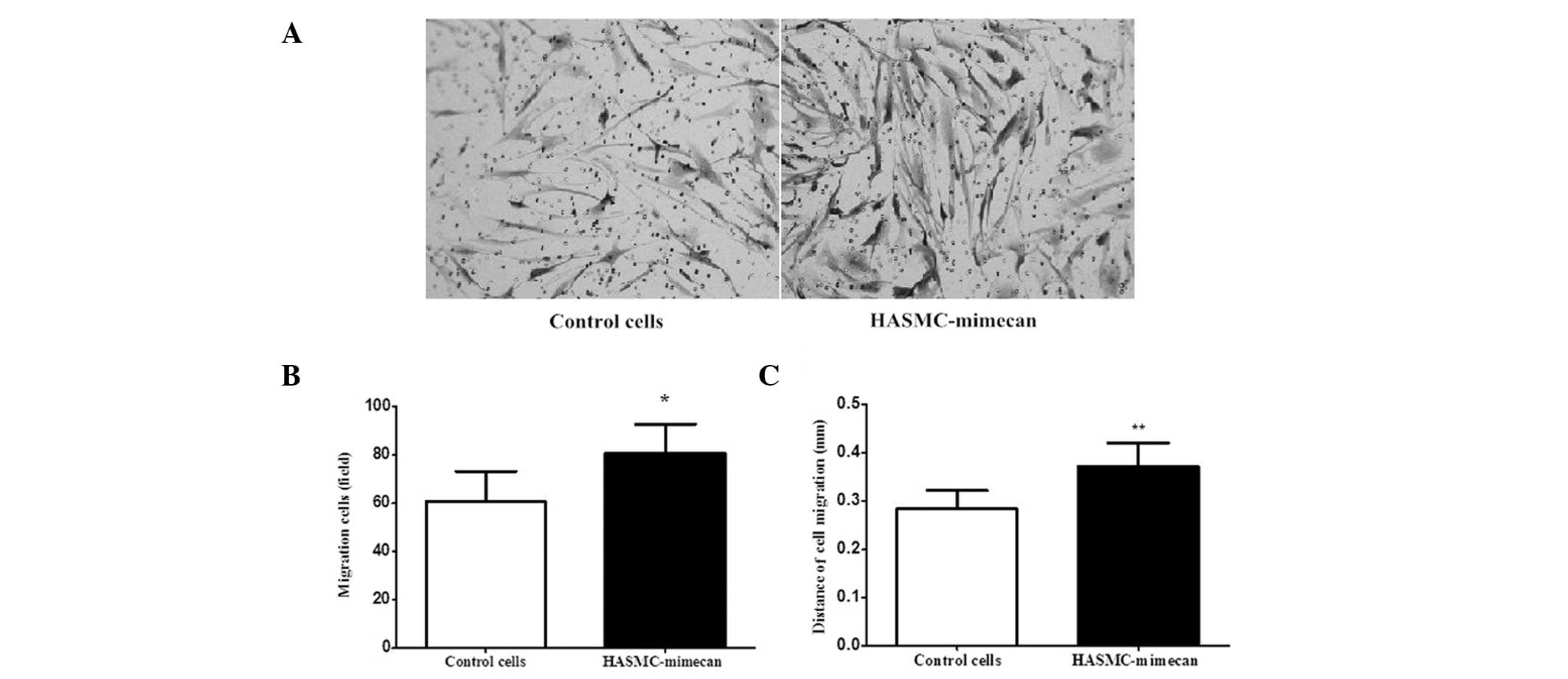
Mimecan enhances HASMC migration
The effect of mimecan on the migration of HASMCs was
evaluated by the Transwell migration assay and sterile cloning
cylinders migration assay, respectively. The Transwell migration
assay demonstrated that mimecan enhances HASMCs migration.
Overexpression of mimecan increased the basal migration of HASMCs
1.34-fold (82 cells/field) compared with that of control cells (61
cells/field) (Fig. 4A and B). The
sterile cloning cylinder migration assay demonstrated the migration
distance of HASMC-mimecan or control cells horizontally. As shown
in Fig. 4C, the migration distance
of HASMC-mimecan (0.37 mm) was greater than that of control cells
(0.28 mm).
Discussion
The present study first established a cell model
stably overexpressing mimecan in HASMCs using a lentivirus system.
It provided a new cell model to investigate the function of mimecan
in VSMCs. The HASMCs used in the present study were purchased from
Cascade Biologics and have been used in a number of previous
studies to investigate VSMCs (15,16).
The SLRP gene family has been shown to be involved
in the regulation of collagen fibrillogenesis, ECM assembly, cell
growth, migration and adhesion (8,17). It
has been proven that mimecan, similar to other SLRP family members,
has a role in regulating collagen fibrillogenesis, which is a
component of ECM (18). Moreover,
the biological scaffolds of ECM are essential for cell
proliferation, adhesion and migration. Shanahan et al
(9) and Scholz et al
(19) reported that mimecan mRNA
expression increases after VSMC proliferation ceases. This result
suggests that mimecan is associated with VSMC proliferation. It is
also reported that knockdown of mimecan enhances primary cultured
VSMC proliferation induced by FBS or Ang II (20). In addition, a large number of
cytokines, including fibroblast growth factor-2, transforming
growth factor-β, platelet-derived growth factor, Ang II and
oncostatin M, inhibit mimecan expression, and MCP-1 enhances the
effect of these cytokines on mimecan (9,11).
Consistent with the previous observations, the present study
clearly indicates that proliferation was significantly inhibited in
the HASMCs that overexpressed mimecan. The functions and phenotypes
of SMCs in naturally occurring lesions presented spatial-temporal
patterns and dynamic aspects (21).
VSMCs from atherosclerosis lesions at an early stage and vessel
walls following balloon injury reveal high proliferation rates,
while VSMCs from plaques at a late stage reveal low proliferation
rates (22). Therefore, we concluded
that if mimecan is involved in the formation of atherosclerosis
through effects on HASMC proliferation, it mainly plays a role in
the early stage.
Although numerous studies have observed that mimecan
is associated with VSMC proliferation (9–11), it
was not known whether mimecan was directly related to the apoptosis
of VSMCs. In the present study, we investigated the effect of
mimecan on HASMC apoptosis using Annexin Ⅴ-PE staining of PS and
observed that the apoptosis rate of HASMCs overexpressing mimecan
was elevated compared with control cells. The apoptosis of VSMCs
plays a subtle role in the development of atherosclerosis by
decreasing the thickness of the neointima and fibrous cap (23). The fibrous cap consists of VSMCs and
ECM. The proportion of apoptotic VSMCs is increased in the unstable
plaque compared with the stable plaque. Loss of VSMCs by apoptosis
leads to a thin fibrous cap of plaque by decreasing collagen
biosynthesis, which increases the risk of plaque rupture (24). Therefore, it seems reasonable to
speculate that increased mimecan expression may accelerate the
formation of unstable atherosclerotic plaques by promoting VSMC
apoptosis. However, the apoptosis of VSMCs can interrupt neointimal
formation at defined time points and reverse the artery restenosis
(25). Additionally, it has been
confirmed that the consequences of VSMC apoptosis depend on the
location of VSMCs in plaques and the stages of the atherosclerotic
plaques. The complexity of VSMC apoptosis effects on
atherosclerotic plaques may have led to the different results in
the previous studies.
Migration of medial VSMCs into the intima is one of
the key points in the development of atherosclerosis and vascular
injury. A previous study indicated that mimecan plays a role in SMC
migration. A different study demonstrated that mimecan protein
accumulates at the front edge of migrating SMCs in atherosclerosis
lesions (10). In the present study,
Transwell cell culture chamber and sterile cloning cylinder
migration assays were used to further confirm that mimecan promotes
VSMC migration. The migration assays demonstrated that the
overexpression of mimecan enhanced HASMC migration ability.
Matrix metalloproteinases (MMPs), which are produced
by VSMCs and macrophages, play a role in vascular remodeling
(26). MMPs, in particular MMP-2 and
MMP-9, contribute to the pathogenesis of atherosclerosis by
facilitating VSMC migration via matrix disruption (27). Numerous studies have demonstrated
that MMP-2 and MMP-9 participate in the development of
atherosclerosis by regulating the proliferation and migration of
VSMCs (28–32). A knockout study demonstrated that
MMP-9 is required for neointimal and arterial lesion formation by
regulating the migration and proliferation of VSMCs (26,28).
Decorin, a member of the SLRP family, affects the production of
MMPs. It has been demonstrated that overexpression of decorin
increases protein levels of MMP-2 (33). The structure of mimecan is similar to
decorin, as both of them are members of the SLRP family. The most
salient feature is that decorin has ten LRRs, and mimecan has 6
LRRs. Therefore, we hypothesize that mimecan may regulate cell
migration by affecting MMPs in a similar to decorin. Further study
is required to investigate whether MMPs mediate the effect of
mimecan on VSMC migration.
SMCs in the human aorta have long been known to be
able to express unique contractile proteins, ion channels and
signaling molecules, which are necessary for the cells' contractile
function, and the SMCs have a low rate of proliferation and
synthetic activity (34). In
response to changes in the local microenvironment, SMCs undergo
more subtle changes in phenotype and function, including
proliferation, secretion, migration and differentiation (35). Changes in the local microenvironment
affect vascular development, atherosclerosis formation and vascular
rupture. During vascular development, VSMCs exhibit a high rate of
proliferation, migration and production of ECM components,
including collagen, elastin and proteoglycans that make up a major
portion of the vessel wall, acquiring high contractile capabilities
(17). These changes are known as
ʻphenotypic modulationʼ (34). In
special conditions, including arterial wall injury or
atherosclerosis, VSMCs, which have a low mitogenic activity level
in the tunica media region of the blood vessel wall, may undergo
progression from a contractile to a synthetic phenotype and begin
proliferating in response to various growth factors or cytokines
(36,37).
The phenotypic state of SMCs is likely to be
extremely complex as the functional role of SMCs changes during
arteriogenesis or atherosclerosis. Animal experiments have also
demonstrated that the expression and location of mimecan are
different in VSMCs with different phenotypes during arteriogenesis
(9–11). Taken together, we speculate that
mimecan may play various roles in different stages of
atherosclerosis through its involvement in the phenotypic
modulation of VSMCs. However, we did not investigate the effect of
mimecan on VSMCs in vivo, and further studies will be
required to characterize molecular mechanisms of mimecan in VSMC
function and atherosclerosis.
Acknowledgements
This study was funded by grants to FLC from the
National Science Foundation of China (no. 30870954 and 81270908)
and the Foundation for Science and Technology from the Medical
College of Shanghai Jiao Tong University (no. YZ1054).
References
|
1
|
Ross R: The pathogenesis of
atherosclerosis: a perspective for the 1990s. Nature. 362:801–809.
1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lusis AJ: Atherosclerosis. Nature.
407:233–241. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rakesh K and Agrawal DK: Cytokines and
growth factors involved in apoptosis and proliferation of vascular
smooth muscle cells. Int Immunopharmacol. 5:1487–1506. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Selzman CH, Miller SA, Zimmerman MA,
Gamboni-Robertson F, Harken AH and Banerjee A: Monocyte chemotactic
protein-1 directly induces human vascular smooth muscle
proliferation. Am J Physiol Heart Circ Physiol. 283:H1455–H1461.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bentz H, Chang RJ, Thompson AY, Glaser CB
and Rosen DM: Amino acid sequence of bovine osteoinductive factor.
J Biol Chem. 265:5024–5029. 1990.PubMed/NCBI
|
|
6
|
Bentz H, Nathan RM, Rosen DM, Armstrong
RM, Thompson AY, Segarini PR, Mathews MC, Dasch JR, Piez KA and
Seyedin SM: Purification and characterization of a unique
osteoinductive factor from bovine bone. J Biol Chem.
264:20805–20810. 1989.PubMed/NCBI
|
|
7
|
Iozzo RV: The family of the small
leucine-rich proteoglycans: key regulators of matrix assembly and
cellular growth. Crit Rev Biochem Mol Biol. 32:141–174. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Iozzo RV: The biology of the small
leucine-rich proteoglycans. Functional network of interactive
proteins. J Biol Chem. 274:18843–18846. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shanahan CM, Cary NR, Osbourn JK and
Weissberg PL: Identification of osteoglycin as a component of the
vascular matrix. Differential expression by vascular smooth muscle
cells during neointima formation and in atherosclerotic plaques.
Arterioscler Thromb Vasc Biol. 17:2437–2447. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernandez B, Kampmann A, Pipp F,
Zimmermann R and Schaper W: Osteoglycin expression and localization
in rabbit tissues and atherosclerotic plaques. Mol Cell Biochem.
246:3–11. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kampmann A, Fernandez B, Deindl E, Kubin
T, Pipp F, Eitenmuller I, Hoefer IE, Schaper W and Zimmermann R:
The proteoglycan osteoglycin/mimecan is correlated with
arteriogenesis. Mol Cell Biochem. 322:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tasheva ES, Maki CG, Conrad AH, et al:
Transcription activation of bovine mimecan by p53 through an
intronic DNA binding site. Biochim Biophys Acta. 1517:333–338.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Long CJ, Roth MR, Tasheva ES, Funderburgh
M, Smit R, Conrad GW and Funderburgh JL: Fibroblast growth factor-2
promotes keratan sulfate proteoglycan expression by keratocytes in
vitro. J Biol Chem. 275:13918–13923. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tasheva ES: Analysis of the promoter
region of human mimecan gene. Biochim Biophys Acta. 1575:123–129.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park SH, Koo HJ, Sung YY and Kim HK: The
protective effect of Prunella vulgaris ethanol extract against
vascular inflammation in TNF-α-stimulated human aortic smooth
muscle cells. BMB Rep. 46:352–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yisireyili M, Saito S, Niwa T, et al:
Indoxyl sulfate-induced activation of (pro)renin receptor promotes
cell proliferation and tissue factor expression in vascular smooth
muscle cells. PLoS One. 9:e1092682014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Svensson L, Oldberg A and Heinegård D:
Collagen binding proteins. Osteoarthritis Cartilage. 9:(Suppl A).
S23–S28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tasheva ES, Koester A, Paulsen AQ, Garrett
AS, Boyle DL, Davidson HJ, Song M, Fox N and Conrad GW:
Mimecan/osteoglycin-deficient mice have collagen fibril
abnormalities. Mol Vis. 8:407–415. 2002.PubMed/NCBI
|
|
19
|
Scholz D, Ito W, Fleming I, Deindl E,
Sauer A, Wiesnet M, Busse R, Schaper J and Schaper W:
Ultrastructure and molecular histology of rabbit hind-limb
collateral artery growth (arteri0genesis). Virchows Arch.
436:257–270. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gu XS, Lei JP, Shi JB, Lian WL, Yang X,
Zheng X and Qin YW: Mimecan is involved in aortic hypertrophy
induced by sinoaortic denervation in rats. Mol Cell Biochem.
352:309–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Owens GK, Kumar MS and Wamhoff BR:
Molecular regulation of vascular smooth muscle cell differentiation
in development and disease. Physiol Rev. 84:767–801. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon D, Reidy MA, Benditt EP and
Schwartz SM: Cell proliferation in human coronary arteries. Proc
Natl Acad Sci USA. 87:4600–4604. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bochaton-Piallat ML, Gabbiani F, Redard M,
Desmouliere A and Gabbiani G: Apoptosis participates in cellularity
regulation during rat aortic intimal thickening. Am J Pathol.
146:1059–1064. 1995.PubMed/NCBI
|
|
24
|
Bauriedel G, Hutter R, Welsch U, Bach R,
Sievert H and Luderitz B: Role of smooth muscle cell death in
advanced coronary primary lesions: implications for plaque
instability. Cardiovasc Res. 41:480–488. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Durand E, Mallat Z, Addad F, Vilde F,
Desnos M, Guerot C, Tedgui A and Lafont A: Time courses of
apoptosis and cell proliferation and their relationship to arterial
remodeling and restenosis after angioplasty in an atherosclerotic
rabbit model. J Am Coll Cardiol. 39:1680–1685. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: the
good, the bad, and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
27
|
Bendeck MP, Zempo N, Clowes AW, Galardy RE
and Reidy MA: Smooth muscle cell migration and matrix
metalloproteinase expression after arterial injury in the rat. Circ
Res. 75:539–545. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Abedi H and Zachary I: Signalling
mechanisms in the regulation of vascular cell migration. Cardiovasc
Res. 30:544–556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho A and Reidy MA: Matrix
metalloproteinase-9 is necessary for the regulation of smooth
muscle cell replication and migration after arterial injury. Circ
Res. 91:845–851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Galis ZS, Johnson C, Godin D, Magid R,
Shipley JM, Senior RM and Ivan E: Targeted disruption of the matrix
metalloproteinase-9 gene impairs smooth muscle cell migration and
geometrical arterial remodeling. Circ Res. 91:852–859. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johnson JL, Dwivedi A, Somerville M,
George SJ and Newby AC: Matrix metalloproteinase (MMP)-3 activates
MMP-9 mediated vascular smooth muscle cell migration and neointima
formation in mice. Arterioscler Thromb Vasc Biol. 31:e35–e44. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zempo N, Koyama N, Kenagy RD, Lea HJ and
Clowes AW: Regulation of vascular smooth muscle cell migration and
proliferation in vitro and in injured rat arteries by a synthetic
matrix metalloproteinase inhibitor. Arterioscler Thromb Vasc Biol.
16:28–33. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haj AL, Zen A, Lafont A, Durand E,
Brasselet C, Lemarchand P, Godeau G and Gogly B: Effect of
adenovirus-mediated overexpression of decorin on
metalloproteinases, tissue inhibitors of metalloproteinases and
cytokines secretion by human gingival fibroblasts. Matrix Biol.
22:251–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owens GK: Regulation of differentiation of
vascular smooth muscle cells. Physiol Rev. 75:487–517.
1995.PubMed/NCBI
|
|
35
|
Somlyo AP and Somlyo AV: Ca2+ sensitivity
of smooth muscle and nonmuscle myosin II: modulated by G proteins,
kinases, and myosin phosphatase. Physiol Rev. 83:1325–1358. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chamley-Campbell J, Campbell GR and Ross
R: The smooth muscle cell in culture. Physiol Rev. 59:1–61.
1979.PubMed/NCBI
|
|
37
|
Schwartz CJ, Valente AJ, Sprague EA,
Kelley JL, Cayatte AJ and Mowery J: Atherosclerosis. Potential
targets for stabilization and regression. Circulation.
86:III117–III123. 1992.PubMed/NCBI
|