Introduction
Cerebral arteriovenous malformation (cAVM) is the
major cause of intracranial hemorrhage and seizures in young and
middle-aged adults, and can seriously threaten their quality of
life and life expectancy (1,2). Due to the minimal harm inflicted on
patients, endovascular embolization therapy has become an important
method for the treatment of cAVM, and the selection of an embolic
material is a major determinant towards the treatment efficacy.
The ideal cAVM embolic material should have a number
of characteristics. Firstly, the material should be liquid, since
this enables easy control, good dispersibility and ensures a
sufficient injection time. Secondly, the material should exhibit a
permanent embolism effect. Finally, the material should be
non-toxic. Therefore, a non-adhesive liquid embolic material is
ideal. In recent years, this particular material had received
considerable attention in the field of endovascular treatment
(3).
Therefore, the present study investigated
chitosan/β-glycero-phosphate (C/GP) as the base material. The C/GP
solution is liquid at room temperature, but forms a hydrogel at
37.0°C (body temperature). In addition, the pH of the C/GP solution
is compatible with the physiological requests. These two materials
have been previously demonstrated to be safe and non-toxic
(4); however, the combination of
C/GP has not been utilized in previous studies as a non-adhesive
temperature-sensitive liquid embolic material to embolize cAVM. In
the current preliminarily study, the embolization efficacy of C/GP
towards the basicranial REM was investigated in a swine model of
cAVM, and the pathological changes of the REM tissues were assessed
6 weeks after the embolization. The aim of the present study was to
provide a novel liquid embolic material for use in cAVM
treatment.
Materials and methods
Preparations prior to the animal
experiment surgery
In total, 24 domestic swines (Duroc pigs from the
Animal Experimental Center of Jilin University, Jilin, China) were
selected as the experimental animals. All animal experimentation
protocols were conducted in accordance with the policies set by the
Chancellor of Jilin Universitys Animal Research Committee (Jilin,
China) and the National Research Council (USA) Committee for the
Update of the Guide for the Care and Use of Laboratory Animals
(5). The pigs weighed between 25 and
30 kg, were aged 3–4 months and no restriction was set on gender.
The animals were preoperatively fasted for 12 h, after which 0.5 mg
atropine hydrochloride (Shanghai Sangon Biological Engineering Co.
Ltd., Shanghai, China) was intramuscularly (IM) injected 30 min
prior to the surgery. In order to prevent infection, 320,000 IU
penicillin (Shanghai Sangon Biological Engineering Co. Ltd.) was
also IM injected 30 min prior to the surgery. For anesthesia,
ketamine hydrochloride (5 mg/kg body weight; Fujian Gutian
Pharmaceutical Industry Co. Ltd., Fuzhou, China) was IM injected,
and sumianxin II (0.3 ml/kg; Shanghai Sangon Biological Engineering
Co. Ltd.) was also IM injected. When the swine became unconscious
and the eyelash reflex disappeared, 1% ketamine hydrochloride in
glucose solution was intravenously injected in a dropwise manner,
and the injection rate was adjusted accordingly during the surgery
to maintain the animals under satisfactory anesthesia. Vital signs
of the animals, including the blood pressure, heart rate and
breathing rate, were monitored with a multipurpose polygraph
(Guangdong Biolight Meditech Co. Ltd., Zhuhai, China).
Establishment of the swine cAVM
model
Under the microscope (Leica Microsystems Co. Ltd.,
Beijing, China), the porcine REM vessels resembled human
arterioles. A cross-section image revealed that the REM vessels had
the same histological structures as human arterioles, including a
vascular intima, a smooth muscle layer, a vascular adventitia and
connective tissues among the vessels. The diameters of the vessels
varied between 70 and 275 µm, with the average diameter of an REM
vessel being 154 µm (6). Massoud
et al (7) previously reported
that the average diameter of an REM vessel, which varies due to
differences in size and weight of the swine, was 328 µm, while the
average vessel diameter in cAVM was 265 µm; these numbers are very
similar. According to the REM situation, the experiment was divided
into two groups. In the low-flow group, one side of the REM was
maintained in the natural state and microvascular anastomosis was
not performed (Fig. 1A). In the
high-flow group, the ascending pharyngeal artery (AP) of one side
of the REM was set as the blood-supplying artery, and through
microsurgical techniques, the distal end of the contralateral
common carotid artery (CCA) was connected with the proximal end of
the internal jugular vein (IJV) via microanastomosis, which
subsequently changed the direction of the blood flow. An
angiography examination of one side of the CCA revealed that the
blood flow went from the AP, through the REM, into the anastomosed
AP on the opposite side, and then through the CCA, the anastomotic
stoma and finally into the IJV (Fig.
1B).
Preparation of the C/GP solution
A 1-ml sample of concentrated hydrochloric acid
(37.5%), containing 0.012 mol hydrochloric acid, was added to
deionized water to reach a total liquid volume of 120 ml. The
formulation was prepared immediately prior to usage. With regard to
the preparation of 2% (w/v) chitosan, the chitosan used was
produced by Biosyntech, Inc. (Laval, QC, Canada), with the
following specifications: Molecular weight, 308 kDa; degree of
deacetylation, 94%; viscosity, 140 mPa.s. In total, 5 g chitosan
was dissolved in 0.1 M hydrochloric acid, and well-mixed at room
temperature to produce a final solution of 250 ml. The solution was
subsequently sterilized at 121°C with high pressure steam for 20
min, followed by storage at 4°C for future use. An 8% (w/v) β-GP
solution was produced by Sigma-Aldrich (Oakville, ON, Canada). In
total, 8 g β-GP was diluted with deionized water to form a 100-ml
solution, which was subsequently disinfected and sterilized with a
0.2-µm filter (Chengdu Ailaite Technology Co. Ltd., Chengdu,
China). The solution was finally stored at 4°C for future use. The
2% (w/v) chitosan and 8% (w/v) β-GP solutions were mixed at an
equal volume (1:1) and then tantalum powder (Sigma-Aldrich) or
Omnipaquem (General Electric Pharmaceutical (Shanghai) Ltd.,
Shanghai, China) was added. The final mixture was refrigerated at
4°C. The solution was prepared immediately prior to usage.
Procedures of the C/GP REM
embolization surgery
Under anesthesia, the animals were laid in a supine
position on a double C-arm digital subtraction angiography (DSA)
table (Siemens Co. Ltd., Beijing, China). A 6F catheter sheath
(Terumo Holding Co. Ltd., Shanghai, China) was punctured into and
kept in the femoral artery, which was beneath the concave area of
the vastus medialis and pectineus. A Y-shaped valve and a three-way
irrigation system (Terumo) was used for the bolus injection of 70
U/kg heparin. A 5F catheter (Cook Medical, Bloomington, IN, USA)
was selectively inserted into one side of the CCA (low-flow group:
direct embolism on one side of the REM) or the contralateral CCA
towards the anastomotic vessel (high-flow group; embolism on two
sides of the REM following successfully built cerebral
arteriovenous malformation models with common carotid artery and
internal jugular vein anastomosis). A high-pressure syringe (Medrad
Inc., Indianola, PA, USA) was used to inject 7 ml contrast agent
Ultravist 300 (Bayer AG, Leverkusen, Germany) for the DSA
examination, with the injection flow rate set as 4 ml/sec. The DSA
examination was performed to assess the condition of the AP, REM
and associated arteries. The 6F flat guiding catheter was then
replaced in order to enable the head of the catheter to reach the
proximal end of the REM-AP. Subsequently, a Tracker 18 (Target
Therapeutics, Inc., Fremont, CA, USA) microcatheter was guided by
the tip-modified microwire to selectively enter the REM through the
AP. After manually injecting the contrast agent for the DSA
examination, the C/GP solution was injected via the microcatheter.
The injection flow rate was 0.6–1 ml/min, and the injected dose was
1.5–2 ml; thus, the injection time varied between 1.5 and 3.0 min.
In order to observe the degree of embolization, DSA was conducted
with the injection of contrast agent following fluoroscopic
observation of the embolization of embolic agent and the flow
direction with hand push contrast agent ‘smoking’ during the
injection of embolic agent. If the REM did not exhibit complete
thrombosis, the embolic agent was continuously injected until the
REM was shown to be totally thrombosed and without any
visualization on the angiography examination. Following the
embolization therapy, the microcatheter was slowly withdrawn.
Postoperative angiography examinations were performed at 2 and 6
weeks after embolization to assess for signs of
revascularization.
Post-processing following the
embolization treatment
At 6 weeks after the embolization, the animals
underwent an angiography examination, after which a lethal dose of
sodium pentobarbital (Shanghai XiTang Biological Technology Co.
Ltd., Shanghai, China) was intravenously injected. The brain
tissues, including the REM, were completely removed. The specimens
were fixed in 10% formalin for 48 h, washed three times with 0.01 M
phosphate-buffered saline solution and placed in 37°C incubators
for preservation. The specimens were then fixed in neutral
formaldehyde solution for a week. Following conventional
dehydration, paraffin embedding, slicing (slice thickness, 5 mm)
and hematoxylin and eosin (HE) staining, the specimens were
histologically analyzed.
Results
Gelatinization time and the ratio of
material
The average gelatinization time of the 1:1 C/GP
solution at 37°C was 120 sec. As a developer, 1 g tantalum powder
or 1 ml Omnipaquem was added to each 10-ml sample of C/GP solution.
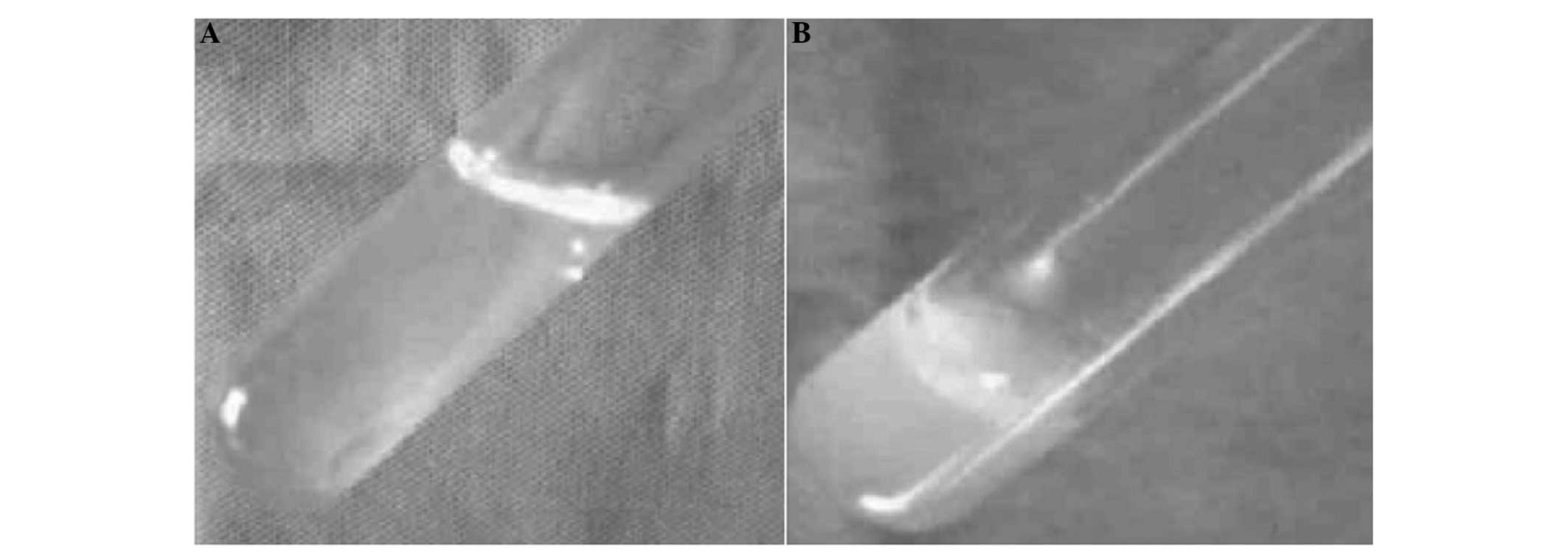
The C/GP material was liquid at temperatures of <37°C and became
a gel at 37°C (Fig. 2A and B).
Postoperative observations
In the low-flow group, all 12 swines survived the
surgery, without wound infections and hemiplegia. In the high-flow
group, 11 swines survived the surgery; however, one pig died of
convulsion and respiratory depression during the embolization
procedure. In an additional case, the pig exhibited postoperative
contralateral facial paralysis, masticatory muscle weakness and
extreme weight loss following embolization of the REM and external
carotid artery.
Histological results
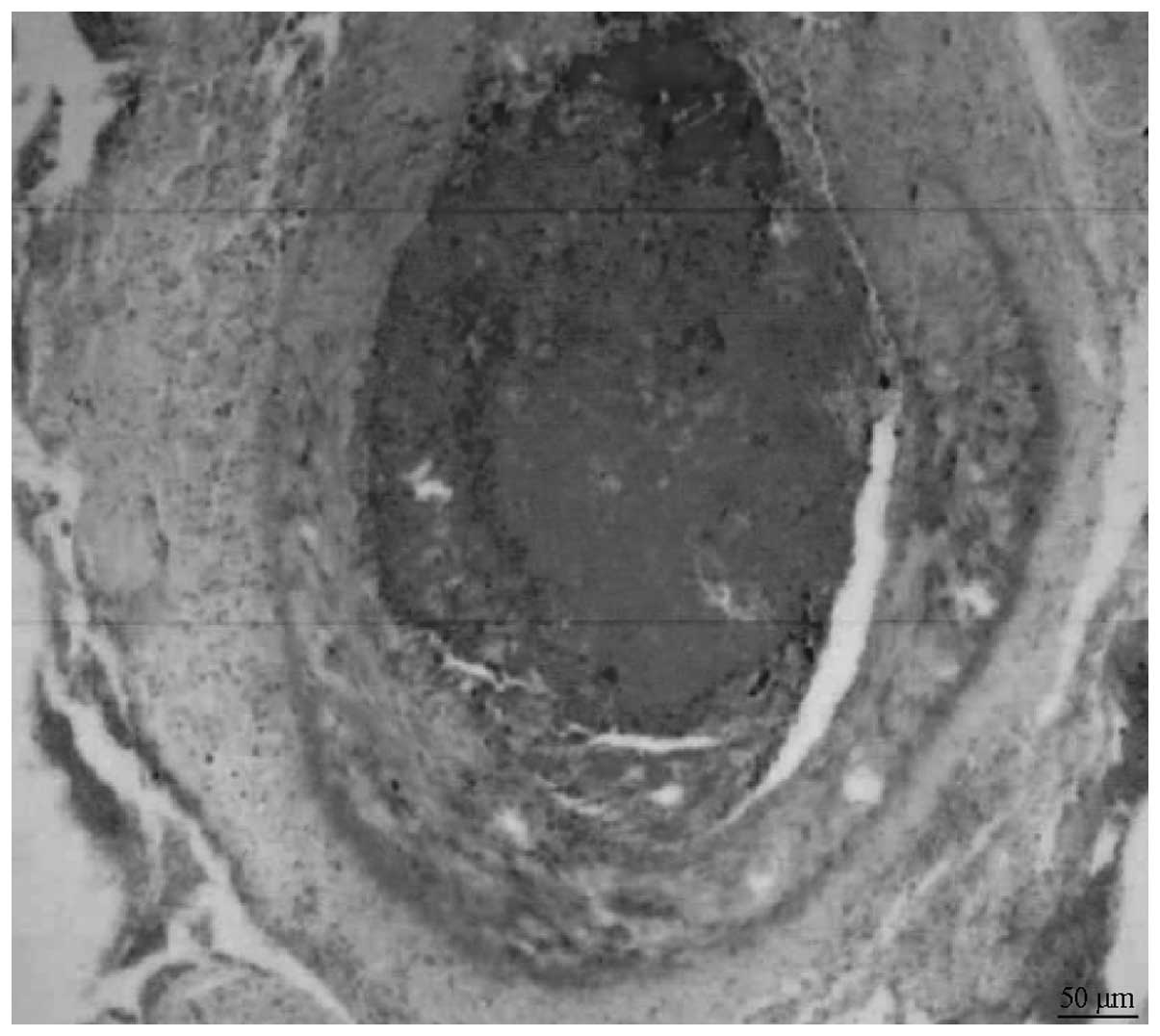
REM histological results were assessed 6 weeks after
the embolization. The results revealed that the C/GP gel filled the
REM vascular cavity and that the tunica intima was integral.
Furthermore, no significant signs of inflammation were observed
(Fig. 3). General histological
observations revealed that the bilateral AP, the structures of the
REM and the internal carotid artery were consistent with that
observed by DSA. No abnormal changes occurred in the brain tissue
of the low-flow group. The embolized side of the REM was completely
embolized, and was comparatively harder and paler compared with the
contralateral side of the REM. The emboli appeared jelly-like. The
REM was easily stripped from the sphenotic foramen at the skull
base. By contrast, the high-flow group exhibited a comparatively
harder and paler REM, with the emboli appearing jelly-like.
Under the light microscope, the REM specimens
revealed an even gel dispersion inside the vascular lumen,
indicating that the vessel lumen was thrombosed completely, and the
hydrogel was located inside the lumen. No signs of inflammation
were observed around the vessels. In addition, the smooth muscle
layer remained intact, the vascular intima was clearly visible, and
no desquamation or vessel wall necrosis was found to have
occurred.
Imaging results
DSA examination prior to the embolization revealed
that the AP of the 24 swines originated from the CCA. The terminal
branches of the AP formed an oval-shaped basicranial REM at the
location of the cavernous sinus, and the REM reconverged into the
internal carotid artery inside the cranium, which then formed the
branches to supply the brain tissues. In addition, the two
branches, ramusanastomoticus (RA) and arteria anastomotica (AA),
extended from the porcine external carotid artery and were also
involved in the blood supply of the REM.
Angiographic performance at weeks 2
and 6 after the embolization
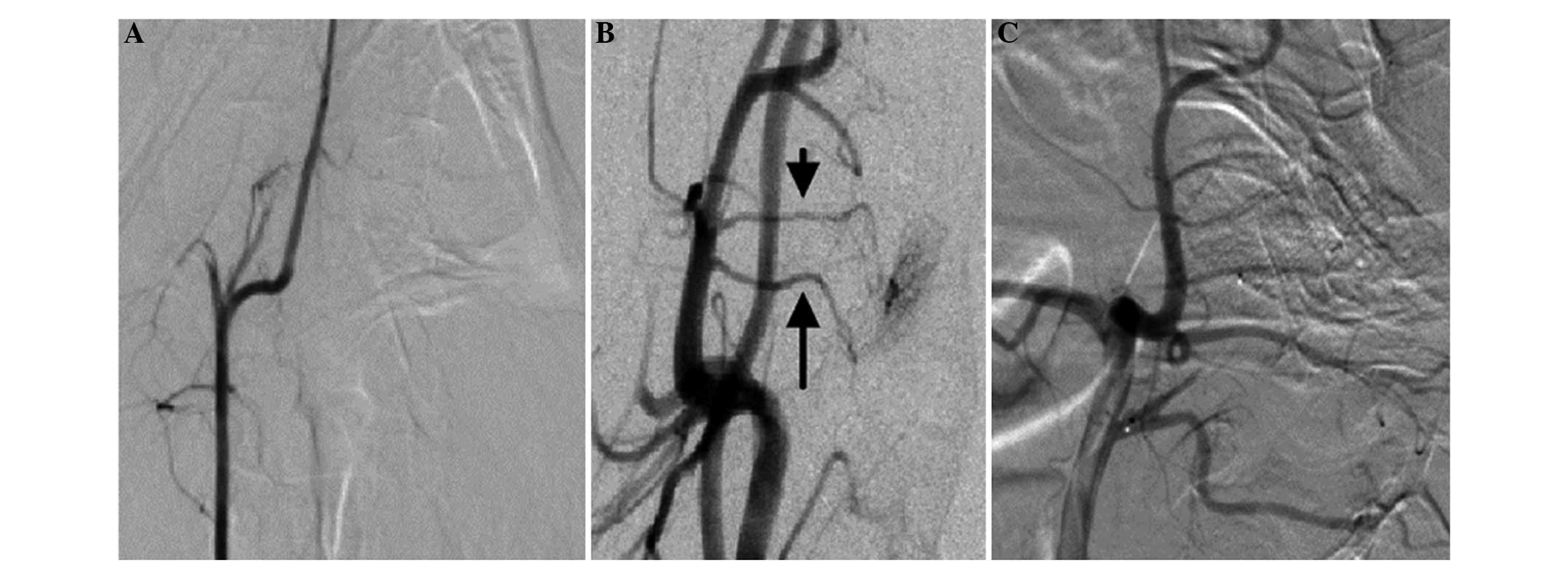
In nine cases from the low-flow group, completely no
development of the REM was observed in the angiographic examination
following the C/GP embolization (Fig.
4A). Although three cases exhibited an occluded AP, the region
of the AP near the original segment of the REM continued to display
the RA after the embolization, and the AA supplied the blood to the
REM (Fig. 4B). In addition,
following the super-selective re-embolism using the Tracker 10
microcatheter towards the RA, visualization of the REM in the
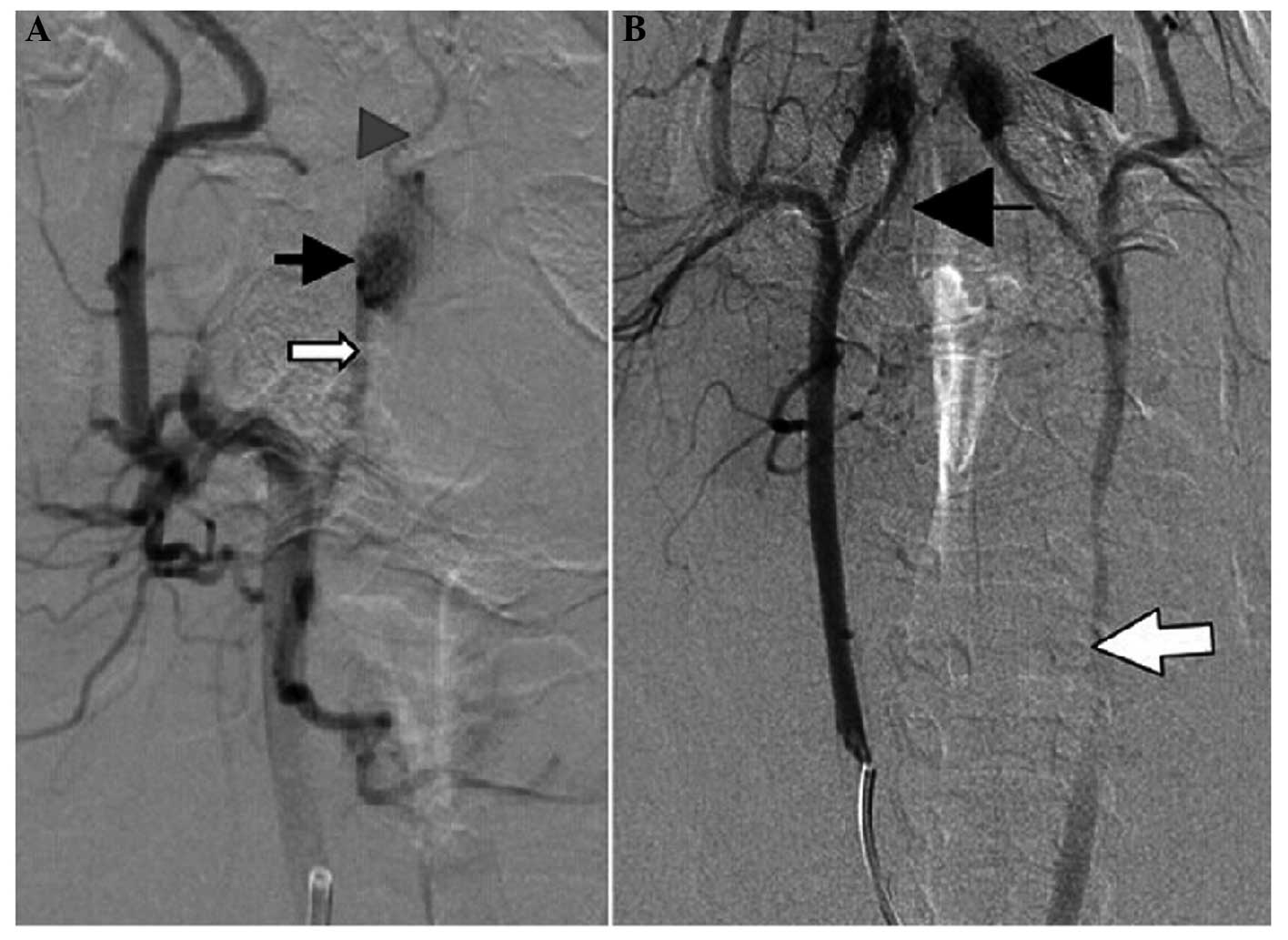
angiography examination did not develop completely (Fig. 4C). All the 12 swines in the high-flow
group exhibited images showing successful establishment of the cAVM
model prior to the embolization, while after the C/GP-dual
embolization, the REM was not visible on the images. In addition,
no revascularization was observed at weeks 2 and 6 following the
embolization.
Perioperative situations
Injection of the embolic agent through the
microcatheter exhibited no resistance, with easy bolus intravenous
push. In addition, the time was controlled between 1.5 and 3 min,
with the total dosage varying between 0.8 and 1.5 ml. Following
completion of the embolization and the withdrawal of the
microcatheter, no adhesion occurred between the tips of the
microcatheter and the embolic agent.
Discussion
cAVM is a common congenital cerebrovascular
maldevelopment, in which the arteries and veins are directly
connected without capillaries, which subsequently leads to a series
of hemodynamic disturbances. The condition is most common in young
adults aged between 20 and 40 years. The main clinical
manifestations include recurrent intracranial hemorrhage, epilepsy,
headaches and neurological deficits. The annual incidence of
cerebral hemorrhage is 2–3%, with the mortality rate between 1 and
2% (8–11).
To date, the treatment of AVM has included lesion
excision, endovascular embolization and stereotactic radiotherapy;
however, each method has limitations (12,13).
Among the various therapies, endovascular treatment exhibits the
least trauma to the patient; however, the embolic material is the
major factor that restricts the treatment efficacy. An ideal
embolic material should have a number of characteristics. Firstly,
the material should be sterile, non-toxic, non-carcinogenic,
non-deformative and non-mutative. In addition, a liquid material is
desirable, since this can be easily controlled, have good
dispersibility and can ensure a sufficient injection time for
surgical ease. Finally, the material should prevent
revascularization from occurring easily, and should exert permanent
thrombotic effects. However, the embolic materials currently used
in clinical practice do not fully meet the aforementioned
criteria.
Various embolic materials are currently used in
clinical practice. One particular embolic material is polyvinyl
alcohol polymer (PVA) (14).
However, a principal drawback of this material is the requirement
of a large diameter catheter for delivery; thus, PVA is unable to
penetrate and reach the ideal location super-selectively. In
addition, adhesive liquid embolic materials (15–18),
including cyano acid isobutyl fatty acid and its derivatives, are
most commonly used in clinical practice. However, their limitations
include adhering easily to the high-molecular microcatheter, which
subsequently blocks the catheter. In certain cases, this may result
in the breakage of the microcatheter inside the cranium.
Furthermore, non-adhesive liquid embolic materials (19,20) can
be used, which do not adhere to the catheter wall. These materials
include CAP, EVAL, Eudragit-E and HEMA-co-MMA. The most commonly
used ONYX glue is a solution of EVAL, DMSO and tantalum powder
mixed at certain percentages, which is necessary to achieve the
correct embolization technique. However, dimethyl sulfoxide is an
organic solvent that has been identified to exert potential
reproductive toxic effects towards the vascular system (21).
Liquid embolic materials that are non-adhesive with
non-organic solvents appear to have the better long-term
developmental prospects, and hydrogel has such characteristics.
Hydrogel is a class of polymer that swells due to the absorption of
water. The hydrogel is able to retain water, while not dissolving.
In addition, hydrogel has very similar properties to body tissues,
while exhibiting little irritation to the surrounding tissues, as
well as good tissue compatibility. However, typical hydrogel is in
a liquid state at high temperatures and a solid state at low
temperatures. Therefore, it may not be possible to successfully
import hydrogel into the aneurysm cavity, and importation may lead
to possible liquefaction of the emboli, resulting in associated
syndromes. Accordingly, a pioneer study in 2000 by Chenite et
al (22) was the first to
describe a C/β-GP-based hydrogel. The authors used a C/GP solution
as the gel system to develop a novel injectable neutral chitosan
solution. This solution had a physiological pH, exhibited a liquid
state at room temperature and was able to surround the live cells
and therapeutic proteins when injected into the body. Furthermore,
the solution was able to form a biodegradable gel at the injection
site under body temperature. Molecules within the hydrogel formed
chains through different intermolecular interaction forces,
including van der Waals forces, hydrophobic interactions and
hydrogen bonding. Following comparison of chitosan gels prepared
with different molecular-weight chitosans, a higher degree of
deacetylation was considered to result in a faster gel-forming
speed, while the molecular weight was shown to have no impact
(23). When the concentration of
chitosan and the degree of deacetylation remained unchanged,
increasing the GP concentration resulted in the gel temperature
decreasing and the pH increasing. In addition, a higher degree of
deacetylation in the C/GP material exhibited a better
biocompatibility (24).
Wang et al (25) were the first to apply a
temperature-sensitive C/β-GP solution for renal artery
embolization, and good results were achieved. Through the use of an
enzymatic digestion method, the authors successfully prepared an
aneurysm model in nine Chinese rabbits. Three weeks after the
modelling, the embolic material was used to embolize the aneurysm
model. With bilateral femoral artery entry, a balloon was inserted
into the right femoral artery, while a microcatheter was inserted
into the left femoral artery. After placing the microcatheter into
the aneurysm cavity, a vascular map was constructed, and the
balloon was directed across the aneurysmatic neck. The balloon was
subsequently filled to completely block the aneurysmatic neck.
Next, the embolic material was slowly injected via the
microcatheter to completely thrombose the aneurysm. At 4 weeks
after the surgery, an angiography examination was conducted and the
rabbits were sacrificed. Tissue samples were collected for HE
staining and pathological analysis was conducted. The angiography
examination revealed that all nine rabbits had undergone a
successful embolization of the in vivo aneurysm. The entire
thrombosis process was clearly visible, and after thrombosis, the
aneurysm was observed to be completely filled with the emboli
material, and the angiography image had developed well. In
addition, there was no ectopic embolization, tube gluing or
catheter obstruction during the embolization process. The rabbits
were in a good condition postoperatively, and had no special
complications. The postoperative 4-week angiography revealed no
revascularization.
In the present study, the chitosan specifications
were as follows: Viscosity, 130 mPa.s; molecular weight, 80,400 Da;
degree of deacetylation, 94%. A solution of 2% chitosan and 8% β-GP
was mixed at different proportions. The optimal ratio was
determined to be 1:1, from which the pH value was 7.28 and the
mechanical strength was up to 14 kPa; thus, the cell survival rate
was the highest at this ratio, reaching 89.0±2.7%. These
characteristics demonstrated the safety and efficacy of this
specific liquid embolic material. In addition, previous in
vitro and in vivo animal experiments have verified the
safety and efficacy of this material (26). The 1:1 C/GP solution had an average
gelatinization time of 120 sec at 37°C; thus, the solution was
determined to be suitable for the embolization therapy of cAVM.
Chitosan is known to have good biocompatibility,
antibacterial, antimicrobial, antiviral and anticancer properties,
as well as being biodegradable (27–29).
Furthermore, chitosan has been approved by the Food and Drug
Administration (FDA) for application as a wound dressing. GP is an
organic compound that is found in a natural form inside the human
body. The FDA have approved the use of GP for intravenous therapy.
In the present study, there was one case of mortality in the
high-flow group. In this case, it was considered that the embolic
agent may have been injected into the brain through the internal
carotid artery, and the postoperative anatomical histology
confirmed that the bilateral internal carotid arteries were
ectopically embolized. An additional case exhibited ipsilateral
masticatory muscle weakness, of which a possible explanation may be
that ipsilateral embolization of the external carotid artery led to
the ischemia of the ipsilateral face. The general histological
examination following the embolization revealed no abnormal changes
in the brain tissues. Furthermore, light microscopy examination
revealed that the REM lumen was filled with the gel substance, the
vascular intima was clearly visible, and the smooth muscle layers
were intact. There was no desquamation or vessel wall necrosis, or
any inflammation observed in the surrounding vessels, indicating
that C/GP was non-toxic to the blood vessels. Following the
addition of C/GP to the tantalum powder, the angiography images
developed well; however, since the tantalum power is metallic, it
may destroy the slice blade for the histological section. If the
C/GP solution was added to a liquid developer, such as Omnipaque,
there was no such risk towards the section knife.
The speed of the C/GP injection was required to be
maintained at an adequate pace, and it was essential that the
injection speed was controlled. A fast injection speed may have
resulted in the C/GP not depositing inside the cAVM prior to
forming the hydrogel, while draining into the other major blood
vessels. However, a slow injection speed may have lead to the
premature deposition of hydrogel inside the catheter, subsequently
blocking the catheter. In the present study, a flow rate of 0.6–1
ml/min was selected for the bolus injection, and each dosage was
0.8–1.5 ml, with the injection time set as 1.5–3 min. Using these
parameters, good results were achieved.
In conclusion, the temperature-sensitive liquid
embolic material, C/GP, exhibited a number of advantages, including
its non-adhesive, complete REM embolization, non-toxic and
biocompatible properties, in addition to its ability to easily pass
through different specifications of microcatheters. Furthermore,
C/GP was not shown to cause any adverse reactions and was found to
be relatively soft following solidification. Therefore, the current
preliminary study indicated that the application of C/GP was
feasible for cAVM treatment. As an ideal material for cAVM
embolization, C/GP may have broad application prospects.
References
|
1
|
Aoun SG, Bendok BR and Batjer HH: Acute
management of ruptured arteriovenous malformations and dural
arteriovenous fistulas. Neurosurg Clin N Am. 23:87–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gross BA and Du R: Natural history of
cerebral arteriovenous malformations: a meta-analysis. J Neurosurg.
118:437–443. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jabbour MN, Elder JB, Samuelson CG, et al:
Aberrant angiogenic characteristics of human brain arteriovenous
malformation endothelial cells. Neurosurgery. 64:139–148. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chenite A, Chaput C, Wang D, et al: Novel
injectable neutral solutions of chitosan form biodegradable gels in
situ. Biomaterials. 21:2155–2161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Research Council (USA) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press; Washington D.C., USA: 2011
|
|
6
|
Lee DH, Wriedt CH, Kaufmann JC, Pelz DM,
Fox AJ and Vinuela F: Evaluation of three embolic agents in pig
rete. AJNR Am J Neuroradiol. 10:773–776. 1989.PubMed/NCBI
|
|
7
|
Massoud TF, Vinters HV, Chao KH, Viñuela F
and Jahan R: Histopathologic characteristics of a chronic
arteriovenous malformation in a swine model: preliminary study.
AJNR Am J Neuroradiol. 21:1268–1276. 2000.PubMed/NCBI
|
|
8
|
da Costa L, Wallace MC, Ter Brugge KG,
OKelly C, Willinsky RA and Tymianski M: The natural history and
predictive features of hemorrhage from brain arteriovenous
malformations. Stroke. 40:100–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Choi JH, Mast H, Hartmann A, Marshall RS,
Pile-Spellman J, Mohr JP and Stapf C: Clinical and morphological
determinants of focal neurological deficits in patients with
unruptured brain arteriovenous malformation. J Neurol Sci.
287:126–130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabriel RA, Kim H, Sidney S, et al:
Ten-year detection rate of brain arteriovenous malformations in a
large, multiethnic, defined population. Stroke. 41:21–26. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim H, Pawlikowska L, Chen Y, Su H, Yang
GY and Young WL: Brain arteriovenous malformation biology relevant
to hemorrhage and implication for therapeutic development. Stroke.
40:(Suppl). S95–S97. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colombo F, Cavedon C, Casentini L,
Francescon P, Causin F and Pinna V: Early results of CyberKnife
radiosurgery for arteriovenous malformations. J Neurosurg.
111:807–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Natarajan SK, Ghodke B, Britz GW, Born DE
and Sekhar LN: Multimodality treatment of brain arteriovenous
malformations with microsurgery after embolization with onyx:
single-center experience and technical nuances. Neurosurgery.
62:1213–1226. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sorimachi T, Koike T, Takeuchi S, et al:
Embolization of cerebral arteriovenous malformations achieved with
polyvinyl alcohol particles: Angiographic reappearance and
complications. AJNR Am J Neuroradiol. 20:1323–1328. 1999.PubMed/NCBI
|
|
15
|
Lieber BB, Wakhloo AK, Siekmann R and
Gounis MJ: Acute and chronic swine rete arteriovenous malformation
models: effect of ethiodol and glacial acetic acid on penetration,
dispersion and injection force of N-butyl 2-cyanoacrylate. AJNR Am
J Neuroradiol. 26:1707–1714. 2005.PubMed/NCBI
|
|
16
|
Natarajan SK, Born D, Ghodke B, Britz GW
and Sekhar LN: Histopathological changes in brain arteriovenous
malformations after embolization using Onyx or N-butyl
cyanoacrylate. Laboratory investigation. J Neurosurg. 111:105–113.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Starke RM, Komotar RJ, Otten ML, et al:
Adjuvant embolization with N-butyl cyanoacrylate in the treatment
of cerebral arteriovenous malformations: outcomes, complications
and predictors of neurologic deficits. Stroke. 40:2783–2790. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strozyk D, Nogueira RG and Lavine SD:
Endovascular treatment of intracranial arteriovenous malformation.
Neurosurg Clin N Am. 20:399–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dudeck O, Jordan O, Hoffmann KT, et al:
Intrinsically radiopaque iodine-containing polyvinyl alcohol as a
liquid embolic agent: evaluation in experimental wide-necked
aneurysms. J Neurosurg. 104:290–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Panagiotopoulos V, Gizewski E, Asgari S,
Regel J, Forsting M and Wanke I: Embolization of intracranial
arteriovenous malformations with ethylene-vinyl alcohol copolymer
(Onyx). AJNR Am J Neuroadiol. 30:99–106. 2009. View Article : Google Scholar
|
|
21
|
Murayama Y, Viñuela F, Ulhoa A, et al:
Nonadhesive liquid embolic agent for cerebral arteriovenous
malformations: preliminary histopathological studies in swine rete
mirabile. Neurosurg. 1998.43:1164–1175. View Article : Google Scholar
|
|
22
|
Chenite A, Chaput C, Wang D, et al: Novel
injectable neutral solutions of chitosan form biodegradable gels in
situ. Biomaterials. 21:2155–2161. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Casettari L, Cespi M, Palmieri GF and
Bonacucina G: Characterization of the interaction between chitosan
and inorganic sodium phosphates by means of rheological and optical
microscopy studies. Carbohydr Polym. 91:597–602. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hsiao MH, Larsson M, Larsson A, Evenbratt
H, Chen YY, Chen YY and Liu DM: Design and characterization of a
novel amphiphilic chitosan nanocapsule-based thermo-gelling biogel
with sustained in vivo release of the hydrophilic anti-epilepsy
drug ethosuximide. J Control Release. 161:942–948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Xu N, Luo Q, et al: In vivo
assessment of chitosan/β-glycerophosphate as a new liquid embolic
agent. Interv Neuroradiol. 17:87–92. 2011.PubMed/NCBI
|
|
26
|
Lajud SA, Han Z, Chi FL, et al: A
regulated delivery system for inner ear drug application. J Control
Release. 166:268–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Khodaverdi E, Tafaghodi M, Ganji F, Abnoos
K and Naghizadeh H: In vitro insulin release from thermosensitive
chitosan hydrogel. AAPS PharmSciTech. 13:460–466. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Wang H, Wang Y, et al: The
influence of chitosan hydrogel on stem cell engraftment, survival
and homing in the ischemic myocardial microenvironment.
Biomaterials. 33:3093–3106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Alinaghi A, Rouini MR, Johari Daha F and
Moghimi HR: Hydrogel-embeded vesicles, as a novel approach for
prolonged release and delivery of liposome, in vitro and in vivo. J
Liposome Res. 23:235–243. 2013. View Article : Google Scholar : PubMed/NCBI
|