Introduction
Autoimmune pancreatitis (AIP) is a type of chronic
pancreas-specific inflammation mediated by an autoimmune
inflammatory reaction (1). Yoshida
et al (2) summarized its
clinical features as follows: i) Increased serum γ-globulin or
immunoglobulin G4 levels and presence of autoantibodies; ii)
pancreatic enlargement; iii) occasional association with stenosis
of the lower bile duct and other autoimmune diseases; iv) notable
response to steroid therapy and v) histological findings of
lymphoplasmacytic sclerosing pancreatitis (LPSP). Serum IgG4 levels
have been frequently observed to be elevated in patients with AIP
(3), indicating the presence of a
systemic disease with abnormal IgG4 expression in the pancreas
(4). The similar clinicopathological
characteristics that AIP and PaC share often result in the
misdiagnosis and unnecessary surgical treatment of patients with
AIP (1). Since AIP responds markedly
to steroid therapy, differentiating AIP from PaC is important in
order to avoid unnecessary pancreatic resection. In the present
study, the clinical data from 12 patients with AIP who were
misdiagnosed with PaC were investigated for the purpose of
improving the diagnosis and treatment of AIP.
Patients and methods
Patients and pancreatic tissue
specimens
Between January 2003 and December 2012, 271 patients
with PaC underwent surgical treatment at the Department of
Hepato-Pancreato-Biliary Surgery, Sun Yat-Sen Memorial Hospital,
Sun Yat-Sen University (Guangzhou, China). Pancreatitis was
identified histopathologically and pancreatic tissue specimens were
obtained from 16 patients. The 16 patients were male with a mean
age of 53.94±12.63 years (range, 25–75 years). None of the patients
had received chemotherapy or radiation therapy prior to the radical
tumor resection. The paraffin-embedded pancreatic tissue specimens
from each case were cut into 4-µm sections consecutively. Routine
histological examination was performed with hematoxylin and eosin
(HE) staining in the Department of Pathology. AIP was diagnosed
based on the Asian Diagnostic Criteria for AIP (5).
The present study was approved by the Ethics
Committee of the Sun Yat-Sen Memorial Hospital and is in accordance
with the Declaration of Helsinki of 1975. Written informed consent
was obtained from either the patients or their guardian.
Immunohistochemistry
The immunohistochemical staining of the tissue
sections for IgG4, using anti-IgG4 antibodies (ab109493; Abcam,
Cambridge, UK) was completed according to the instructions defined
in the Power Vision Two-Step kit (ZSG, Beijing, China) at the
Medical Research Center of Sun Yat-Sen Memorial Hospital.
The staining was independently evaluated by two
investigators who were unaware of the clinical data. The
immunostained sections were first scanned under a light microscope
at low magnification (x40), and five non-overlapping fields were
then examined at a final magnification of x400. The final results
were calculated by dividing the rate of IgG4+ plasma
cells in the 10 high-power fields (HPFs; magnification, x400) by 10.
The positive cell rate was divided into four grades: Score 0, 0%
per 1 HPF; score 1, 20% per 1 HPF; score 2, 20–50% per 1 HPF and
score 3, ≥50% per 1 HPF (6). When
the assessment of the two observers differed, agreement was reached
by using a double-headed microscope. A negative result was defined
as a score of 0 and a positive result as a score of 3.
Pancreatic imaging
A single radiologist, blinded to the clinical
diagnosis, examined computed tomography (CT) (16/16, 100%) or
magnetic resonance imaging (MRI) (6/16, 37.5%) scans of the 16
patients with pancreatitis and recorded the presence or absence of
the following pancreatic abnormalities: Presence of a low-density
mass with or without pancreatic ductal dilatation; pancreatic duct
cutoff; diffuse glandular enlargement without ductal dilatation
(>4 mm in diameter) or cutoff; focal glandular enlargement
without ductal dilatation or cutoff; and diffuse pancreatic
atrophy.
Follow-up
The 16 patients with chronic pancreatitis were
followed up for 12–138 months (mean, 45 months). At each follow-up
visit, clinical and laboratory data were collected and an abdominal
ultrasound or CT scan was performed. The deadline of follow-up was
October 2013.
Results
IgG4 expression in pancreatic
tissues
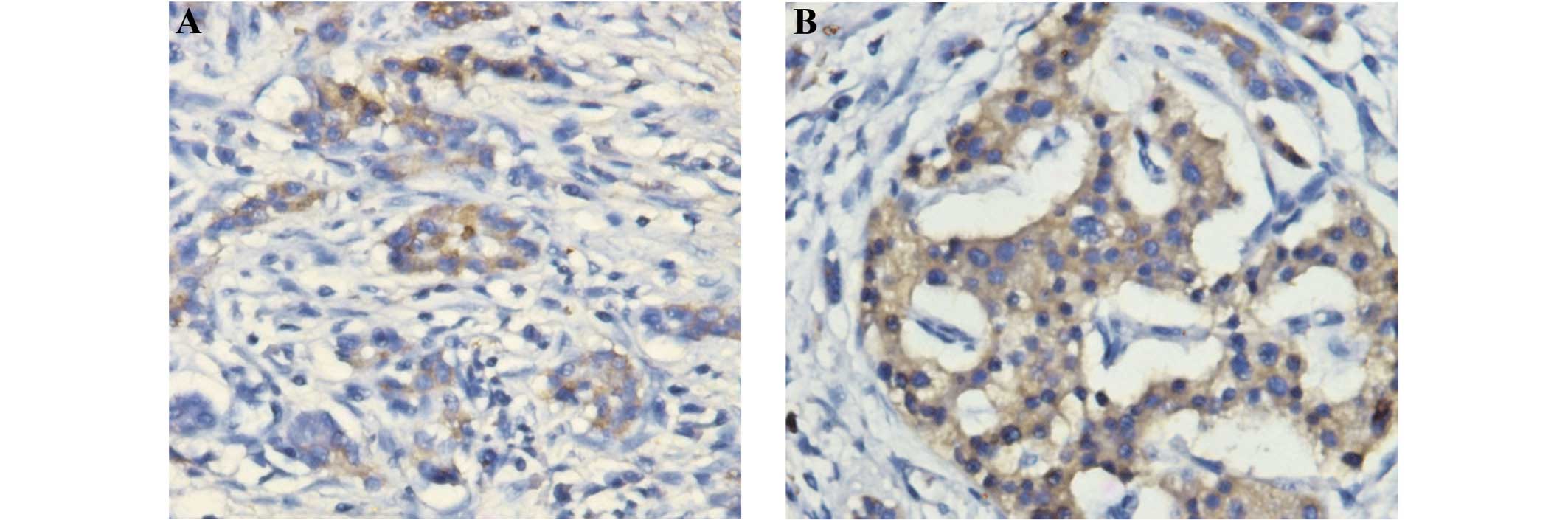
In the immunohistochemical study, positive staining
of IgG4 proteins was observed in the plasma cells (Fig. 1). The results, as evaluated by the
two investigators, were identical for all slides. High IgG4
expression (scores 2 or 3) was observed in 6 out of the 16
pancreatitis samples (37.5%), while moderate IgG4 expression (score
1) was also observed in 6 out of the 16 pancreatitis samples
(37.5%); the combination of the staining and imaging results led to
these 12 patients being diagnosed with IgG4-positive AIP. Four
cases scored 0.
Clinicopathological data
All 12 patients with AIP underwent
pancreatoduodenectomy. The incidence rate of AIP was 4.43%
(12/271). The general characteristics of these patients are
summarized in Table I. Six patients
presented with emaciation (6/12, 50.0%), 7 with jaundice (7/12,
58.3%) and 7 with abdominal pain (7/12, 58.3%). Three patients had
a history of diabetes, with 1 of them also suffering from chronic
jaw gland inflammation. Of the included patients, 12 patients had
an elevated level of γ-glutamyltransferase (>40 IU/l) and 9 had
an elevated level of carbohydrate antigen 19-9 (CA19-9) (>37 and
<200 U/ml) preoperatively; however, the blood and urine amylase
levels of the 12 patients were normal. One patient exhibited no
autoantibodies, while the other 11 patients were not examined for
the presence of autoantibodies due to discharge from the hospital.
The 12 patients with pathological confirmation of AIP all had
resected specimens. The majority of the specimens were gray-yellow
or gray-white, with either a fragile or a firm texture. HE staining
showed microscopically detectable chronic pancreatitis: 8 specimens
exhibited pancreatic fibrosis, 3 had pancreatic duct expansion, 11
exhibited plasma cell infiltration into the pancreatic tissues and
1 specimen did not show the inflammatory cell infiltration. The
histological findings of the 12 cases of AIP indicated LPSP.
 | Table I.Demographic characteristics and
clinical features of the 12 patients with autoimmune
pancreatitis. |
Table I.
Demographic characteristics and
clinical features of the 12 patients with autoimmune
pancreatitis.
|
|
|
| Clinical
features | Laboratory
examinationd |
|---|
|
|
|
|
|
|
|---|
| Case no. | Age (years) | IgG4 expression | Duration
(days)a | A/J/Eb | Other
symptoms/diabetesc | TBIL (µmol/l) | DBIL (µmol/l) | CA724 r-GGT
(U/l) | CA199 (U/ml) | CA125 (U/l) | (U/l) | CEA (µg/ml) | AFP (µg/l) |
|---|
| 1 | 53 | Strong | 15 | +/+/– | –/– | 115.9 | 115.8 | 395 | 6.8 | 196.9 | 10.7 | 4.1 | 4.35 |
| 2 | 67 | Strong | 75 | +/–/– | – | 11.3 | 7.3 | 309 | 1.5 | 11.4 | 5.4 | 3.2 | 3.63 |
| 3 | 70 | Strong | 75 | +/+/+ | Anorexia/– | 91.1 | 67.4 | 1,392 | 1.42 | 60.1 | 7.9 | 1.7 | 8.00 |
| 4 | 57 | Strong | 8 | –/–/+ | –/– | 7.4 | 2.6 | 42 | 0.7 | 10.8 | 14.6 | 2.2 | 4.80 |
| 5 | 58 | Strong | 9 | +/+/– | –/– | 204.9 | 196.7 | 319 | 7.0 | 197.8 | 11.1 | 3.5 | 1.30 |
| 6 | 59 | Strong | 15 | +/+/– | –/– | 40.0 | 34.9 | 278 | 0.8 | 59.1 | 37.8 | 1.9 | 3.56 |
| 7 | 25 | Moderate | 69 | +/–/+ | Anorexia/– | 16.1 | 8.5 | 279 | – | 17.5 | 16.2 | 3.7 | 2.80 |
| 8 | 45 | Moderate | 8 | –/–/– | –/– | 20.4 | 11.6 | 606 | 1.5 | 42.9 | 11.5 | 2.7 | 2.00 |
| 9 | 44 | Moderate | 9 | –/–/– | –/Diabetes | 22.1 | 12.8 | 329 | 2.9 | 50.1 | 30.0 | 0.1 | 1.50 |
| 10 | 44 | Moderate | 15 | –/+/+ | –/Diabetes | 109.4 | 83.8 | 2,251 | 2.3 | 45.9 | 24.8 | 2.7 | 3.32 |
| 11 | 64 | Moderate | 9 | +/+/+ | Low fever/– | 239.2 | 160.8 | 48 | – | 37.4 | 58.2 | 0.8 | 2.80 |
| 12 | 52 | Moderate | 30 | –/+/+ | –/Diabetes | 82.2 | 66.3 | 600 | – | 59.9 | 9.3 | 3.4 | 1.77 |
Imaging characteristics
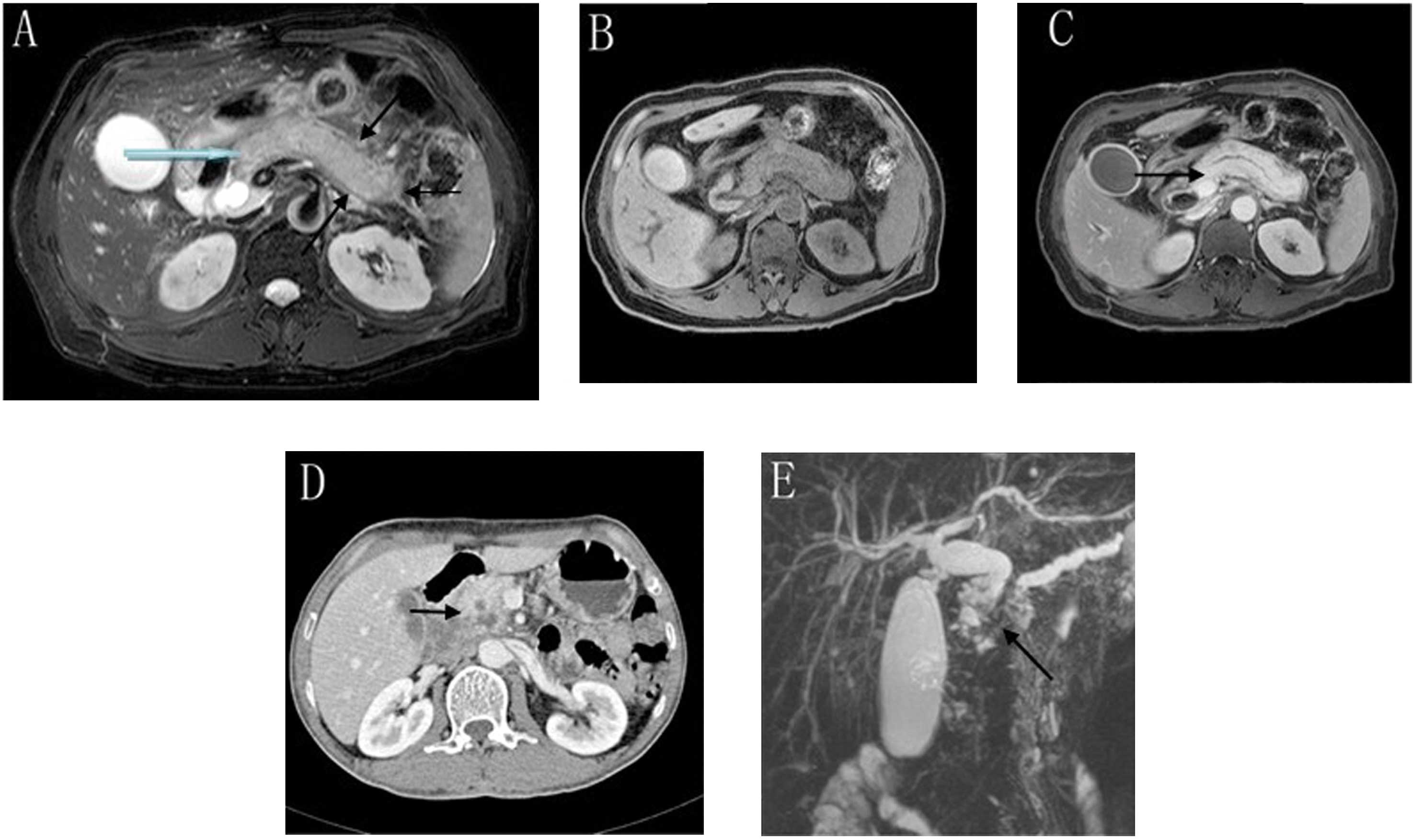
A number of the CT and MRI findings of pancreatitis
are presented in Table II and
Fig. 2. Out of the 12 patients with
AIP, 11 exhibited diffuse pancreatic enlargement and focal
pancreatic enlargement in the head of the pancreas (11/12, 91.7%);
1, pancreatic diminution (1/12, 8.3%); 3, pancreatic duct expansion
(3/12, 25.0%); 4, ‘sausage-like’ pancreatic changes (4/12, 33.3%);
and 5, ‘halo’ sign pancreatic changes (5/12, 41.7%). During the
scanning period of the CT imaging it was observed that the edges of
the 12 pancreatic tissue specimens were flat and that the density
of the specimens was uniform, without liquefaction necrosis or
calcification. Through the enhanced scanning it was observed that
the 12 pancreatic lesion areas were unequally intensified in a
‘snowflake’ pattern, and from the arterial- to the portal- and
delayed-phase imaging, the pancreatic tissues were observed to
exhibit progressively increased pancreatic lesion areas. Magnetic
resonance cholangiopancreatography (MRCP) demonstrated that the
major clinical manifestations of the patients were biliary
obstruction, common bile duct, bile duct and gallbladder wall
thickening. Four of the patients exhibited distal pancreatic duct
expansion. No metastasis or infiltration of adjacent organs was
identified in any of the 12 patients.
 | Table II.Imaging characteristics of the 16
patients. |
Table II.
Imaging characteristics of the 16
patients.
|
|
| Immunoglobulin
G4 |
|---|
|
|
|
|
|---|
| Computed tomography
findings | N | Positive (n) | Negative (n) |
|---|
| Diffuse enlargement
of the pancreas |
|
|
|
|
Yes | 11 | 11 | 0 |
| No | 5 | 1 | 4 |
| Focal enlargement
in head of pancreas |
|
|
|
|
Yes | 11 | 11 | 0 |
| No | 5 | 1 | 4 |
| Low-density
mass |
|
|
|
|
Yes | 7 | 7 | 0 |
| No | 9 | 5 | 4 |
| Diffuse pancreatic
atrophy |
|
|
|
|
Yes | 5 | 1 | 4 |
| No | 11 | 11 | 0 |
| Diffuse enlargement
of the pancreas with ductal dilatation |
|
|
|
|
Yes | 3 | 3 | 0 |
| No | 9 | 9 | 0 |
| Diffuse pancreatic
atrophy with ductal dilatation |
|
|
|
|
Yes | 0 | 0 | 0 |
| No | 4 | 0 | 4 |
| Capsule-like rim
around pancreas |
|
|
|
|
Yes | 0 | 0 | 0 |
| No | 0 | 0 | 0 |
Prognosis and response to
steroids
During the follow-up, 12 patients suffered from
postoperative intermittent abdominal pain; 7 of these patients
required treatment with painkillers. Following the surgery, 7
patients had an elevated level of total bilirubin. In 1 patient the
level of serum IgG4 continuously declined for 11 months after he
had received the metacortandracin hormone treatment (0.6 mg/kg),
and the abnormal enlargement of the salivary gland was
reversed.
Discussion
AIP is a type of chronic pancreas-specific
inflammation caused by an autoimmune inflammatory reaction.
Kasashima et al (7) proposed
that AIP, apart from being a type of pancreatitis, was also a type
of pancreatic injury occurring as a consequence of systemic
disease. The manifestation of this injury as AIP results in it
being easily misdiagnosed as PaC. Thus, numerous patients have
undergone unnecessary surgical treatment. AIP and PaC have similar
clinicopathological characteristics, such as abdominal pain,
jaundice and weight loss (8). AIP
typically occurs in patients >55 years old (9). The term ‘AIP’ is considered to
encompass two different subtypes of the disease. The histological
pattern of type 1 is known as LPSP, and is characterized by
periductal lymphoplasmacytic infiltration, storiform fibrosis and
obliterative venulitis (10,11), while that of type 2 is known as
idiopathic duct-centric pancreatitis, which can be recognized by
neutrophil infiltration and granulocytic epithelial lesions
(12). All 12 cases in the present
study were misdiagnosed preoperatively as PaC, revealing the
frequency of AIP misdiagnosis. The aim of the reflective analysis
performed in this study was to facilitate the identification of and
provide diagnostic strategies for AIP.
IgG4-positive plasma cell infiltration is widely
considered to be the gold standard for AIP diagnosis (13), as was observed in the majority of the
specimens collected in this study. It was observed that the
combination of moderate and higher IgG4 labeling of the plasma
cells reached 100% (12/12) in the patients with AIP. In general,
high IgG4 expression (scores 2 or 3) was observed in 6 out of the
16 pancreatitis samples, while moderate IgG4 expression was also
found in 6 samples. Takahashi et al (13) reported that the accuracy of the
spiral CT in the diagnosis of AIP is 68–76%. The typical imaging
finding in AIP is diffuse enlargement of the pancreas, i.e., the
‘sausage-like’ change. A well-defined capsule-like rim (‘halo’
sign), which is caused by fibrosis surrounding the lesions and can
be observed in 12–40% of patients with AIP (14), is an important imaging characteristic
of the disease and is rarely observed in malignant pancreatic
tumors (15). In certain cases, a
dilated main pancreatic duct can be noted through CT (16). Endoscopic retrograde
cholangiopancreatography is widely used in Japan for the purpose of
investigating obstructive jaundice and is mandatory in the Japanese
criteria (17). MRCP has gained
popularity for being a non-invasive method of obtaining
high-quality images of the pancreaticobiliary tree (18). In the present study, 11 patients
exhibited diffuse pancreatic enlargement and focal pancreatic
enlargement in the head of pancreas (11/12, 91.7%), 1 showed
pancreatic diminution (1/12, 8.3%) and 3 had pancreatic duct
expansion (3/12, 25.0%). During the scanning period, 12 pancreatic
tissue specimens exhibited uniform density, without liquefaction
necrosis and calcification. Enhanced scanning showed that the 12
pancreatic lesion areas were unequally intensified in a
‘snowflake-like’ shape. From the arterial phase to the portal
phase, the pancreatic lesion area progressively increased. Four
patients (33.3%) exhibited pancreatic ‘sausage-like’ changes and 5
patients (41.7%) experienced ‘halo’ sign changes. No metastasis or
adjacent organ invasion, which can be used to differentiate AIP
from PaC and facilitate its identification, was found in any of the
12 cases.
In the current study, 12 preoperative patients with
AIP were misdiagnosed with PaC and underwent pancreatoduodenectomy,
demonstrating that it was difficult to distinguish pancreatic
cancer from AIP in the clinical setting; however, the serology,
imaging and pathological features of AIP are unique. In terms of
serology, the serum CA199 level of the 12 patients with AIP was
<200 U/ml, while its level in patients with PaC is often >200
U/ml (8). On the imaging level, 4
patients (33.3%) exhibited pancreatic ‘sausage-like’ changes and 5
patients (41.7%) showed ‘halo’ sign changes, while there was no
evidence of metastasis or adjacent organ invasion. With regard to
histology, 12 patients had a local stiffness of the pancreas, 11
(91.7%) showed pancreatic plasma cell infiltration and 8 (66.7%)
showed pancreatic fibrosis. In terms of immunology, IgG4 expression
was elevated in 12 out of 12 (100%) patients with AIP; therefore,
from a clinical point of view, patients with a preliminary
diagnosis of PaC, whose serum tumor markers are not high or who
exhibit ‘sausage-like’ or ‘halo’ sign changes on imaging, without
tumor invasion of adjacent organs, should be considered for a
diagnosis of AIP. In such cases, the blood IgG4 levels of the
patients should be measured. A serum IgG4 level of 1,350 mg/l has
high sensitivity and specificity in the diagnosis of AIP (3). At present, glucocorticoids are
considered to be highly efficacious in the long-term treatment of
AIP (16). Prior to glucocorticoid
treatment, it is necessary to completely rule out other types of
pancreatic diseases. Clinical symptoms would improve significantly
after 2–4 weeks through hormone therapy (19). In the present study, hormone therapy
was recommended once patients were diagnosed with AIP. If the
patients have only had a single surgery, symptoms such as abdominal
pain and jaundice are unlikely to be relieved. Following the
metacortandracin treatment, the level of IgG4 in 1 patient with AIP
continuously declined and the abnormal enlargement of the salivary
gland was reversed, thus proving that early glucocorticoid
treatment can alleviate the clinical symptoms of the patient and
improve the prognosis of AIP.
In conclusion, AIP is a rare type of pancreatitis.
As the clinicopathological features of AIP are similar to those of
PaC, misdiagnoses of AIP as PaC are quite common, rendering
unnecessary surgical resection. This study analyzed 12 patients
with AIP in the Sun Yat-Sen Memorial Hospital. It was found that
the serum CA199 levels in those patients were <200 U/ml. In
imaging studies, pancreatic ‘sausage-like’ and ‘halo’ sign changes
were observed, and the mass did not invade the adjacent organs and
blood. From a histological perspective, plasma cells were found to
have infiltrated widely. Immunology showed that cases with high
levels of IgG4 were positive for IgG4 in biopsy. Combining medical
imaging with IgG4 expression to obtain a diagnosis has proven to
have significant practical value, which could reduce the rate of
AIP misdiagnosis and the risk of unnecessary surgery.
Acknowledgements
This study was supported by The Special Research
Foundation of the National Natural Science Foundation of China (no.
81301865).
References
|
1
|
Kamisawa T, Egawa N, Nakajima H, Tsuruta
K, Okamoto A and Kamata N: Clinical difficulties in the
differentiation of autoimmune pancreatitis and pancreatic
carcinoma. Am J Gastroenterol. 98:2694–2699. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yoshida K, Toki F, Takeuchi T, Watanabe S,
Shiratori K and Hayashi N: Chronic pancreatitis caused by an
autoimmune abnormality. Proposal of the concept of autoimmune
pancreatitis. Dig Dis Sci. 40:1561–1568. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hamano H, Kawa S, Horiuchi A, et al: High
serum IgG4 concentrations in patients with sclerosing pancreatitis.
N Engl J Med. 344:732–738. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kamisawa T, Takuma K, Egawa N, Tsuruta K
and Sasaki T: Autoimmune pancreatitis and IgG4-related sclerosing
disease. Nat Rev Gastroenterol Hepatol. 7:401–409. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Otsuki M, Chung JB, Okazaki K, et al:
Research Committee of Intractable Pancreatic Diseases provided by
the Ministry of Health, Labour and Welfare of Japan and the Korean
Society of Pancreatobiliary Diseases: Asian diagnostic criteria for
autoimmune pancreatitis: Consensus of the Japan-Korea Symposium on
Autoimmune Pancreatitis. J Gastroenterol. 43:403–408. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aoki S, Nakazawa T, Ohara H, et al:
Immunohistochemical study of autoimmune pancreatitis using
anti-IgG4 antibody and patients' sera. Histopathology. 47:147–158.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kasashima S, Zen Y, Kawashima A, Endo M,
Matsumoto Y and Kasashima F: A new clinicopathologic entity of
IgG4-related inflammatory abdominal aortic aneurysm. J Vasc Surg.
49:1264–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghazale A, Chari ST, Smyrk TC, et al:
Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and
in distinguishing it from pancreatic cancer. Am J Gastroenterol.
102:1646–1653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zamboni G, Lüttges J, Capelli P, et al:
Histopathological features of diagnostic and clinical relevance in
autoimmune pancreatitis: a study on 53 resection specimens and 9
biopsy specimens. Virchows Arch. 445:552–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Park DH, Kim MH and Chari ST: Recent
advances in autoimmune pancreatitis. Gut. 58:1680–1689. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Notohara K, Levy MJ, Chari ST and
Smyrk TC: IgG4-positive plasma cell infiltration in the diagnosis
of autoimmune pancreatitis. Mod Pathol. 20:23–28. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Chari S, Smyrk TC, et al:
Autoimmune pancreatitis (AIP) type 1 and type 2: an international
consensus study on histopathologic diagnostic criteria. Pancreas.
40:1172–1179. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takahashi N, Fletcher JG, Fidler JL, Hough
DM, Kawashima A and Chari ST: Dual-phase CT of autoimmune
pancreatitis: A multireader study. AJR Am J Roentgenol.
190:280–286. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kubota K, Iida H, Fujisawa T, et al:
Clinical significance of swollen duodenal papilla in autoimmune
pancreatitis. Pancreas. 35:e51–e60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Finkelberg DL, Sahani D, Deshpande V and
Brugge WR: Autoimmune pancreatitis. N Engl J Med. 355:2670–2676.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamisawa T, Wakabayashi T and Sawabu N:
Autoimmune pancreatitis in young patients. J Clin Gastroenterol.
40:847–850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okazaki K, Kawa S, Kamisawa T, et al:
Research Committee of Intractable Diseases of the Pancreas:
Clinical diagnostic criteria of autoimmune pancreatitis: Revised
proposal. J Gastroenterol. 41:626–631. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vaishali MD, Agarwal AK, Upadhyaya DN,
Chauhan VS, Sharma OP and Shukla VK: Magnetic resonance
cholangiopancreatography in obstructive jaundice. J Clin
Gastroenterol. 38:887–890. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chari ST, Takahashi N, Levy MJ, et al: A
diagnostic strategy to distinguish autoimmune pancreatitis from
pancreatic cancer. Clin Gastroenterol Hepatol. 7:1097–1103. 2009.
View Article : Google Scholar : PubMed/NCBI
|