Introduction
Premature ovarian failure (POF) is a typical
pathological disease of the reproductive system in aging females
(1–5). Patients with POF are <40 years old
and commonly have the features of amenorrhea, hypoestrogenism and
high levels of gonadotrophin (1–5). These
patients also exhibit symptoms of the menopause, such as hot
flushes, night sweats and vaginal dryness (1–5).
Furthermore, patients with POF are usually infertile due to a lack
of mature, healthy follicles or as a result of the remaining
follicles not responding to stimulation (1–5);
therefore, POF is also known as primary ovarian insufficiency
(1–5). At present, the number of patients with
POF is increasing; however, the factors contributing to POF are
largely unknown.
Tripterygium glycosides (TGs) are components
of the Chinese herb Tripterygium wilfordii Hook. f., also
known as Huangteng (6–8). TGs possess various biological
activities and can be extracted from T. wilfordii for use in
the treatment of various diseases, such as lupus, cancer,
rheumatoid arthritis and nephritic syndrome (6–8).
Previous studies have demonstrated that drug resistance and
toxicity of TGs can coexist (6–8). Other
reports have indicated that TGs induce liver injury and
dysfunction, glutathione depletion and reduce antioxidant enzymes
in BALB/C mice (6–8). It is therefore necessary to identify
methods of reducing the toxicity of TGs during the development of
novel pharmaceuticals and clinical treatments utilizing the effects
of TGs.
Serine/threonine kinase 11 (Stk11), also known as
LKB1, is a serine/threonine kinase of 433 amino acids that can
function as a tumor suppressor (9–11). Stk11
maintains tissue homeostasis in vivo, but Stk11 somatic
mutations can be found in lung and cervical cancer (9–11). In
addition, heterozygous germline mutations in Stk11 induce
Peutz-Jeghers syndrome (9–11). Stk11 functions in an active complex
with a pseudokinase STE20-related kinase adapter protein (STRADa or
STRADb) and the scaffold protein MO25 (MO25a or MO25b) (9–11).
Several substrates of Stk11 have been identified, including p53,
phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and
dual-specificity protein phosphatase PTEN and
serine/threonine-protein kinase 11-interacting protein.
Furthermore, Stk11 activates the catalytic subunit of the adenosine
monophosphate-activated protein kinase (AMPK) as well as several
other kinases with T-loop activation domains similar to AMPK
(9–11). Studies have additionally shown that
heterozygous Stk11-knockout mice (Stk11+/–) exhibit
earlier tumor formation, increased tumor incidence and a reduced
lifespan (9–11).
The aim of the present study was to investigate the
molecular mechanism underlying the nuclear function of Stk11 in
association with p53 following TG treatment. Furthermore, the study
aimed to determine whether Stk11 has a direct role in the
activation of p21/WAF1 transcription to induce POF following TG
treatment in rats.
Materials and methods
Animals and TG treatment
Hebetic female Sprague Dawley (SD) rats (n=40)
between four and five weeks of age were obtained from the Animal
Research Center, Shanghai University of Traditional Chinese
Medicine (Shanghai, China). The study was approved by the Animal
Ethics Committee of Shanghai University of Traditional Chinese
Medicine in compliance with the Experimental Animal Regulations of
the National Science and Technology Commission of China (permit no.
TCMUAE2013003). All rats were kept for 14 days, three per cage, in
a temperature-controlled colony room with a standard light-dark
cycle and provided food and water ad libitum. All treatment
steps were performed as previously described (3–5). In
brief, the animals were divided into five groups: A blank control
group [healthy, wild-type (WT) SD rats; n=8] that was not treated
with TGs; a negative control group (healthy, WT SD rats; n=8)
treated with saline; and an experimental group (healthy, WT SD
healthy rats; n=24) that was subdivided into three groups for
treatment with different doses (low, medium and high; n=8/dose) of
TGs (Huangshi Feiyun Pharmaceutical Co., Ltd., Huangshi, China)
dissolved in solution. The low-, medium- and high-dose TGs were
administered every day by intraperitoneal injection (25, 50 or 75
mg/kg body weight in 100 µml saline solution, respectively).
Following treatment, the experiments using the animal model were
conducted within 14 days. All of the rats were sacrificed by carbon
dioxide inhalation on day 15.
RNA extraction and analysis by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
All steps were performed as previously described
(5). In brief, total RNA from each
cell was isolated using TRIzol™ reagent (Invitrogen Life
Technologies, Carlsbad, CA, USA) according to the manufacturer's
instructions. Reverse transcription of the RNA samples into cDNA
was conducted using the ReverTra Ace-α First Strand cDNA Synthesis
kit (Toyobo, Osaka, Japan). RT-qPCR was performed using a realplex
4 real-time PCR detection system from Eppendorf Co. Ltd. (Hamburg,
Germany) with SYBR® Green Realtime PCR Master Mix (Toyobo)
detection dye. RT-qPCR amplification was carried out over 40 cycles
comprising denaturation at 95°C for 15 sec and annealing at 58°C
for 45 sec. Quantification of the target cDNA was performed using
the relative quantification method. Gene expression relative to a
control (calibrator) was calculated using a comparative threshold
cycle (Ct), and steady-state mRNA levels are expressed as an n-fold
difference compared with the calibrator. For each sample, the
target gene Ct values were normalized using the following formula:
ΔCt = Ctgenes - Ct18S rRNA. To calculate the
relative expression levels, the following formula was used: ΔΔCt =
ΔCttreated group - ΔCtcontrol group. The
values used to plot the relative marker expression were determined
using the expression 2−ΔΔCt, and the mRNA levels were
calibrated based on the levels of 18S rRNA. Gene-specific primers,
as shown in Table I, were used to
amplify each cDNA.
 | Table I.Sequences of the reverse
transcription-quantitative polymerase chain reaction primers. |
Table I.
Sequences of the reverse
transcription-quantitative polymerase chain reaction primers.
| Gene product | Primer sequences
(5′→3′) |
|---|
| Stk11 | F:
CGGGCAACCTGCTCCTCACCACCAA |
|
| R:
CGGCAGGTGTCATCCACAGCGAAAGGG |
| p53 | F:
ATGACTGAGGTCGTGAGACGCTGCCC |
|
| R:
GGAGCCAGGCCGTCACCATCAGAGC |
| p21 | F:
AGCAGTTGAGCCGCGATTGCGATGCG |
|
| R:
GAGCGCATCGCAATCGCGGCTCAAC |
| 18S rRNA | F:
TGCGGAAGGATCATTAACGGA |
|
| R:
AGTAGGAGAGGAGCGAGCGACC |
Histopathological analysis
Coronary artery tissues were stained with
hematoxylin-eosin (HE) for analysis as previously described
(5,12). Briefly, all fresh tissues were washed
three times with phosphate-buffered saline (PBS), fixed with 4%
paraformaldehyde (Sigma-Aldrich, St. Louis, MO, USA) for 30 min,
dehydrated through a graded series of ethanol, cleared in xylene
and embedded in paraffin. Serial 6-µm sections were then cut and
stained with HE.
Immunohistochemistry
Immunohistochemical analysis was performed as
previously described (5,12). Briefly, all fresh tissues were washed
three times with PBS, fixed with 4% paraformaldehyde
(Sigma-Aldrich) for 30 min, dehydrated through a graded series of
ethanol, cleared in xylene and embedded in paraffin. Serial 6-µm
sections were then cut and rinsed with 3% phosphate buffer, prior
to microwave antigen retrieval. The primary polyclonal antibodies
(Table II) were added and incubated
with the sample for 45 min at 37°C. Following incubation, the
secondary antibody conjugated to horseradish peroxidase (Santa Cruz
Biotechnology, Inc.) was added and incubated with each sample for
45 min at 37°C. The avidin-biotin complex chromogenic reagent
(Santa Cruz Biotechnology, Inc) was used for the secondary antibody
reaction and color detection. PBS (pH 7.4) was used as a negative
control in the place of primary antibody. Five randomly selected
fields of view (magnification, x200) from each tissue section were
observed and analyzed using Intel® Integrated Performance
Primitives software (Intel Corp., Santa Clara, CA, USA).
 | Table II.Primary polyclonal antibodies, their
source and dilutions. |
Table II.
Primary polyclonal antibodies, their
source and dilutions.
| Antibodies | Source | Application
(dilution) |
|---|
| Goat anti-rat
Stk11 | Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA | ICH (1:200) |
| Rabbit anti-rat p53
Ser15-pho | Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA | ICH (1:200) |
| Rabbit anti-rat
p21 | Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA | ICH (1:200) |
| Rabbit anti-rat
activated caspase-3 | Cell Signaling
Technology, Inc., Danvers, MA, USA | ICH (1:200) |
| Rabbit anti-rat p53
Ser15-pho | Cell Signaling
Technology, Inc., Danvers, MA, USA | ChIP (1:1000) |
Chromatin immunoprecipitation (ChIP)
assays
Primary antibodies (Table II) and non-specific rabbit
immunoglobulin G (Upstate Biotechnology, Inc., Lake Placid, NY,
USA) negative control antibodies were used for the ChIP experiments
as described previously (9). In
brief, the cells were scraped from the bottom of the culture dish,
and were fixed with 1% formaldehyde for 30 min at 37°C and then
quenched with 125 mM glycine for 10 min at room temperature to
establish DNA-protein cross-links. The samples were sonicated on
ice to create 200–1,000-bp chromatin fragments and incubated with
antibodies at 4°C overnight. PCR amplification was performed under
the following conditions: 33 cycles with denaturation at 95°C for
30 sec, annealing at 55°C for 30 sec and extension at 72°C for 30
sec.
ELISA assay
The rat estradiol (E2) and follicle stimulating
hormone (FSH) ELISA kit (Westang Bio, Shanghai, China) was used to
determine the levels of E2 or FSH in rat plasma, according to the
manufacturer's instructions. Briefly, 100 µl rat E2 or FSH
standardarded to 0, 50, 300, 1,000, and 5,000 pg/ml, or 0, 1.56,
3.12, 6.25, 12.5, 25, 50, and 100 ng/ml, were added to anti-E2 FSH
antibody precoated microtest wells and incubated for 60 min.
Following three washes, with ELISA washing solution, the
horseradish peroxidase-conjugated detection antibodies were added,
followed by the substrate solution. The absorbance was determined
at a wavelength of 450 nm using a microplate reader (BioTek Synergy
Mx; Biotek Instruments, Inc., Winooski, VT, USA).
Statistical analysis
Each experiment was performed at least three times.
Data are presented as the mean ± standard error and were analyzed
using the Student's t-test when appropriate. Differences were
considered significant at P<0.05.
Results
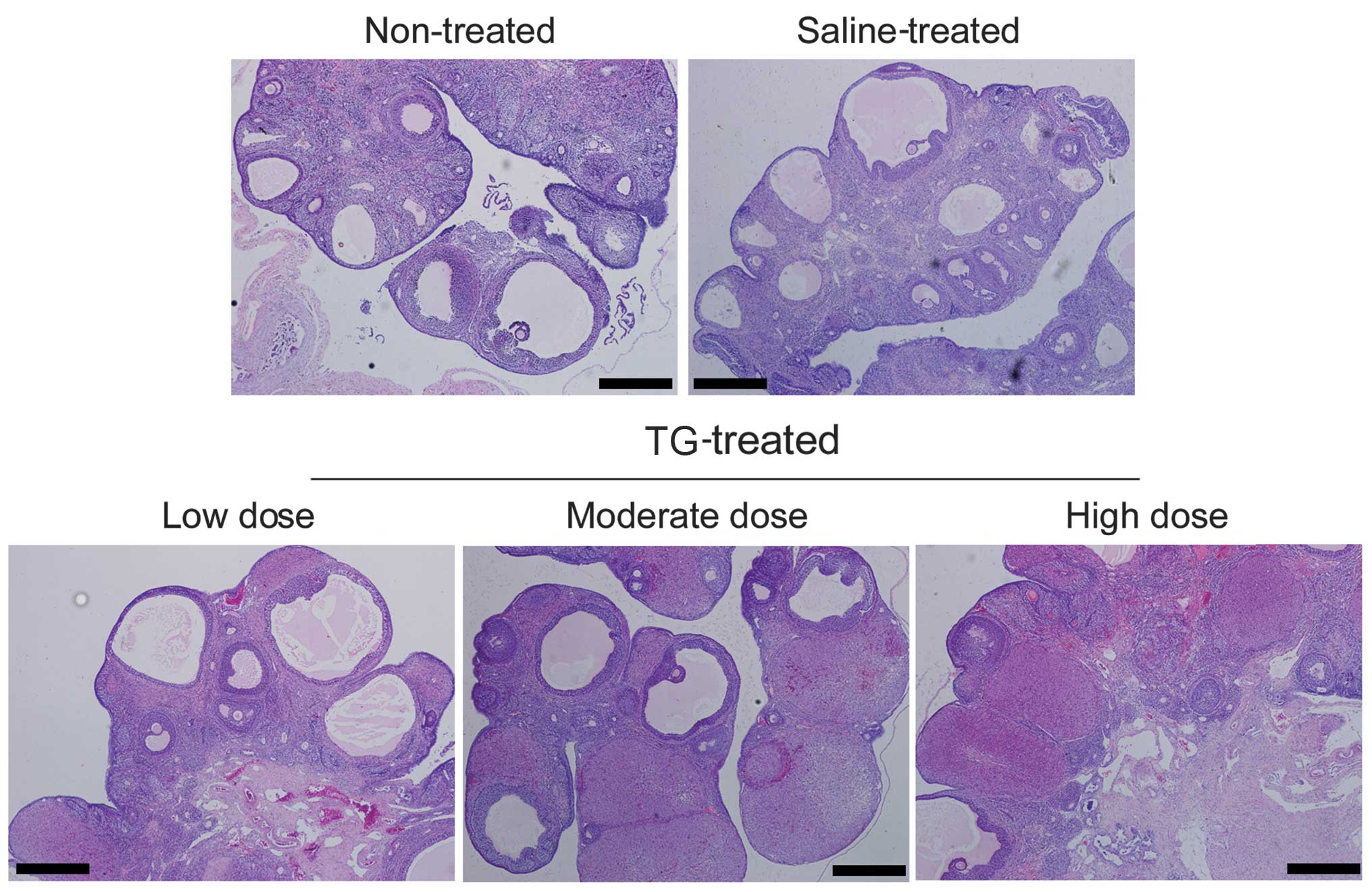
TGs induce POF in rats
Pathology results from the treated rats indicated
that TGs affect ovary function. The untreated rat ovaries exhibited
an intact structure, and the ovarian interstitial cells were
appropriately loose for ovulation (Fig
1). In the TG-treated rats, however, the number of atretic
follicles was increased, and the ovarian granuloma cells were
observed to be undergoing significant apoptosis. Furthermore,
increasing the dose of TGs caused the rat ovarian granuloma cells
to become more closely distributed and the number of mature
follicular cells to decrease significantly. The number of apoptotic
cells was observed to have increased significantly, and there was
significant dysplasia in the corpus luteum. In addition, the
interstitial space was generally visible at multiple bleeding
sites, and inflammatory cell infiltration into the ovaries was
observed in the TG-treated groups (Fig.
1). These results suggest that TGs induce damage and POF in
rats.
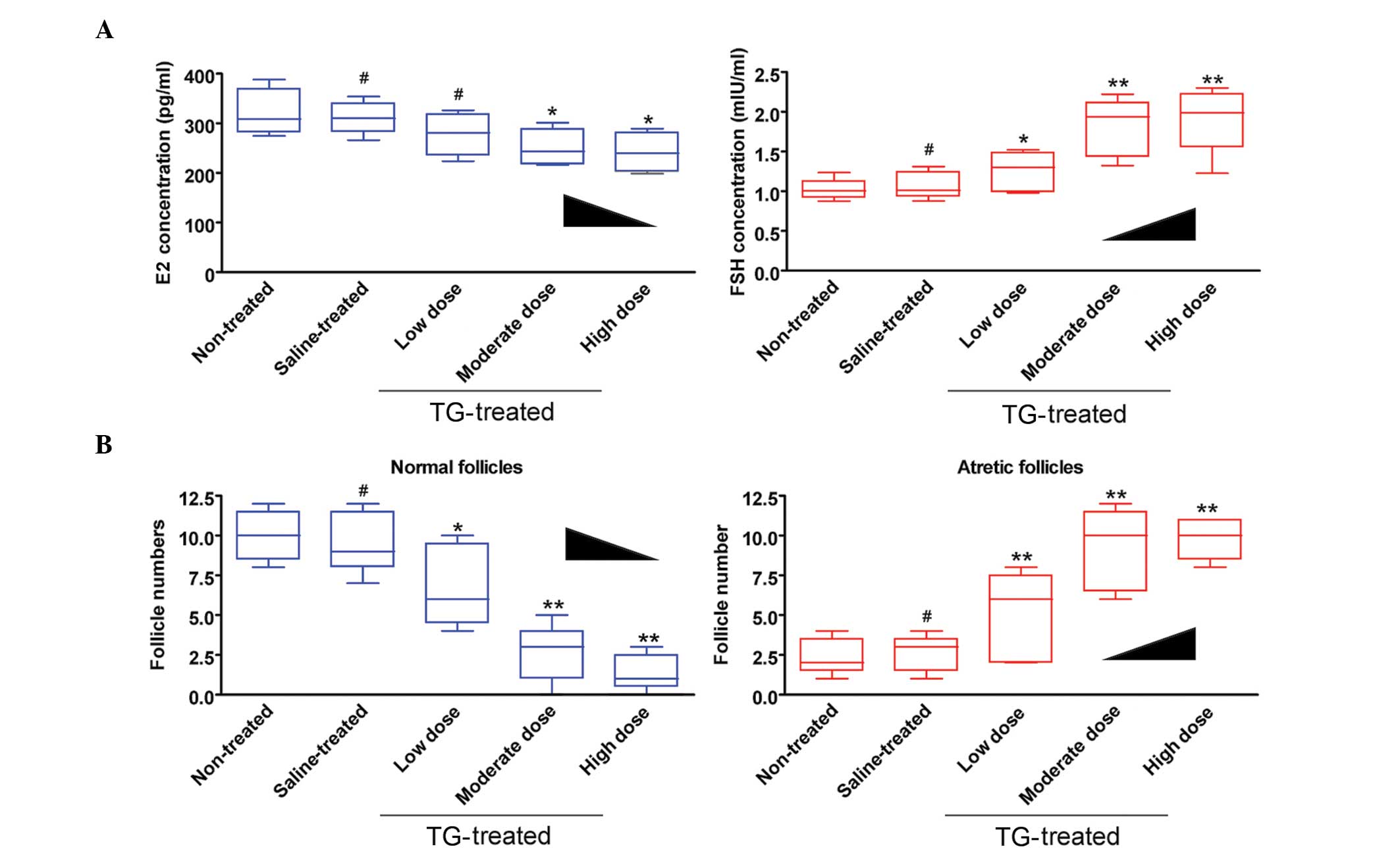
TGs affect rat follicle maturation and
hormone secretion
To characterize follicle maturation and hormone
secretion, the plasma estradiol (E2) and follicle-stimulating
hormone (FSH) levels, as well as follicle pathologies, were
examined in mice. ELISA showed that the plasma E2 levels were
decreased but the FSH levels were increased in the medium-dose
TG-treated-group compared with those in the no treatment (blank
control) and saline-treated (negative control) groups (Fig. 2). Furthermore, the number of
follicles (atretic or normal) at every stage was counted in each
group. The results revealed a significantly lower number of normal
follicles but a higher number of atretic follicles in the
TG-treated group compared with the non-treated and saline groups
(Fig. 2 and Table III). These results suggest that TGs
induce ovarian hormonal dysregulation and follicle maturation.
 | Table III.Hormone assay and follicle count. |
Table III.
Hormone assay and follicle count.
|
|
|
| Tripterygium
glycoside-treated |
|---|
|
|
|
|
|
|---|
| Parameter | No treatment | Saline-treated | Low-dose | Medium-dose | High-dose |
|---|
| E2 (pg/ml) |
320.00±17.23 |
310.80±11.98 |
278.10±16.91 |
250.10±14.65 |
241.30±14.76 |
| FSH (mIU/ml) |
1.02±0.05 |
1.06±0.06 |
1.26±0.09 |
1.83±0.14 |
1.92±0.15 |
| Normal follicles
(n) |
10±1 |
10±1 |
7±1 |
3±1 |
1±1 |
| Atretic follicles
(n) |
2±1 |
3±1 |
5±1 |
9±1 |
10±1 |
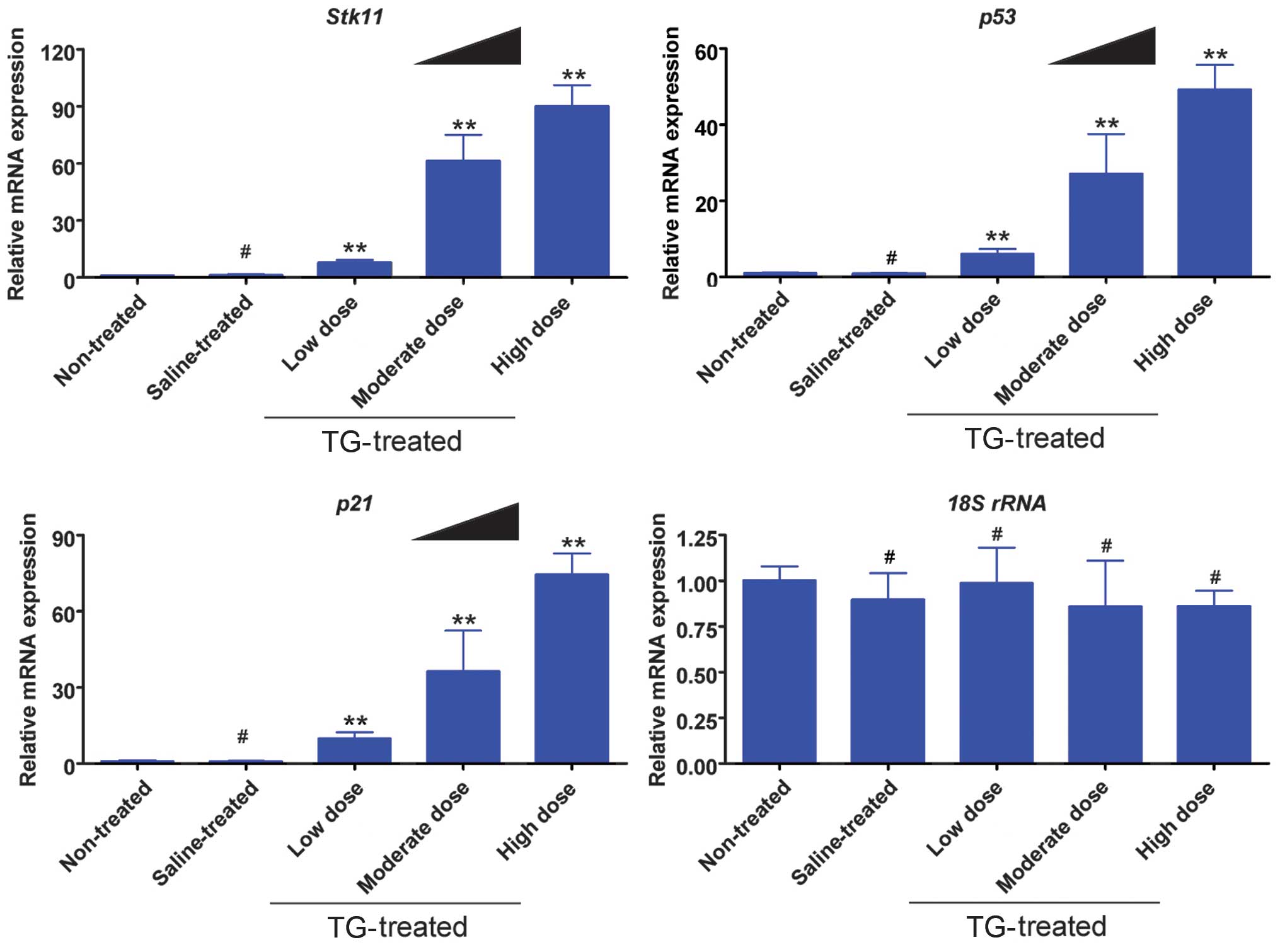
TGs induce mRNA expression of the
Stk11-p53-p21 signaling pathway in ovaries
The results from the RT-qPCR analysis indicated that
the mRNA expression levels of Stk11, p53 and p21 were elevated
significantly in the TG-treated groups compared with those in the
non-treated and saline groups (Fig.
3). Furthermore, the TG concentration was positively correlated
with gene expression.
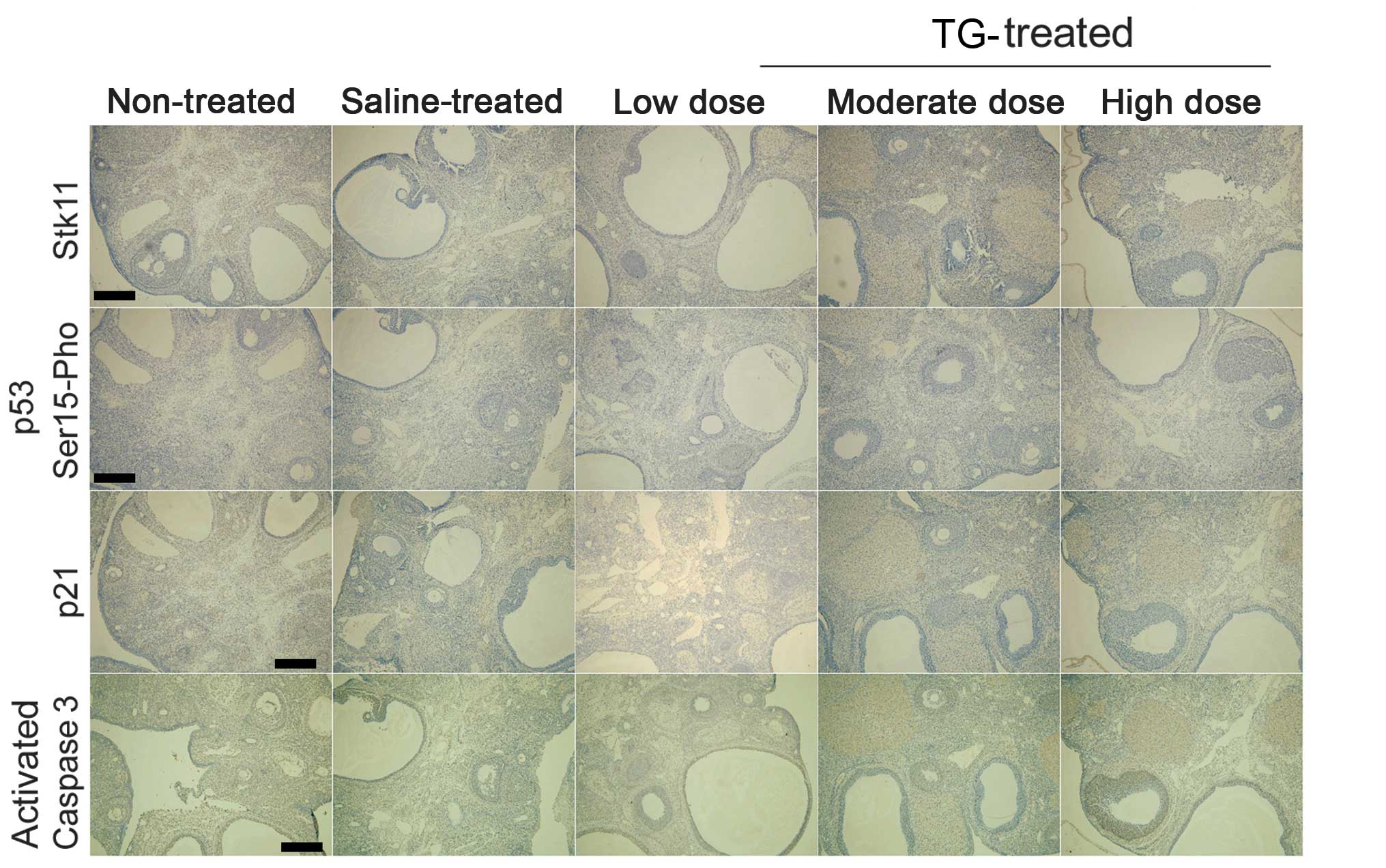
TGs induce protein expression of the
Stk11-p53-p21 signaling pathway in ovaries
Immunohistochemistry showed that the protein
expression levels of Stk11, p53 and p21 were significantly higher
in the TG-treated mice compared with those in the non-treated and
saline groups (Fig. 4). Furthermore,
Serine 15 phosphorylation of p53 was also significantly enhanced in
the TG-treated groups. In addition, increased TG concentration was
correlated with increased caspase-3 activation in the TG-treated
groups (Fig. 4). These results
indicate that TGs stimulate the Stk11-p53-p21 signaling pathway and
induce apoptosis in ovaries.
TGs induce the transcriptional
activity of p53
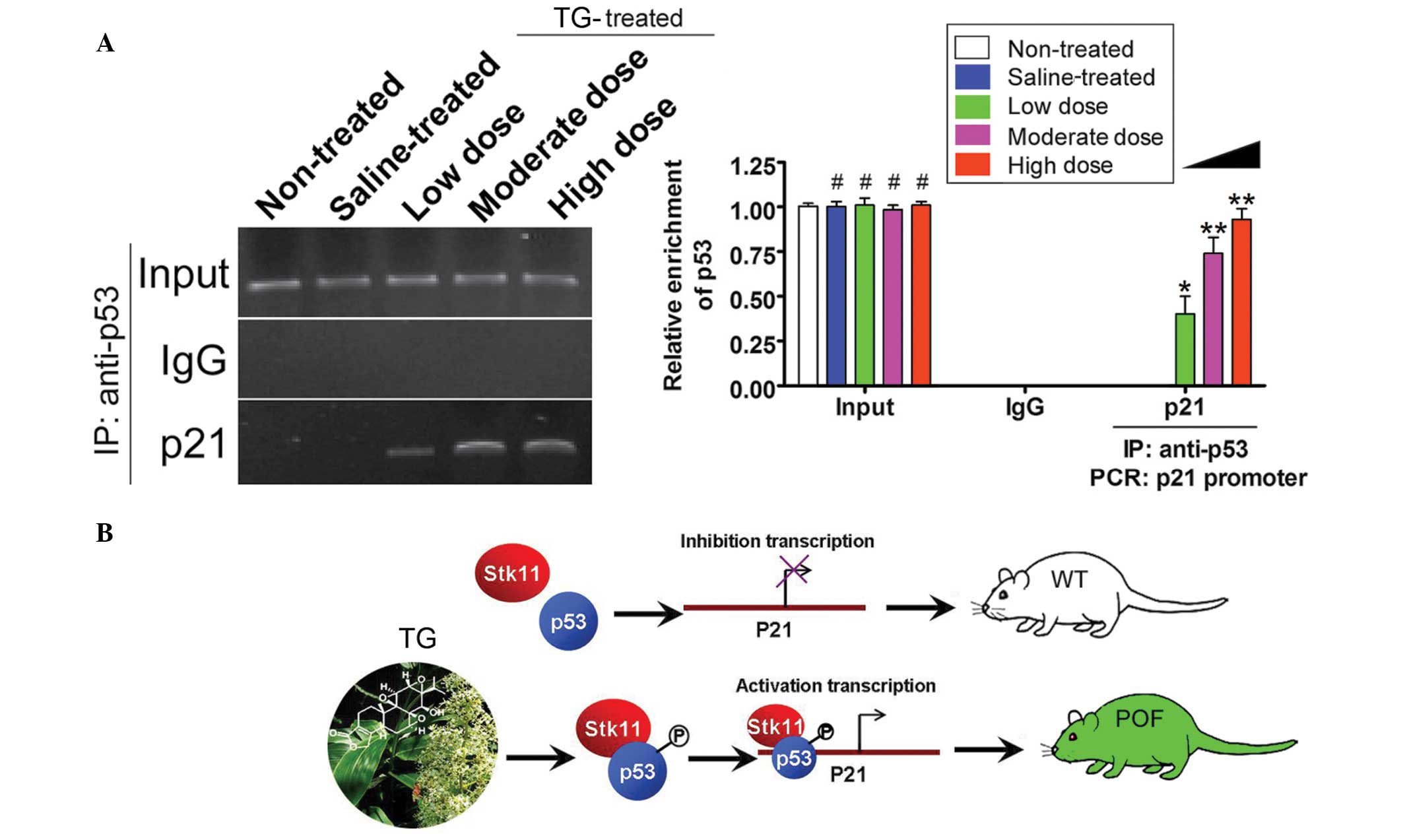
ChIP-PCR assays were used to determine the activity
of the transcription factors p53 and p21. The p21 promoter
fragments were not amplified by PCR when p53 was immunoprecipitated
from the non-treated and saline groups (Fig. 5); however, in the TG-treated groups,
the p21 promoter fragment was amplified following p53
immunoprecipitation (Fig. 5).
Furthermore, this PCR product was increased with the increasing
concentrations of TGs. These results therefore showed that TGs
induce the transcriptional activity of p53.
Discussion
POF disrupts ovarian function, which causes
infertility in females, but the mechanism regulating this is not
clear (3–5). The cause of POF can be roughly divided
into two categories: Congenital factors, such as genetic mutations,
and environmental factors, including infection, inflammation,
chemical drugs or radiation injury (3–5). TG is a
common anti-allergy drug, which can be used for the treatment of
rheumatoid arthritis, primary glomerular nephropathy, nephritic
syndrome, lupus nephritis, purpura, lupus erythematosus, subacute
and chronic severe hepatitis, and chronic active hepatitis. TG can
also be used for the treatment of allergic cutaneous vasculitis,
dermatitis, eczema, psoriasis, arthritis, leprosy, Behçet's
syndrome, recurrent aphthae and ankylosing spondylitis (6–8).
Although TG has certain curative effects and cost advantages in the
treatment of these diseases, the side effects are becoming
increasingly prevalent (6–8).
In the present study, the effect of different TG
concentrations was assessed in female rats. It was found that,
regardless of dose, TGs induced ovarian dysfunction and failure.
The secretion of estrogen decreased in the TG-treated rats, as well
as the feedback to pituitary secretion, such as gonadotropin,
caused by increased FSH and luteinizing hormone. Continued
treatment of TG caused ovarian tissue damage, granuloma cell
swelling and necrosis, inflammatory cell infiltration and bleeding.
The apoptosis of ovarian granuloma cells in the TG-treated rats was
particularly evident. Based on these findings, the mechanism
underlying the TG-induced granuloma cell apoptosis was investigated
by characterizing the activity of the Stk11-p53-p21 signaling
pathway.
Cell apoptosis or cell cycle arrest is activated
when the Stk11-p53-p21 signaling pathway is expressed (9). In the present study, it was found that
TG treatment in rats caused significant ovarian granuloma cell
apoptosis or necrosis. Furthermore, the expression of the
Stk11-p53-p21 signaling pathway in the TG-treated rat ovaries was
detected. TGs not only stimulated the expression of the
Stk11-p53-p21 signaling pathway in the rat ovarian tissue,
particularly ovarian granuloma cells, but also induced the
transcriptional activity of p53, which bound the promoter of p21
(9). When p53 binds this promoter,
transcription of the p21 gene is notably enhanced (9). Activated p53 could therefore play a
role in the transcription and expression of p21 following TG
treatment. This may explain the toxicity of TGs and how they induce
apoptosis and necrosis. In conclusion, TGs induce apoptosis and
necrosis within the ovary to initiate POF via p53 activation and
Stk11-p53-p21 signaling.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (grant nos. 81273794 and
81202811), and project funded by the China Postdoctoral Science
Foundation (grant no. 2014M550250), and the Shanghai Municipal
Health Bureau Fund (grant no. 20124320).
References
|
1
|
Beck-Peccoz P and Persani L: Premature
ovarian failure. Orphanet J Rare Dis. 1:92006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vujović S, Kanazir S, Ivović M, et al:
Collagen type I alpha 1 gene polymorphism in premature ovarian
failure. Srp Arh Celok Lek. 141:344–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu T, Huang Y, Guo L, Cheng W and Zou G:
CD44+/CD105+ human amniotic fluid mesenchymal stem cells survive
and proliferate in the ovary long-term in a mouse model of
chemotherapy-induced premature ovarian failure. Int J Med Sci.
9:592–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Qin W, Huang Y, Zhao Y and Wang J:
Induction of estrogen-sensitive epithelial cells derived from
human-induced pluripotent stem cells to repair ovarian function in
a chemotherapy-induced mouse model of premature ovarian failure.
DNA Cell Biol. 32:685–698. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu T, Huang Y, Zhang J, et al:
Transplantation of human menstrual blood stem cells to treat
premature ovarian failure in mouse model. Stem Cells Dev.
23:1548–1557. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Shen F, Guan C, et al: Activation of
Nrf2 protects against triptolide-induced hepatotoxicity. PLoS One.
9:e1006852014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XJ, Jiang ZZ and Zhang LY: Triptolide:
progress on research in pharmacodynamics and toxicology. J
Ethnopharmacol. 155:67–79. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou J, Xi C, Wang W, et al:
Triptolide-induced oxidative stress involved with Nrf2 contribute
to cardiomyocyte apoptosis through mitochondrial dependent
pathways. Toxicol Lett. 230:454–466. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeng PY and Berger SL: LKB1 is recruited
to the p21/WAF1 promoter by p53 to mediate transcriptional
activation. Cancer Res. 66:10701–10708. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vaahtomeri K and Mäkelä TP: Molecular
mechanisms of tumor suppression by LKB1. FEBS Lett. 585:944–951.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ollila S and Mäkelä TP: The tumor
suppressor kinase LKB1: lessons from mouse models. J Mol Cell Biol.
3:330–340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shen DZ, Xin SL, Chen C and Liu T: Effect
of atorvastatin on expression of TLR4 and NF-κB p65 in
atherosclerotic rabbits. Asian Pac J Trop Med. 6:493–496. 2013.
View Article : Google Scholar : PubMed/NCBI
|