Introduction
Autogenous arteriovenous fistula (AVF) is considered
the best type of vascular access for maintenance hemodialysis
(1). In patients with chronic renal
insufficiency, immature AVFs are observed when significant
anatomical features cause a failure to mature (2), and intimal hyperplasia seriously
affects the long-term efficacy (3).
Routine preoperative upper extremity mapping with ultrasound
increases not only the AVF construction rate, but also the
likelihood of maturation (4). To
predict the function of AVFs, intraoperative ultrasound
transit-time flow measurements gained at surgery and postoperative
follow-up with duplex ultrasonography have been found to be helpful
(5). Animal models of fistula can be
used for observation of the changes in the local flow dynamics of
arteries and pathological dynamic changes of venous
arterialization, and to investigate intervention effects. Large and
small animals have advantages and disadvantages in modeling.
Fistula models using large animals such as dogs and pigs, which
have vascular structures similar to those of humans and are simple
to establish, provide a satisfactory simulation of local
hemodynamics. It is also easy to perform external stent
intervention and the monitoring of some hemodynamic data in such
models. However, large animals have high feeding costs and a
complex process of anesthesia, and it can be difficult to establish
a mixed model of disease, such as with diabetes or chronic renal
failure. Small animal models do not have these deficiencies
(6–9), and the wide use of gene knockout rats
in recent years is particularly important, as it provides an
important platform for the study of the mechanisms of the
occurrence and development of diseases. The establishment of an
arteriovenous fistula model in animals with chronic renal failure
by a cuff technique has been reported (10), but the experimental results are often
influenced by the technology proficiency level of the practitioner.
A rat model of arterial bypass grafting has suggested that the
hemodynamic and morphological changes associated with a distal AVF
may potentiate graft patency and function (11). In the present study, an AVF model was
established using a cuff technique following 5/6 nephrectomy.
Materials and methods
Experimental animals
A total of 40 specific pathogen-free healthy male
Sprague-Dawley (SD) rats, 4 weeks old, weighing 150–160 g were
purchased from Shanghai Silaike Experimental Animal Co. Ltd.
(Shanghai, China). The rats were fed in the laminar laboratory of
the Experimental Animal Center of Wenzhou Medical College (Wenzhou,
China), with a feeding temperature of 25°C, relative humidity of
70% and a 12/12 h light/dark cycle. The study was carried out in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of National Research Council
(12). The animal use protocol has
been reviewed and approved by the Institutional Animal Care and Use
Committee (IACUC) of Wenzhou Medical University.
Cuff
A 20 G disposable venous indwelling needle catheter
(B. Braun Melsungen AG, Melsungen, Germany), cut into 2.5-mm
segments was used.
Establishment of the 5/6 nephrectomy
rat model
Following a week of adaptive feeding, random digital
expression methods were used to randomly divide the rats into an
experimental group (n=20) and sham surgery group (n=20), and the
modeling method was conducted according to the previously reported
method (13,14).
Establishment of the rat AVF
model
A week after the establishment of the 5/6
nephrectomy rat model, the rats of the experimental group were
anesthetized by the intraperitoneal injection of 300 mg/kg 10%
chloral hydrate aqueous solution. The neck skin was shaved, and a
~1.5 cm oblique incision was made in the neck slightly to the right
of center. Following the freeing and resection of the right
submandibular gland and right sternocleidomastoid muscle, the right
external jugular vein injury was separated by a noninvasive
technique. The branches were ligated with an 8-0 suture, an ~8-mm
vein bridge length was dissociated, and the lumen was flushed with
physiological saline (containing heparin sodium 5,000 U/l). Then,
~8 mm right common carotid artery was separated, and following
ligation of the proximal end to block the blood flow with a
noninvasive vascular clamp, the artery was cut in the middle point.
The vessel wall at the broken end of the artery was passed through
a disposable intravenous indwelling needle catheter; with gentle
pulling, the artery was flipped outwards to sheathe the catheter
vessel, and was then inserted into the reversed vein bridge.
Ligation with 5-0 suture was twice performed, and the incision was
sutured. In the control group, the same incision position was
adopted, and following the freeing and resection of the right
submandibular gland and right sternocleidomastoid muscle, the right
external jugular vein and the right carotid artery were separated
by a noninvasive technique, and the incision was sutured. The
experimental animals were sent to the breeding center for
recovery.
Specimen collection and treatment
All rats were fed with a standard pellet diet, with
free access to food and water. On days 7 and 28 after the
establishment of the AVF model, rats from the two groups were
sacrificed (10 rats from each group at each time-point). Referring
to the anastomotic stoma as the midpoint, 1 cm venous and arterial
tissue was excised to be fixed with 4% paraformaldehyde overnight
and embedded in paraffin prior to the preparation of 5-µm serial
sections that were observed by an AX70 light microscope (Olympus
Corp., Tokyo, Japan). The rats were sacrificed following the
drawing of blood from the inferior vena cava; the blood was
retained for analysis.
Determination of renal function
The dynamic changes of blood creatinine and urea
nitrogen levels were monitored using an 7180 automatic biochemical
analyzer (Hitachi High-Technologies Corp., Tokyo, Japan).
Histomorphology
The intimal tissues were subjected to hematoxylin
and eosin staining, and collagen and elastin double staining with
Victoria Blue B and Ponceau S staining solution (Fuzhou Maixin
Biotechnology Development Co. Ltd., Fuzhou, China). Image Pro-Plus
6 software (Media Cybernetics, Inc., Rockville, MD, USA) was used
for image analysis. The tunica intima and tunica media membrane
thickness was respectively calculated, and the area of intimal
hyperplasia was the area of the internal elastic lamina minus the
area of the lumen.
Immunohistochemistry
The sections were routinely dewaxed and hydrated,
and then subjected to 120°C antigen retrieval for 10 min.
Endogenous peroxidase activity was quenched with 3%
H2O2 for 10 min, and then immune bovine serum
was added for 10 min for blocking. The sections were then incubated
with primary antibody (monoclonal mouse anti-rat α-SMA antibody,
1:300, cat. no. 555018; Abcam, Cambridge, UK), at 4°C overnight.
The sections were washed three times with phosphate-buffered saline
(Sigma-Aldrich, St Louis, MO, USA), 5 min each time. After warming
in a 37°C incubator for 20 min, the secondary antibody (horseradish
peroxidase-conjugated polyclonal goat anti-mouse IgG, cat. no.
B-14029; Beijing Sequoia Jinqiao Biological Technology Co. Ltd.,
Beijing, China) was added and the sections were incubated at 37°C
for 30 min. The sections were then developed with
3,3′-diaminobenzidine, counterstained with hematoxylin,
differentiated with 0.1% hydrochloric acid in alcohol, and flushed
with water. The sections were then routinely dehydrated, mounted
with transparent neutral gum and observed in Q550CW image
acquisition system (Leica Science Lab, Berlin, Germany). In each
vessel organization, three non-consecutive slices were observed and
10 high magnification (x200) fields of view were randomly selected
in each section. The positive immunohistochemical staining signal
of the selected view was subjected to image analysis, using Image
Pro-Plus 6 image analysis software (MediaCybernetics, Inc.,
Rockville, MD, USA). The percentage of the positively stained area
in relation to the total field area in each field, namely the α-SMA
relative positive area (%) was calculated, and a mean value was
then calculated.
Statistic analysis
Using SPSS software, version 16.0 (SPSS, Inc.,
Chicago, IL, USA) the measurement data were represented as mean ±
standard deviation. After testing for homogeneity of variance,
single factor analysis of variance was performed for significance
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparison of renal function
The creatinine and urea nitrogen levels of the
experimental group on days 7 and 28 after modeling were
significantly increased compared with those in the sham surgery
group in the same time-point (P<0.05; Table I).
 | Table I.Comparison of renal function in the
rats after surgery. |
Table I.
Comparison of renal function in the
rats after surgery.
|
| BUN (mmol/l) | SCr (µmol/l) |
|---|
|
|
|
|
|---|
| Time-point | Experimental
group | Sham surgery
group | Experimental
group | Sham surgery
group |
|---|
| Day 7 |
13.37±1.07a |
6.32±1.12 |
61.25±3.09a |
36.25±2.85 |
| Day 28 |
15.12±1.42a |
7.36±1.85 |
56.89±3.41a |
39.56±3.03 |
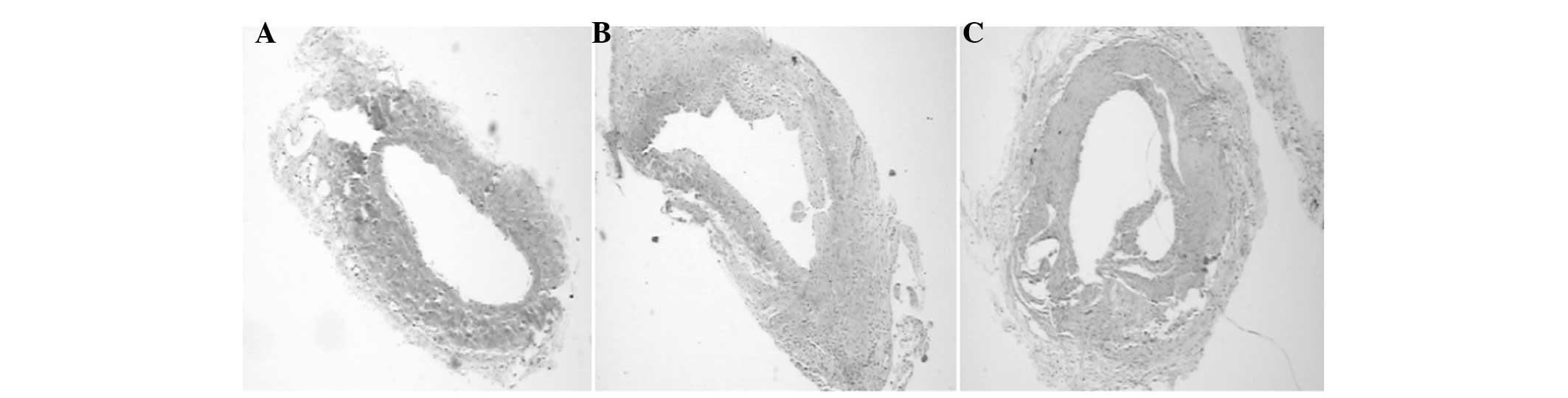
Observation of histomorphology
On day 7 after arteriovenous fistula surgery in the
experimental group, active smooth muscle cell proliferation was
visible in the tunica membrane at the venous end, with significant
intimal thickening, polypoid hyperplasia protruding into the
cavity, and large amounts of smooth muscle cells and collagen
fibers deposited in the hyperplastic tissue. On day 28, the above
pathological changes were more evident, with the lumen being more
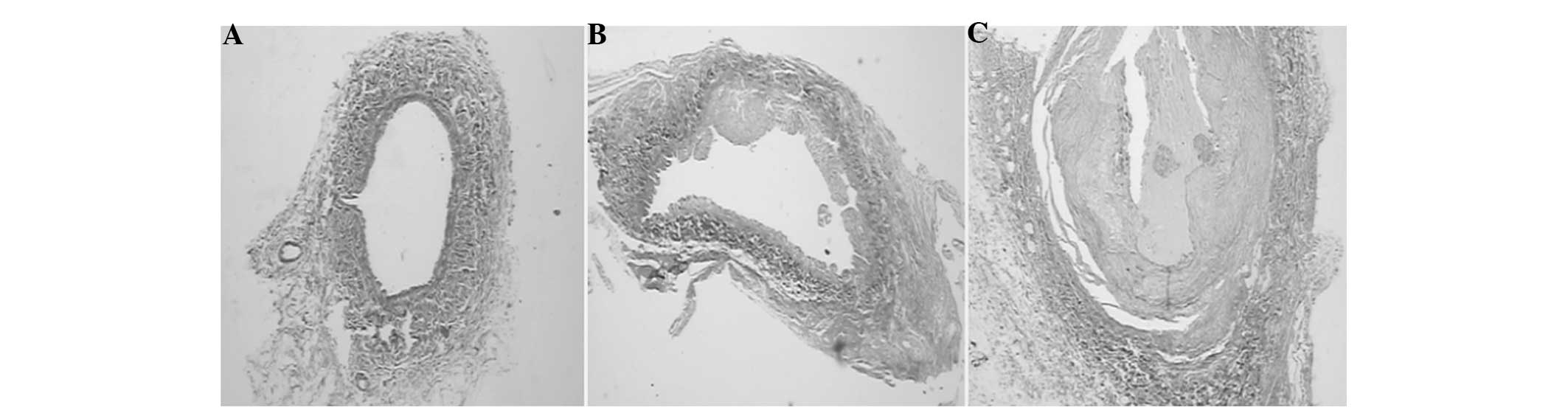
stenotic, but still able to allow the flow of blood. With double
collagen and elastin staining, it was observed that the internal
elastic layer was discontinuous with polypoid hyperplasia, and
greater amounts of collagen fibers were deposited in the vein near
the anastomotic stoma in the experimental group on days 7 and 28.
By contrast, in the sham surgery group, the internal elastic layer
was observed to be normal and continuous. Image analysis showed
that the tunica intima and tunica media thickness and the
neointimal area of the experimental group was gradually increased
in week 1 and week 4, compared with those in the sham surgery
group; the difference was statistically significant (P<0.05;
Table II, Figs. 1–3).
 | Table II.Vein pathological changes near the
anastomotic stoma following surgery of each group of rats. |
Table II.
Vein pathological changes near the
anastomotic stoma following surgery of each group of rats.
|
| Day 7 | Day 28 |
|---|
|
|
|
|
|---|
| Variable | Experimental
group | Sham surgery
group | Experimental
group | Sham surgery
group |
|---|
| Tunica intima
thickness (µm) |
38.22±1.39a |
7.01±1.40 |
69.50±5.34a |
8.34±1.22 |
| Tunica media
thickness (µm) |
50.51±2.65a |
21.82±2.25 |
46.14±6.59a |
23.92±2.26 |
| Hyperplastic area
(µm2) |
19.20±1.30a |
2.39±1.56 |
22.88±3.35a |
3.46±1.71 |
| α-SMA-positive
membrane area (%) |
6.33±0.86a |
4.33±1.51 |
7.05±1.32a |
4.89±1.13 |
Immunohistochemistry
In the tunica intima and tunica media, the
α-SMA-positive areas near the anastomotic stoma in the experimental
group were increased in size compared with those in the sham
surgery group at the same time-point (P<0.05); in the
experimental group, α-SMA positive areas near the anastomotic stoma
increased with time (P=0.52).
Discussion
An AVF remains the consensus-recommended vascular
access for individuals requiring hemodialysis (15). It can cause the dilatation of native
veins (16). Provision of a reliable
and durable vascular access for hemodialysis continues to be a
challenge for clinicians, with a retrospective review showing an
overall primary failure rate of 5.5% (17). Approach through the radial artery
distal is an effective and safe method for the salvage of
nonmaturing radiocephalic arteriovenous fistulas (18). Inflow stenosis is frequently
associated with fistula dysfunction (19). An animal model of autogenous fistula
can simulate the dynamic changes in the local hemodynamics of an
internal fistula and venous arterialization pathology, and
facilitate the observation of the effects of intervention measures.
Large and small animal models have advantages and disadvantages. In
dogs, perianastomotic pannus has been found to be primarily
composed of intimal smooth muscle cells, and neointimal thickening
was significantly reduced in prosthetic arteriovenous fistulae
(20). Various small animal models
have been reported in the non-Chinese literature (8,21–23).
However, this type of modeling is complex and the success rate is
low, and has not been reported previously in China. In the present
study, a simplified model of autologous vascular fistula in rats
with chronic renal failure was created, laying a foundation for
further study of failure mechanisms and interventions.
An anastomosis method is often used in the
establishment of the models, but the experimental results can be
affected by the technology proficiency level of the practitioner.
Application of a cuff technique in the anastomosis of blood vessels
has been introduced in organ transplantation animal models
(3,24). The present study used a cuff
technique to establish an AVF model in rats subjected to 5/6
nephrectomy, and may be considered to improve on previous animal
models. The model has the following characteristics: i) routine
modeling requires three surgical procedures and causes great trauma
to the animal, while the current method causes minimal trauma,
involves an simple dissection technique, with the anastomotic blood
vessel being similar to that in the clinic, and is easy for the
rats to endure; ii) anastomosis is conducted using a cuff technique
without microsurgical suture, and has a short surgery time, a
consistent anastomotic diameter and a high success rate (there were
no failed cases in the experimental group); iii) the experimental
results are less affected by the surgical technique and
proficiency, with a low anastomotic blood leakage occurrence rate,
high success rate of vein grafting and high fistula patency rate;
however, attention must be paid when inserting the artery into the
vein bridge, which requires two ligations, to avoid vein stump
aneurysm and the creation of turbulence; iv) the disposable venous
indwelling catheter used in the experimental group was a common
clinical catheter, the specification of which may be selected
according to the vascular caliber. It contacts only the carotid
artery adventitia, that is, the carotid artery and external jugular
vein anastomosis is connected between the intima, which does not
readily cause thrombosis.
The 5/6 nephrectomy rat model was established
successfully in this study. The blood urea nitrogen and creatinine
levels of the experimental group had increased on day 7 after 5/6
nephrectomy compared with those in the sham surgery group. On day
28 after the surgery, the blood urea nitrogen and creatinine values
decreased slightly in the experimental group, but remained
significantly higher than those in the sham surgery group. This
indicates that acute decompensation may gradually transition to
residual renal compensation, indicating an improved simulation of
chronic renal insufficiency 28 days after surgery.
It is currently considered that the important
pathological changes of intimal hyperplasia are the increase of
vascular smooth muscle cells and extracellular matrix (13,25–28). The
present study showed that there was intimal hyperplasia of the
fistula in the first week after surgery, and the pathological
features included smooth muscle cell proliferation, the
accumulation of collagen fibers, extensive polypoidal hyperplasia,
and evident stenosis in the fourth week after surgery. Staining
showed that the elastic layer was not continuous following fistula
surgery, suggesting that vessel fracture promotes smooth muscle
cell migration to the intima. Immunohistochemistry showed that
expression of α-SMA in the experimental group gradually increased
with time. The α-SMA-positive area of the tunica intima and tunica
media in the vein near the anastomotic stoma of the experimental
group increased significantly compared with that in the sham
operation surgery group at the same time-point in weeks 1 and 4,
indicating that the expression of α-SMA may increase with increased
intimal hyperplasia, consistent with previous studies (7,29–31).
In summary, a rat model of autologous arteriovenous
fistula with chronic renal failure was successfully established by
a cuff technique. This was indicated by the pathological changes to
the vein, which were smooth muscle cell proliferation, evident
collagen fiber deposition, and the expression of α-SMA, by the
appearance of the vessel and renal function. This model appears to
provide a very good simulation of the pathological processes of
chronic renal insufficiency with fistula. The establishment of the
model is simple and easy to master, and can eliminate the effect of
different anastomotic techniques on experimental research. Thus, it
provides an ideal experimental animal model of early and metaphase
chronic renal insufficiency for research into intimal hyperplasia,
and is worthy of promotion.
Acknowledgements
This work is supported by the Fund of Science and
Technology Bureau of Wenzhou City (grant no. Y20110129).
References
|
1
|
Gowda A, Pavan M and Babu K: Vascular
access profile in maintenance hemodialysis patients. Iran J Kidney
Dis. 8:218–224. 2014.PubMed/NCBI
|
|
2
|
Han M, Kim JD, Bae JI, Lee JH, Oh CK, Ahn
C and Won JH: Endovascular treatment for immature autogenous
arteriovenous fistula. Clin Radiol. 68:e309–e315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kokubo T, Ishikawa N, Uchida H, et al: CKD
accelerates development of neointimal hyperplasia in arteriovenous
fistulas. J Am Soc Nephrol. 20:1236–1245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kakkos SK, Haddad GK, Stephanou A, Haddad
JA and Shepard AS: Routine preoperative venous and arterial mapping
increases both, construction and maturation rate of upper arm
autogenous arteriovenous fistulae. Vasc Endovascular Surg.
45:135–141. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Usta E, Elkrinawi R, Salehi-Gilani S, et
al: Risk factors predicting the successful function and use of
autogenous arteriovenous fistulae for hemodialysis. Thorac
Cardiovasc Surg. 61:438–444. 2013.PubMed/NCBI
|
|
6
|
Chang CJ, Chen CC, Hsu LA, et al:
Degradation of the internal elastic laminae in vein grafts of rats
with aortocaval fistulae: potential impact on graft vasculopathy.
Am J Pathol. 174:1837–1846. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan CY, Chen YS, Ma MC and Chen CF:
Remodeling of experimental arteriovenous fistula with increased
matrix metalloproteinase expression in rats. J Vasc Surg.
45:804–811. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Castier Y, Lehoux S, Hu Y, Foteinos G,
Tedgui A and Xu Q: Characterization of neointima lesions associated
with arteriovenous fistulas in a mouse model. Kidney Int.
70:315–320. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Caplice NM, Wang S, Tracz M, Croatt AJ,
Grande JP, Katusic ZS and Nath KA: Neoangiogenesis and the presence
of progenitor cells in the venous limb of an arteriovenous fistula
in the rat. Am J Physiol Renal Physiol. 293:F470–F475. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang A, Wang Y, Han G, Truong L and Cheng
J: Chronic kidney disease accelerates endothelial barrier
dysfunction in a mouse model of an arteriovenous fistula. Am J
Physiol Renal Physiol. 304:F1413–1420. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin F, Dardik H, Pangilinan A, Robinson J,
Chuy J and Wengerter K: Remodeling and suppression of intimal
hyperplasia of vascular grafts with a distal arteriovenous fistula
in a rat model. J Vasc Surg. 34:701–706. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals8th.
National Academies Press; Washington: 2011
|
|
13
|
Hoch H, Stegbauer J, Potthoff SA, Hein L,
Quack I, Rump LC and Vonend O: Regulation of renal sympathetic
neurotransmission by renal α(2A)-adrenoceptors is impaired in
chronic renal failure. Br J Pharmacol. 163:438–446. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sivanesan S, How TV, Black RA and Bakran
A: Flow patterns in the radiocephalic arteriovenous fistula: an in
vitro study. J Biomech. 32:915–925. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jennings WC and Taubman KE: Alternative
autogenous arteriovenous hemodialysis access options. Semin Vasc
Surg. 24:72–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Georgakarakos EI, Kapoulas KC, Georgiadis
GS, Tsangaris AS, Nikolopoulos ES and Lazarides MK: An overview of
the hemodynamic aspects of the blood flow in the venous outflow
tract of the arteriovenous fistula. J Vasc Access. 13:271–278.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Benna S, Deardon D, Hamilton D and
El-Enin H: Long-term outcome of upper limb autogenous arteriovenous
fistulas for hemodialysis access. Saudi J Kidney Dis Transpl.
24:109–114. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsieh MY, Lin L, Tsai KC and Wu CC: Radial
artery approach to salvage nonmaturing radiocephalic arteriovenous
fistulas. Cardiovasc Intervent Radiol. 36:957–963. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Caeiro F, Carvalho D, Cruz J, Ribeiro
Santos J and Nolasco F: Efficacy of percutaneous transluminal
angioplasty on dysfunctional fistulae because of inflow stenosis. J
Vasc Access. 14:231–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gentile AT, Mills JL, Gooden MA, et al:
Vein patching reduces neointimal thickening associated with
prosthetic graft implantation. Am J Surg. 176:601–607. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paszkowiak JJ and Dardik A: Arterial wall
shear stress: observations from the bench to the bedside. Vasc
Endovascular Surg. 37:47–57. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Corpataux JM, Haesler E, Silacci P, Ris HB
and Hayoz D: Low-pressure environment and remodelling of the
forearm vein in Brescia-Cimino haemodialysis access. Nephrol Dial
Transplant. 17:1057–1062. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guzman RJ, Krystkowiak A and Zarins CK:
Early and sustained medial cell activation after aortocaval fistula
creation in mice. J Surg Res. 108:112–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizuta T, Nakahara K, Shirakura R, Fujii
Y, Kawaguchi A, Minami M and Kawashima Y: Total nomicrosuture
technique for rat lung transplantation. J Thorac Cardiovase Surg.
102:159–160. 1991.
|
|
25
|
Roy-Chaudhury P, Sukhatme VP and Cheung
AK: Hemodialysis vascular access dysfunction: a cellular and
molecular viewpoint. J Am Soc Nephrol. 17:1112–1127. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kelly B, Melhem M, Desai P, et al:
Perivascular paclitaxel polymers reduce neointimal hyperplasia and
venous stenosis in PTFE arteriovenous grafts: a possible solution
for hemodialysis vascular access dysfunction. J Am Soc Nephrol.
15:25A2004.
|
|
27
|
Kuji T, Masaki T, Goteti K, et al:
Efficacy of local dipyridamole therapy in a porcine model of
arteriovenous graft stenosis. Kidney Int. 69:2179–2185. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Janardhanan R, Yang B, Vohra P, et al:
Simvastatin reduces venous stenosis formation in a murine
hemodialysis vascular access model. Kidney Int. 84:338–352. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Muto A, Fitzgerald TN, Pimiento JM, et al:
Smooth muscle cell signal transduction: implications of vascular
biology for vascular surgeons. J Vasc Surg. 45:(Suppl A). 15–24.
2007. View Article : Google Scholar
|
|
30
|
Roy-Chaudhury P, Miller M, Reaves A, et
al: Adventitial fibroblasts contribute to venous neointimal
hyperplasia in PTFE dialysis grafts. J Am Soc Nephrol.
12:301A2001.
|
|
31
|
Motwani JG and Topol EJ: Aortocoronary
saphenous vein graft disease: pathogenesis, predisposition and
prevention. Circulation. 97:916–931. 1998. View Article : Google Scholar : PubMed/NCBI
|