Introduction
Peripheral nerve injury leads to the damage of
axons, where neurons lose the basis of survival and gradually
degenerate or disappear as a result of the interruption of axonal
transport. Regeneration of the injured peripheral nerve is
dependent on the vitality of the central neurons and the nerve
regeneration microenvironment, while the death of neurons
exacerbates the peripheral nerve injury (1). Therefore, the key to preventing or
reducing the occurrence of degenerative cell death may be the
protection of the motor neuron cell body following peripheral nerve
injury. Thus, it is necessary to identify methods of protecting
motor neurons and to develop a novel repair technique.
Insulin-like growth factor-1 (IGF-1), identified
more than 30 years ago as a type of biologically active peptide
with a role in the central and peripheral nervous systems, exhibits
neurotrophic and neural regulatory effects (2). Studies on IGF-1 have predominantly
focused on diseases such as diabetes, osteoporosis and Alzheimer's
disease (3–5). A number of animal studies have
investigated the role of IGF-1 in the treatment of peripheral nerve
injuries (6–8), including injuries involving the facial
nerves and sciatic nerve. However, studies investigating the
effects of IGF-1 on the pathological changes of spinal motor
neurons following peripheral nerve injury are less common. As the
half-life of IGF-1 is short and repeated transfusions are required,
genetic engineering may be performed to solve the problem of
long-term IGF-1 supply. In the present study, IGF-1 was applied to
the area of sciatic nerve injury in order to observe the protective
effects on spinal cord neurons.
With advances in basic research, cell
transplantation, tissue engineering technology and genetic
engineering technology, the analysis of peripheral nerve repair has
been enriched and developed. Numerous nutritional factors have been
found to be associated with nerve regeneration, including
brain-derived neurotrophic factor (9), glial cell line-derived neurotrophic
factor (10), ciliary neurotrophic
factor (11) and IGF-1. IGF-1, a
multifunctional biological peptide that has a role in the central
and peripheral nervous systems, exhibits neurotrophic and neural
regulatory effects. At present, the majority of studies have
focused on the central nervous system (12), while there is limited literature
regarding the promotion of peripheral nerve regeneration by IGF-1
following peripheral nerve injury and its effect on the
pathological changes of spinal motor neurons. The present study
investigated the protective effects of IGF-1 on spinal motor
neurons following peripheral nerve injury by applying transgenic
IGF-1 to a sciatic nerve injury site.
According to the hypothesis of nerve regeneration in
small gaps (13), with the
appropriate clearance, neural nutrition and chemotaxis can be used
in nerve regeneration to solve the phenomenon of dislocated nerve
growth and to achieve more accurate docking (14,15). In
the present study, a rat model of sciatic nerve crush injury was
developed and plasmid-liposome-mediated human IGF-1 (hIGF-1) was
introduced into the nerve regeneration chamber via epineural
injection. The protective effects of hIGF-1 on the spinal motor
neurons at various time points following peripheral nerve injury
were then observed.
Through the analysis of hIGF-1 expression, apoptosis
detection and transmission electron microscopy (TEM) observations
of motor neurons in the anterior horn of the spinal cord, the
protective effect of internal transgenic hIGF-1 on spinal motor
neurons following peripheral nerve injury was investigated, as well
as the underlying mechanism.
Materials and methods
Experimental animals and reagents
A total of 90 adult male Wistar rats, weighing
200–250 g, were provided by the Experimental Animal Center of Jilin
University (Changchun, China). Positive liposomes (Lipofectamine®;
2 µg/µl) and pcDNA3.1 (1 µg/µl) were purchased from Beijing
Yuanping Hao Biotechnology Ltd (Beijing, China) and pcDNA-hIGF-1 (1
µg/µl) was supplied by Dr Bo Shen from the Public Health School of
Jilin University (Changchun, China). Liposomes and plasmids were
mixed at a mass ratio of 1.5:1. The mouse anti-human IGF-1
polyclonal antibody (#MS-1508; PcAb) and streptavidin-peroxidase
(SP) kit were purchased from the Fujian Maixin Biotechnology
Development Co. (Fuzhou, China) and the terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) kit was
purchased from Wuhan Boster Biological Technology, Ltd. (Wuhan,
China). The current study was approved by the Institutional Animal
Care and Use Committee of the Experimental Animal Center of Jilin
University.
Establishing animal models and
groupings
The rats were randomly divided into three groups:
hIGF-1 treatment, sham-transfected control and blank control groups
(n=30 per group). Ketamine hydrochloride (5%; 130 mg/kg) was
injected intraperitoneally to anesthetize the rats and the right
sciatic nerve was exposed. The sciatic nerve 5 mm below the
piriformis muscle was clamped three times using a clamp of 3 mm
width, and squeezed for 10 sec each time, with observation
conducted at a magnification of x6 under an operating microscope.
Next, 10 µl pcDNA-hIGF-1 (hIGF-1 DNA, 4 µg) and Lipofectamine
transfection reagent was immediately injected into the clamped
epineurium of the rats in the hIGF-1 treatment group. The rats of
the sham-transfected group were injected with a pcDNA3.1,
Lipofectamine and distilled water mixture (10 µl) and the blank
control group was not injected with any substance. The animals were
maintained in a single cage following surgery.
Observation methods
Immunohistochemical staining of hIGF-1 expression
by motor neurons in the anterior horn of the spinal cord
Rats from each group were anaesthetized by the
intraperitoneal injection of 5% ketamine hydrochloride and then
perfused with 4% formaldehyde for whole animal fixation at days 2,
7, 14 and 28 postoperatively. The L4–6 spinal cord segments of the
animals in each group were obtained and placed in 4%
paraformaldehyde in a refrigerator at 4°C overnight. The samples
were embedded in paraffin and IGF-1 expression levels in the L4–6
spinal cord segments were detected by incubation with a primary
mouse anti-hIGF-1 PcAb (1:500) antibody overnight at 4°C followed
by treatment with a goat anti-mouse polyclonal IgG secondary
antibody (#MS-642; 1:1,000; Fujian Maixin Biotechnology Development
Co.) for 1 h at room temperature. The expression levels were
evaluated using an image analysis system (HPIAS-1000; Shimadzu
Corporation, Kyoto, Japan). In this system, the brown or brownish
yellow positive staining was evaluated as a gray scale value. The
SP kit reagent that provided the brown staining was anti-Mouse
HIGF-1 supplied by ~100 µg antiserum lyophilized from a 0.2 µm
filtered solution in phosphate-buffered saline (PBS). These values
were inversely related to the quantity of IGF-1 expressed. Three
samples per group were analyzed at each time point.
Detection of apoptosis of motor neurons in the
anterior horn of the spinal cord
Apoptosis was detected in L4–6 spinal cord motor
neurons using the TUNEL detection kit, following polyformaldehyde
perfusion at days 7, 14 and 21 after surgery. Three samples per
group were analyzed at each time point.
Counting of motor neuron cells of the anterior
horn in the spinal cord and observation of pathological
changes
Spinal cord L4–6 paraffin-embedded sections, which
were perfused with polyformaldehyde at days 2, 7, 14 and 28
following surgery, were double-stained with Marsland and Luxol fast
blue. The form and number of spinal anterior horn cells,
cytoplasmic Nissl bodies and the proliferation of glial cells were
observed using a HPIAS-1000 Color pathological image analysis
system (Shimadzu Corporation). Three samples per group were
analyzed at each time point.
TEM observation
On day 56, L4–6 spinal cord anterior horns were
obtained from five rats in each group and placed in 25 g/l
glutaraldehyde for prefixation and 10 g/l osmium tetroxide for
postfixation. The samples were then fixed in a series of ethanol
dehydrates and epoxy-embedded in epon 812 (Haide Biotechnology Co.,
Ltd., Beijing, China). Ultra-thin sections were cut using an LKB
Ultratome III (Pharmacia, Stockholm, Sweden) and then uranyl
acetate and lead citrate double-staining was performed. Next, the
ultrastructure of the neuropil within the spinal cord anterior horn
was observed using radiography and a JEM-1200EX transmission
electron microscope (Jeol, Tokyo, Japan).
Statistical analysis
Results were statistically analyzed with SPSS
statistical analysis software, version 11.5 (SPSS, Inc., Chicago,
IL, USA), using multiple-sample mean variance analysis. Data are
expressed as the mean ± standard deviation. P<0.05 was
considered to indicate a statistically significant difference.
Results
hIGF-1 expression levels in the motor
neurons in the anterior horn of the spinal cord
Comparison between time points for each
group
The hIGF-1 expression levels in the motor neurons in
the anterior horn of the spinal cord were compared on different
days. A small amount of brown positive product was observed in the
hIGF-1 treatment group at day 2 following the injection of
exogenous hIGF-1 to treat the nerve injury. The quantity of
positively stained product significantly increased by days 7 and 14
when compared with the level at day 2 (P<0.01), and no
statistically significant difference was observed between days 7
and 14 (P>0.05). Peak expression occurred at day 7 and gradually
decreased with time. The quantity of positive expression products
significantly reduced to the level observed at day 2 by day 28
following the injection; a statistically significant difference was
observed when compared with the levels at days 7 and 14
(P<0.01). There was no significant difference (P>0.05) when
compared with the day 2 level.
For the sham-transfected and blank control groups,
positive staining, indicative of IGF-1 expression, was only
observed in glial cells at day 2 and was not detected in the motor
neurons. However, the quantity of positively stained products in
the motor neurons significantly increased at days 7 and 14 when
compared with the level at day 2 (P<0.01); there was no
significant difference between days 7 and 14 (P>0.05). Peak
expression occurred at day 7 and gradually decreased with time. The
expression level at day 28 was significantly lower than the levels
at days 7 and 14, and slightly lower than the level at day 2;
statistically significant differences were observed when comparing
the expression levels at days 2, 7 and 14.
Comparison between groups at each time point
IGF-1 expression levels in the hIGF-1 treatment
group were higher compared with those in the other two groups and
the difference was statistically significant (P<0.01). No
significant difference in IGF-1 expression levels was identified
between the sham-transfected and blank control groups (P>0.05;
Table I).
 | Table I.hIGF-1 protein expression levels in
the spinal cord determined by immunohistochemistry (gray
valuesa). |
Table I.
hIGF-1 protein expression levels in
the spinal cord determined by immunohistochemistry (gray
valuesa).
| Group | Day 2 | Day 7 | Day 14 | Day 28 |
|---|
| Blank control |
158.38±2.070 |
154.23±2.900b |
154.52±2.200b |
161.70±2.030c,d |
| Sham-transfected |
158.04±2.230 |
153.59±2.610b |
156.13±2.110b |
160.98±2.400c,d |
| hIGF-1 treatment |
143.87±2.851e |
133.39±2.111b,e,f |
135.41±2.351b,e,f |
143.69±2.451c–f |
Detection of apoptotic motor neurons
in the anterior horn of the spinal cord
TUNEL staining of apoptotic cell nuclei was brownish
yellow, while normal cell nuclei were blue-violet. The apoptotic
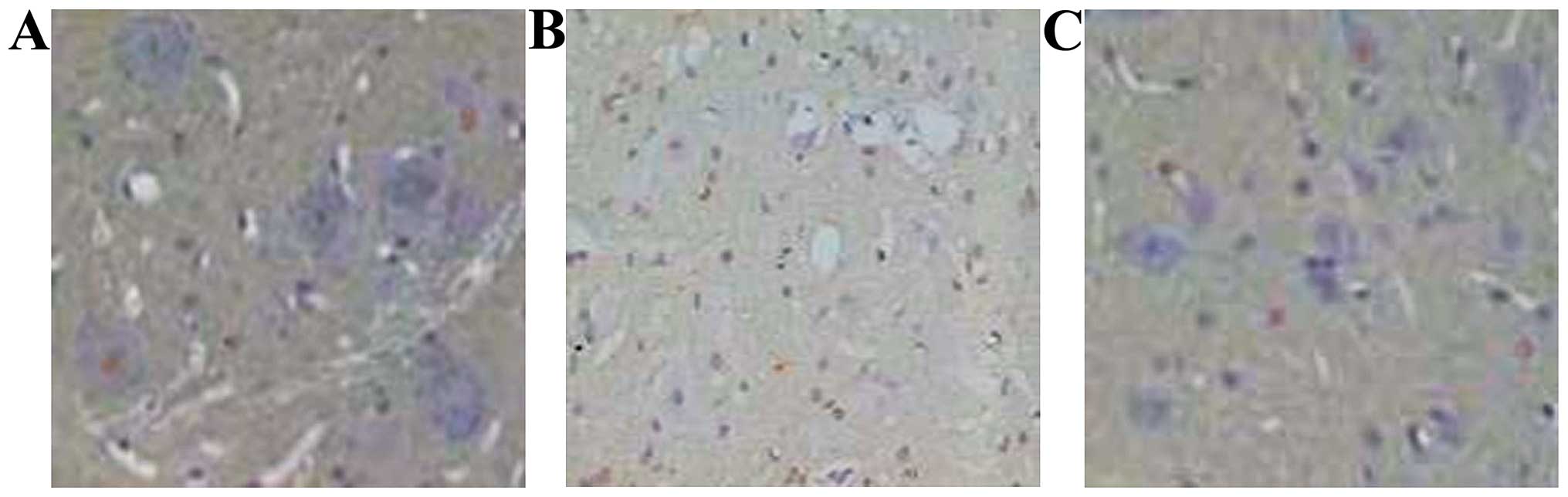
cell counts determined through TUNEL staining (Fig. 1) at different time points, revealed
that the number of motor neuron cells undergoing apoptosis was at a
maximum 7 days after sciatic nerve injury and gradually decreased
by days 14 and 21. The apoptotic cell count in the hIGF-1 treatment
group was lower compared with those in the sham-transfected and
blank control groups and the difference was statistically
significant (P<0.01; Table II).
The apoptotic cells were motor neuron and glial cells.
 | Table II.Number of motor neurons positive for
TUNEL staining in the anterior horn of the spinal cord. |
Table II.
Number of motor neurons positive for
TUNEL staining in the anterior horn of the spinal cord.
| Group | Day 7 | Day 14 | Day 21 |
|---|
| Blank control |
5.60±1.520 |
4.6±1.14 |
3.08±1.320 |
| Sham-transfected |
6.20±1.480 |
5.4±1.52 |
2.72±1.400 |
| hIGF-1 treatment |
2.40±0.551a,b |
2.0±1.01a,b |
1.68±0.891a,b |
Motor neuron cell count in the
anterior horn of the spinal cord and pathological changes
Observations between time points for the
groups
At day 2 following surgery, there was no evident
difference in the number of motor neuron cells in the anterior horn
of the spinal cord compared with the number in the normal spinal
cord side. Nissl bodies in the cytoplasm were clearly visible and
showed coarse granular features. In addition, the nerve cells
exhibited a little swelling and degeneration. Compared with the
count at day 2, the number of motor neuron cells in the anterior
horn gradually decreased between days 7 and 14 following surgery.
In addition, Nissl body particles became thinner, smaller and even
centrally dissolved. Motor neuron cells of the anterior horn showed
deformation and neuronophagia, until ulceration disappeared after
day 14. Compared with the count at day 14, the number of anterior
horn cells in the spinal cord had increased by day 28. The number
of glial cells also increased, satellite phenomena appeared around
the damaged motor neurons and new Nissl body particles were
synthesized in the cytoplasm.
Comparison between groups at each time point
At day 2, there was no significant difference in the
number of motor neurons among the three groups (P>0.05). The
motor neuron cell numbers in the anterior horn of the spinal cord
in the IGF-1 treatment group were increased at the day 7, 14 and 28
time points compared with those in the sham-transfected and control
groups. In addition, in the IGF-1 treatment group the cytoplasmic
Nissl bodies exhibited slightly reduced deformation than that in
the other two groups and motor neuron repair occurred earlier. The
effect on day 28 was particularly superior (Table III).
 | Table III.Number of motor neuron cells in the
anterior horn of the spinal cord. |
Table III.
Number of motor neuron cells in the
anterior horn of the spinal cord.
| Group | Day 2 | Day 7 | Day 14 | Day 28 |
|---|
| Blank control |
20.80±2.390 |
12.00±1.220 |
9.20±1.300 |
12.80±1.480 |
| Sham-transfected |
20.00±2.550 |
13.00±1.000 |
12.40±1.140 |
11.20±1.300 |
| hIGF-1 treatment |
23.20±2.391 |
16.20±1.301a,b |
15.00±1.001a,b |
17.20±1.921a,b |
TEM observation
Neuropil changes were observed in the intramedullary
section of the spinal cord by TEM at day 56 following surgery. In
the hIGF-1 treatment group, the neuropil structure in the
intramedullary section of the spinal cord was normal. However, in
the sham-transfected group, the gap in the neuropil processus was
large, the density of the axon mitochondria had increased and the
myelin sheath of myelinated nerve fibers was loose. Vacuoles were
observed in the neuropil of the blank control group.
Discussion
IGF-1 is a biological peptide with multiple
functions, including neurotrophic and neural regulatory effects.
The peptide is a member of the nerve growth factor family (16). Since Daughaday et al (17) identified this factor to be closely
associated with a growth hormone (GH), research into IGF-1 has
developed. In addition, the emergence of recombinant IGF-1
biological products has developed a broader perspective of its use
in the diagnosis and treatment of disease (18–20).
As neurotrophic factors have a short half-life, the
application of genetic engineering is an effective method to
provide a long-term supply of neurotrophic factors. In 1990, Wolff
et al (21) first used a
direct injection of DNA into muscles to transfer genes, for
exogenous gene expression in skeletal muscle, which was a prelude
to the use of plasmid vectors for gene therapy research.
Reconnection of nerves following peripheral nerve injury is a
necessary condition for the recovery of neurological function. The
success of regeneration following peripheral nerve repair depends
on the vitality of the central motor neurons and a suitable
microenvironment for the renewal of growth. Substances such as
nerve growth factors are important for the regeneration of the
microenvironment. hIGF-1 has been confirmed to promote the
regeneration of peripheral neurons. IGF-1 binds to the IGF-1
receptor on the surface of neurons at the site of injury, improves
neuronal survival and promotes axonal regeneration and synapse
formation, thereby contributing to the regeneration of the nerve
(22).
Appropriately small gaps can use neural nutrition
and chemotaxis to solve neural dislocation growth phenomena with
more accurate docking. Certain studies (23,24) have
reported that 1–3 mm gaps achieve the best regenerative effect,
which is why a 3-mm gap was selected in the present study. A number
of studies have used exogenous-promoting nerve growth factor to
promote peripheral nerve regeneration during the repair of injury
(5,22,25).
However, since the half-life of these factors is very short, the
application of traditional methods requires repeated transfusions
of these molecules and it is difficult to maintain effective
concentrations in specific areas. The experimental application of
genetic engineering involves using a plasmid as a carrier, which
may extend the role of hIGF-1. In addition, compared with viral
vectors, plasmid carriers are able to accommodate large fragments
of DNA, which are transformed into circular DNA following
transformation into target cells, and are not integrated and
replicated. As a result, there is no viral infection or
carcinogenic potential due to the presence of the viral vectors in
the host (26,27).
The results of the present study indicate that
exogenous hIGF-1 administered in the area of the injured sciatic
nerve can be transported to spinal motor neuron cells for
expression, and that peak expression occurs at approximately day 7.
Apoptosis of motor neurons was observed at days 7, 14 and 21
following surgery. Apoptosis occurs following peripheral nerve
injury, and may be the main route of neuronal cell death. The
apoptosis peak occurred at approximately day 7. The numbers of
apoptotic cells in the hIGF-1 treatment group at the various time
points were fewer than those in the sham-transfected and control
groups, indicating that IGF-1 is able to reduce the apoptosis of
motor neurons following peripheral nerve injury and protect motor
neurons. At days 2, 7, 14 and 28 following surgery, the number of
motor neurons in the IGF-1 treatment group was higher compared with
those in the other two groups. The degree of pathological change,
including the central solution of Nissl bodies in neuron cells and
neuronophagia, was lower in the IGF-1 treatment group than that of
the other two groups. In addition, the phenomenon of
oligodendroglia repairing motor neurons was more evident. All the
results demonstrate that IGF-1 has the ability to protect motor
neurons. Neuropil refers to the complex network area formed by the
dendrites and axons of neurons and the neurites of gliocytes in the
grey matter of the central nervous system, which are connected to
each other. The neuropil is an important area for message exchange
among central neurons; thus, injury, repair and regeneration
functions of the spinal cord are accompanied by the degeneration
and rebuilding of the neuropil. At day 56 following surgery, the
ultrastructure of the neuropil in the IGF-1 treatment group was
better than in the other two groups, indicating that the spinal
cord was repaired more effectively.
The mechanism underlying hIGF-1 transfection into
neural tissue cells and the detailed mechanism behind
neuroprotection and nutrition remain unclear. Certain studies
hypothesize that exogenous IGF-1 and −2 play a role in the central
nervous system, primarily through targeted IGF protein-binding to
neurons and glial cells (28).
Nagano et al (29)
hypothesized that the mechanism may involve the phosphoinositide
3-kinase signaling pathway. It has also been hypothesized (30) that the mechanism may function via the
stimulating effects of trauma and other external factors causing
increases in cell membrane permeability and the transport of
exogenous plasmid DNA into the cell via a specific type of cell
membrane transport, particularly pinocytosis. However, whether this
hypothesis is correct requires further study.
The animal experiments in the present study
demonstrate that IGF-1 administered in a liposome-mediated plasmid
as carrier can be taken up by damaged axons and transported to the
spinal neurons. The IGF-1 was found to play the greatest role in
the spinal cord 7–14 days after transfection, with a neurotrophic
effect at the biological level. Thus, this preliminary study on
peripheral nerve injury and the pathological changes of spinal
motor neurons, indicates that following peripheral nerve injury,
the application of exogenous IGF-1 protected spinal motor neuron
function. In future studies, transgenic IGF-1 and biomaterials for
tissue engineering may be combined together to promote nerve
regeneration following peripheral nerve injury, and potentially
provide a new method for nerve generation.
References
|
1
|
Wang SR, Sun ZR, Dai YM, et al: Expression
of ciliary neurotrophic factor of spinal cord motor neuron after
sciatic nerve injury and changes after acupuncture intervention in
rats. Zhongguo Zuzhi Gongcheng Yanjiu. 8:6954–6957. 2004.(In
Chinese).
|
|
2
|
Bothwell M: Insulin and somatemedin MSA
promote nerve growth factor-independent neurite formation by
cultured chick dorsal root ganglionic sensory neurons. J Neurosci
Res. 8:225–31. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Godińez-Gómez R, Trujillo-Hernández B,
Tene CE, et al: Electrophysiological abnormalities in type 2
diabetic patients with reduced levels of insulin-like growth factor
I. J Int Med Res. 34:21–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng JZ, Dong KL, Li GC and Li LM: Effect
of xiaokeling concentration fluid on mRNA expression of
insulin-like growth factor-1 in sciatic nerve of
Streptozotocin-induced diabetic rats. Zhong Nan Da Xue Xue Bao Yi
Xue Ban. 30:49–52. 2005.(In Chinese). PubMed/NCBI
|
|
5
|
Wang W, Teng Y, Zhang MQ, et al: Function
of insulin receptor and insulin-like growth factor receptor in
Alzheimer's disease. Sichuan Shengli Kexue Zazhi. 35:112–115.
2013.
|
|
6
|
Kiryakova S, Söhnchen J, Grosheva M, et
al: Recovery of whisking function promoted by manual stimulation of
the vibrissal muscles after facial nerve injury requires
insulin-like growth factor 1 (IGF-1). Exp Neurol. 222:226–234.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hu WL, Hu LQ, Li SW and Zheng XM:
Reconstruction of erectile reflex circuit by autologous vein graft
combined with use of insulin-like growth factor. Zhonghua Yi Xue Za
Zhi. 84:1280–1282. 2004.(In Chinese). PubMed/NCBI
|
|
8
|
Emel E, Ergün SS, Kotan D, et al: Effects
of insulin-like growth factor-1 and platelet-rich plasma on sciatic
nerve crush injury in a rat model. J Neurosurg. 114:522–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vögelin E, Baker JM, Gates J, Dixit V,
Constantinescu MA and Jones NF: Effects of local continuous release
of brain derived neurotrophic factor (BDNF) on peripheral nerve
regeneration in a rat model. Exp Neurol. 199:348–353. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YC, Oh SJ and Marra KG: Synergistic
lithium chloride and glial cell line-derived neurotrophic factor
delivery for peripheral nerve repair in a rodent sciatic nerve
injury model. Plast Reconstr Surg. 132:251e–262e. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Lineaweaver WC, Oswald T, Chen Z,
Chen Z and Zhang F: Ciliary neurotrophic factor for acceleration of
peripheral nerve regeneration: an experimental study. J Reconstr
Microsurg. 20:323–327. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brinton RD and Wang JM: Therapeutic
potential of neurogenesis for prevention and recovery from
Alzheimer's disease: allopregnanolone as a proof of concept
neurogenic agent. Curr Alzheimer Res. 3:185–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saito I, Oka Y and Odaka M: Promoting
nerve regeneration through long gaps using a small nerve tissue
graft. Surg Neurol. 59:148–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang P, Zhang C, Kou Y, et al: The
histological analysis of biological conduit sleeve bridging rhesus
monkey nerve injury with small gap. Artif Cells Blood Substit
Immobil Biotechnol. 37:101–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang B, Zhang P and Jiang B: Advances in
small gap sleeve bridging peripheral nerve injury. Artif Cells
Blood Substit Immobil Biotechnol. 38:1–4. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zheng WH, Kar S, Doré S and Quirion R:
Insulin-like growth factor-1 (IGF-1): a neuroprotective trophic
factor acting via the Akt-kinase pathway. J Neural Transm Suppl.
60:261–272. 2000.PubMed/NCBI
|
|
17
|
Daughaday WH, Hall K, Salmon WD, Van den
Brande JL and Van Wyk JJ: On the nomenclature of the somatomedins
and insulin-like growth factors. Endocrinology. 121:1911–1922.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rosen CJ and Pollak M: Circulating IGF-I:
New perspectives for a new century. Trends Endocrinol Metab.
10:136–141. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Twigg SM and Baxter RC: Insulin-like
growth factor (IGF)-binding protein 5 forms an alternative ternary
complex with IGFs and the acid-labile subunit. J Biol Chem.
273:6074–6079. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holman SR and Baxter RC: Insulin-like
growth factor binding protein-3: factors affecting binary and
ternary complex formation. Growth Regul. 6:42–47. 1996.PubMed/NCBI
|
|
21
|
Wolff JA, Malone RW, Williams P, et al:
Direct gene transfer into mouse muscle in vivo. Science.
247:1465–1468. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pu SF, Zhuang HX and Ishii DN:
Differential spatio-temporal expression of the insulin-like growth
factor genes in regenerating sciatic nerve. Brain Res Mol Brain
Res. 34:18–28. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Butí M, Verdú E, Labrador RO, et al:
Influence of physical parameters of nerve chambers on peripheral
nerve regeneration and reinnervation. Exp Neurol. 137:26–33. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kou YH, Yin XF, Zhang PX and Jiang BG:
Small-gap bridging technology for peripheral nerve injury repair
and the new sleeve material. Beijing Da Xue Xue Bao. 43:647–651.
2011.(In Chinese). PubMed/NCBI
|
|
25
|
Zhang ZJ, Gao BQ, Liang CY, Liao ZG, Xu JG
and Liu YF: Insulin-like growth factor in the regeneration of
facial nerve following injury. Zhongguo Zhongxiyijiehe Er Bi Yanhou
Ke Zazhi. 12:7–9. 2004.(In Chinese).
|
|
26
|
Gyula A, Shoushu J, Agnes J, et al: Direct
gene transfer and expression into rat heart in vivo. New Biol.
3:711991.PubMed/NCBI
|
|
27
|
Jiao S, Williams P, Berg RK, et al: Direct
gene transfer into nonhuman primate myofibers in vivo. Hum Gene
Ther. 3:21–33. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sizonenko SV, Sirimanne ES, Williams CE
and Gluckman PD: Neuroprotective effects of the N-terminal
tripeptide of IGF-1, glycine-proline-glutamate, in the immature rat
brain after hypoxic-ischemic injury. Brain Res. 922:42–50. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nagano T, Nakamura M, Nakata K, et al:
Effects of substance P and IGF-1 in corneal epithelial barrier
function and wound healing in a rat model of neurotrophic
keratopathy. Invest Ophthalmol Vis Sci. 44:3810–3815. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang SQ, Liu L, Zhang JQ, et al:
Expression of exogenous gene at the site of anastomoses of
peripheral nerve. Tong Ji Yi Ke Da Xue Xue Bao. 33:455–458.
2004.(In Chinese).
|