Introduction
Cisplatin has been widely and effectively used for
chemotherapy against a number of tumor types; however, the drug
induces a series of side effects in various organs, of which the
major organ affected is the kidney (1–3).
Cisplatin triggers the apoptosis of renal tubular epithelial cells,
which is followed by inflammation and fibrosis (4). These alterations predominantly occur in
cells of the proximal tubule, particularly in the epithelial cells
surrounding the S3 segment, since cisplatin accumulates at the
highest concentrations in these areas (5). Previously, multiple mechanisms and
signaling pathways associated with cisplatin-induced tubular cell
death have been illustrated and considered as potential targets for
clinical treatment (6–10).
The identification of a novel and effective
treatment for cisplatin-induced renal injury has been prompted in
this situation. Previous studies have demonstrated that mesenchymal
stem cells (MSCs) have enormous potential to repair the injured
tissues through infusion and paracrine signaling (11–13).
MSCs are undifferentiated adult cells that can be isolated from a
variety of tissues, but primarily from the bone marrow (BM). The
cells emerge from mesenchymal cells that generate connective
tissues, including bone, cartilage and fat, and blood
supply-related organs, such as the vasculature and hematopoietic
systems. MSCs are defined by their adherence to plastic in culture,
multipotentiality (14) and their
ability to be induced and differentiated into renal cells (15). Transplantation of BM-MSCs or other
types of progenitor cells from rodents is a strategy for renal
repair in experimental models of acute kidney injury (AKI)
(16–21), a highly life-threatening clinical
condition (12,14,22).
Currently, adipose-derived (AD)-MSCs have been increasingly
recognized due to their similar capacity to BM-MSCs, exhibiting
multi-differentiation and tissue repair properties. In addition,
the collection of adipose tissue is easier since liposuction is
widely used in clinical practice. Furthermore, it has been
demonstrated that AD-MSCs are able to protect the kidney from renal
failure (18,23–31).
The aim of the present study was to further
investigate the functional effects of AD-MSCs on AKI. To the best
of our knowledge, this is the first study to use human AD-MSCs for
the treatment of animals with AKI. In addition, the current study
applied detailed tracking following transplantation. The
amelioration of renal function was evaluated via several aspects,
including functional recovery at the biochemical level,
reconstruction of the tubule structure with more proliferative
epithelial cells, and the relevance between AD-MSC fusion and
functional restoration of the kidney.
Furthermore, the present study utilized a co-culture
system with two types of cell in a Transwell assay to analyze the
antiapoptotic capacity of AD-MSCs in vitro. The
antiapoptotic functions of AD-MSCs were analyzed with the aim to
provide experimental evidence supporting the therapeutic potential
for replacing MSCs in cell transplantation for human diseases.
Materials and methods
AD-MSC culture
AD-MSCs were isolated from human fat tissue. The
adipose tissue was obtained from abdominal fat collected during
liposuction surgery at Wuhan Mei Ji Yuan Plastic and Esthetic
Surgery Hospital (Wuhan, China). The study was conducted in
accordance with the Declaration of Helsinki, and with approval from
the Ethics Committee of Wuhan University (Wuhan, China). Written
informed consent was obtained from all the participants. Culture
methods were based on a classical method and modified (32). Using liposuction, up to 20 ml adipose
tissue was obtained, which was centrifuged at 800 × g for 5 min,
after which the liquid was removed. The tissue samples were washed
with phosphate-buffered saline (PBS; Sigma-Aldrich, St. Louis, MO,
USA) three times, followed by digestion in 0.075% collagenase
(Sigma-Aldrich), using the same volume as the tissue, for 30 min at
37°C whilst shaking. The sample was neutralized with Dulbecco's
modified Eagle's medium (DMEM; Gibco Life Technologies, Carlsbad,
CA, USA) containing 20% fetal bovine serum (FBS; Gibco Life
Technologies), and centrifuged at 1,000 × g for 15 min. The pellet
was resuspended in PBS and passed through a 70-µm cell strainer.
The cells were cultured in low glucose-DMEM, supplemented with 20%
FBS and antibiotics. After 24 h, the media were removed, and
refreshed every three days until the cells reached >70%
confluence, after which the cells were passaged.
Phenotypic analysis and in vitro
differentiation assays
AD-MSC phenotypic analysis was conducted using
Cytomics FC500 (Beckman Coulter, Brea, CA, USA). The cells were
harvested in passage three, washed in flow cytometry buffer and
incubated for 20 min in flow cytometry buffer containing
fluorescein-conjugated monoclonal antibodies directed against MSC
antigens: phycoerythrin (PE)-conjugated anti-human CD13 (cat. no.
301704), fluorescein isothiocyanate (FITC)-conjugated anti-human
CD90 (cat. no. 328108), allophycocyanin-conjugated anti-human CD44
(cat. no. 338806) and FITC-conjugated anti-human CD105 (cat. no.
323203), and against hematopoietic antigens: APC-conjugated
anti-human CD14 (cat. no. 301808), PE-conjugated anti-human CD34
(cat. no. 343606), PE/Cy5-conjugated anti-human CD45 (cat. no.
304010) and FITC-conjugated anti-human CD71 (cat. no. 334104). All
antibodies were purchased from BioLegend (San Diego, CA, USA).
AD-MSCs were obtained by plastic adhesion. As
aforementioned, AD-MSCs have a similar differentiation capacity to
MSCs derived from bone marrow. To confirm their pluripotency
potential, AD-MSCs were cultured in induction media, which caused
the cells to differentiate into osteoblasts, adipocytes and
chondroblasts [osteoblasts (cat. no. HUXMD-90021), adipocytes (cat.
no. HUXMA-90031) and chondroblasts (cat. no. HUXMA-90041/90042)
differentiation kits; Cyagen Biosciences, Inc., Guangzhou,
China).
Labeling the AD-MSCs and
transplantation following AKI in vivo
The ability of the AD-MSCs to repair the kidney
following injury and the underlying mechanisms associated with cell
localization after transplantation were analyzed. AD-MSCs were
labeled using a PKH-26 Red Fluorescence Cell Linker kit
(Sigma-Aldrich), which is normally used for general cell membrane
labeling according to Morigi et al (33). Next, the cells were transplanted into
the rats who had been subjected to cisplatin-induced AKI. To
confirm the cell viability when labeled with PKH-26 and the dyeing
efficiency, a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) assay was performed, after which the stained cells
were seeded on coverslips and observed under a microscope
(SZ61GFP/TS; Olympus Corporation, Tokyo, Japan).
Male Sprague-Dawley rats (weight, 200–230 g) were
provided with a standard rat chow and free access to water in a
temperature- and humidity-controlled facility. The rats were
subjected to a 12-h light/dark cycle. The study was carried out in
strict accordance with the recommendations in the Guide for the
Care and Use of Laboratory Animals of the National Institutes of
Health. The animal use protocol was reviewed and approved by the
Institutional Animal Care and Use Committee of Wuhan University.
The rats were divided into the following three groups. Group 1
received saline injections and were referred to as the healthy
control group (n=10). Group 2 was the AD-MSC treatment group
(n=15), in which each rat was administered AD-MSCs
(1–2×106 cells/1 ml saline) one day after cisplatin
induction. Only the cells cultured in passage three were used for
grafting. Finally, group 3 was the cisplatin injection group (n=12)
that were subjected to cisplatin-induced AKI, as with the rats in
group 2. However, the rats in group 3 received a saline injection
instead of the AD-MSCs. In all the groups, the cells and reagents
were injected via the tail vein, with the exception of cisplatin
and the first saline injection in group 1, where the reagents were
injected into the peritoneal cavity. The rats in groups 2 and 3
were injected with cisplatin (Sigma-Aldrich) at a concentration of
6 mg/kg body weight. After five days, the rats were sacrificed by
severing the carotid artery, and the kidneys were harvested.
Tissues samples were sectioned for histology, apoptosis and
proliferation and morphometric analyses. Since the AD-MSCs were
obtained from human adipose tissue, the kidney sections of the
animals injected with PKH-26-labeled cells were stained with a
mouse anti-human monoclonal CD105 antibody (cat. no. MCA1557; AbD
Serotec, Kidlington, UK), followed by an anti-mouse Cy5 antibody
(Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA).
Five rats from group 2 were injected with AD-MSCs that had not been
labeled with PKH-26, and the remaining 10 rats in group 2 were
injected with AD-MSCs labeled with PKH-26; however, these sections
were stained with Cy5-anti-human CD105 to detect the location of
the AD-MSCs in the kidney. Samples were counterstained with
FITC-labeled lectin wheat germ agglutinin (WGA; Vector Laboratories
Ltd., Peterborough, UK), while the nuclei were stained with
4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI;
Sigma-Aldrich).
Determination of renal function
All the animals were sacrificed at day five
following cisplatin induction and whole blood samples were
collected. Rats were housed in metabolism cages (Nanjing Bian Zhen
Bio-science Company, Nanjing, China), and the blood serum and urine
were collected every 24 h for determination of renal function by
biochemistry analysis, which included measurements of the serum
blood urea nitrogen (BUN) level (48T/96T; Biofine, Blaine, WA,
USA), the creatinine clearance rate (Ccr) (48T/96T; Shanghai Yanjin
Bio-science Company, Shanghai, China), urinary microalbumin (mALB)
(cat. no. ARB12655; Beijing Ai Ran Bio-tech Ltd., Beijing, China)
and β2 microglobulin (β2 mG) (Shanghai Yuanyan Bio-science Limited
Company, Shanghai, China). These parameters were measured using
standard diagnostic kits with a Clinical Chemistry Analyzer (AU400;
Olympus Corporation).
Renal histology
After the animals were sacrificed, one side of the
kidney tissues were removed by surgery and fixed in formalin. The
tissues were sectioned into 5-µm slices for paraffin embedding,
which was followed by staining with hematoxylin and eosin (H&E)
or periodic acid-Schiff (PAS) reagent. Tubular injury was scored by
grading the percentage of the affected tubules under ten randomly
selected non-overlapping fields (magnification, ×200). The scoring
was conducted as follows: 0, 0%; 1, ≤10%; 2, 11–25%; 3, 26–45%; 4,
46–75%; and 5, 76–100%. To score the injured tubules, whole tubular
numbers per field were considered as standard under ×200
magnification. Thus, the injury was calculated as follows: Injury
score (%) = (number of injured tubules/number of whole tubules) ×
100 (23).
A terminal transferase-mediated dUTP nick-end
labeling (TUNEL) assay (DeadEnd™ Fluorometric TUNEL system; cat no.
G3250; Promega Corporation, Madison, WI, USA) was used to identify
the extent of apoptosis. Furthermore, the number of cells
undergoing proliferation was measured through staining with a
monoclonal anti-proliferating cell nuclear antigen (PCNA) antibody
(cat. no. MA1-16827; Thermo Fisher Scientific, Waltham, MA, USA).
Paraffin-embedded sections were stained with TUNEL and PCNA and
observed under a microscope (LSM 700; Carl Zeiss AG, Oberkochen,
Germany) at ×400 magnification (34). In total, 20 areas were randomly
selected for each slide to determine the result.
The other side of the kidney tissues were embedded
in Tissue-Tek OCT Compound (cat. no. 4583; Sakura Finetek Japan
Co., Ltd., Tokyo, Japan) and fresh-frozen in liquid nitrogen. The
samples were sectioned into 8-µm slices and stored at −80°C. The
sections were subsequently fixed in acetone for 10 min and
incubated with FITC-labeled lectin WGA, which binds membrane
glycoproteins and sialic acid and was used for identifying the
tubular structures. Nuclei were stained with DAPI. In total, 20
sections per mouse were analyzed.
Co-culture with AD-MSCs and injured
NRK-52E cells
A specific rat renal proximal tubular cell line,
NRK-52E (American Type Culture Collection, Manassas, VA, USA), was
used to set up the co-culture model. This cell line had been
previously used as in vitro model (34). The cells were cultivated according to
recommended conditions, comprising DMEM supplemented with 10%
heat-inactivated FBS, 2 mM glutamine (Gibco Life Technologies) and
penicillin/streptomycin (100 U/ml; Gibco Life Technologies).
Cells were divided to three groups and cultured in
six-well dishes. Group 1 NRK-52E cells were cultured without any
treatment, while group 2 NRK-52E cells were induced by cisplatin.
Group 3 NRK-52E cells were treated with cisplatin, in the same
conditions as group 2, but were also co-cultured with AD-MSCs in a
Transwell assay (pore size, 0.4 µm; Costar®; Corning Life Science,
Corning, NY, USA). Cisplatin (100 mM) was added to groups 2 and 3
at a volume of 170 µl over a period of 6 h. In addition, for group
3, AD-MSCs were cultivated in Transwell chambers at a concentration
of 2.5×105/ml in 2 ml media for four days. NRK-52E cells
were lysed after co-culture and the protein was extracted for
western blot analysis. The expression levels of phosphorylated
(p)-p38, B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X protein
(BAX) were analyzed using a standard protocol, which was used to
identify the antiapoptotic mechanisms of AD-MSCs (34). Briefly, the cells were harvested and
lysed in radioimmunoprecipitation assay buffer (Sigma-Aldrich).
Protein concentrations of the lysates were quantified using
Bicinchoninic Acid Protein Assay kit (Pierce Biotechnology, Inc.,
Rockford, IL, USA). Cell lysates were then loaded onto an 12%
SDS-PAGE gel and separated by electrophoresis. Following
electrophoresis, the proteins were transferred to polyvinylidene
fluoride membranes (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
and non-specific binding sites were blocked with 5% nonfat milk in
PBS with 0.1% Tween-20. The membranes were then incubated with the
following primary antibodies: Rabbit polyclonal anti-p-p38 (cat.
no. ab4822; Abcam, Cabridge, MA, USA), rabbit polyclonal anti-BAX
(cat. no. ab7977; Abcam); rabbit monoclonal anti-Bcl-2A1 (cat. no.
ab33862; Abcam) and mouse monoclonal anti-actin (cat. no. A3853;
Sigma-Aldrich) at 4°C overnight. The membranes were then incubated
with HRP-conjugated secondary antibodies (Dako, Glostrup, Denmark)
for 1 h at 4°C, and visualized by enhanced chemiluminescence (GE
Healthcare Life Sciences, Little Chalfont, UK). The blots were
analyzed by ImageJ 1.48 software (National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Data were collected, and analyzed using the t-test and
one-way analysis of variance. Statistical analysis was performed
using SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA), and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Isolation and characterization of
human AD-MSCs
Human AD-MSCs were isolated from human fat tissue
that was obtained from abdominal fat during liposuction surgery.
Cultured cells were adherent to the dish and were shown to be
morphologically flattened and spindle-shaped under a light
microscope (Fig. 1A). The
pluripotency of the AD-MSCs was demonstrated with distinct culture
conditions that enabled the cells to differentiate into
osteoblasts, adipocytes and chondroblasts (Fig. 1B-D).
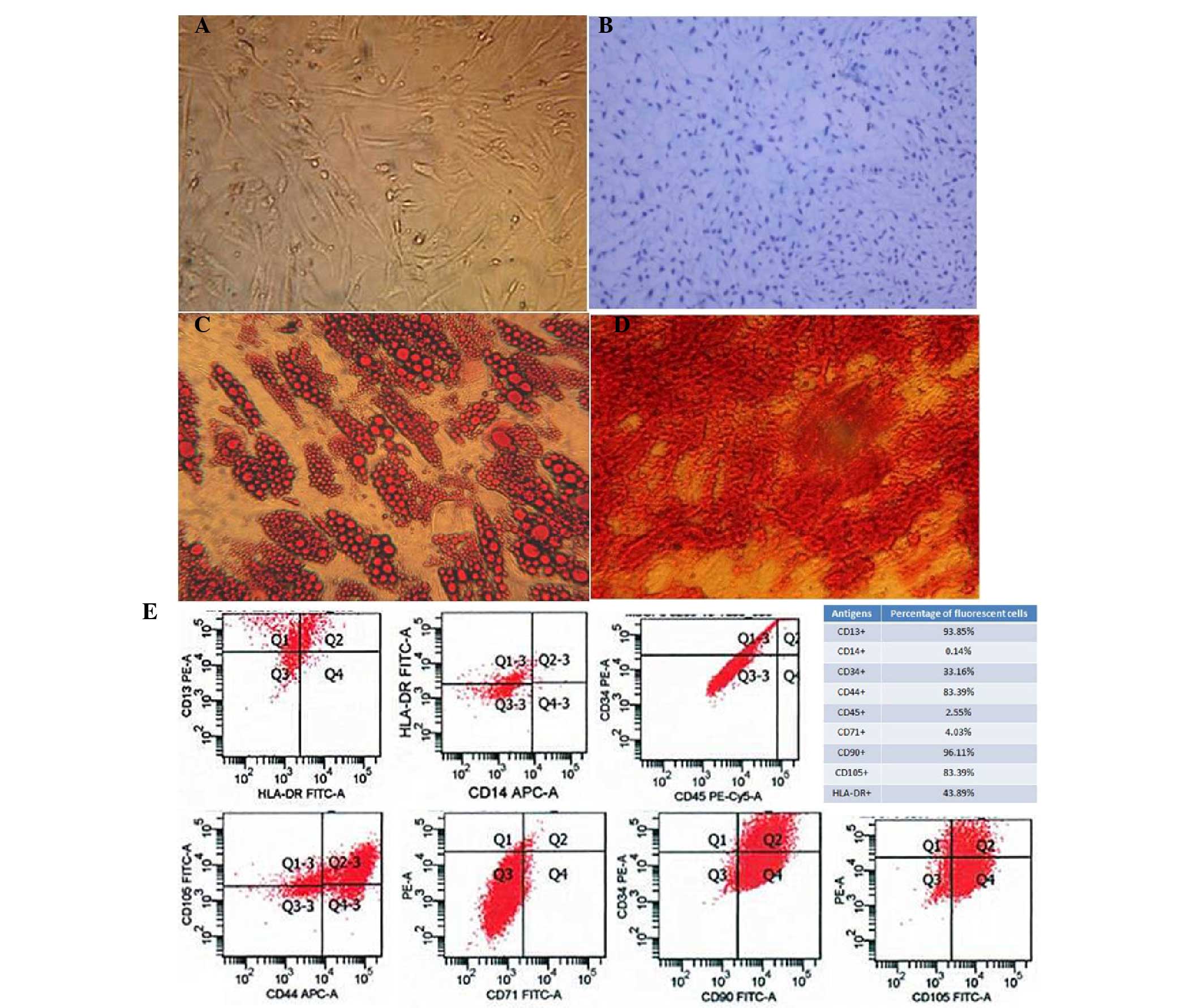 | Figure 1.Characterization of human
adipose-derived mesenchymal stem cells (AD-MSCs). (A) A
representative phase-contrast microscopy image of AD-MSCs showing
typical spindle-shaped morphology (magnification, ×100). (B)
Differentiation of cartilage cells (magnification, ×400). (C)
Adipocyte differentiation (magnification, ×400). (D) Osteogenic
differentiation of AD-MSCs stained with Alizarin Red
(magnification, ×400). (E) Fluorescence-activated cell sorting
characterization of AD-MSCs expressing CD13, CD90, CD44 and CD105,
the commonly used surface markers for MSCs. MSCs were also shown to
express CD14, CD34, CD45 and CD71 at a very low level. FITC,
fluorescein isothiocyanate; PE, phycoerythrin. |
To further characterize the human AD-MSCs,
fluorescence-activated cell sorting analysis was performed to
confirm that the cells endogenously expressed CD13, CD90, CD44 and
CD105, which are the most important surface markers for MSCs.
However, other surface markers, including CD14, CD34, CD45 and
CD71, were rarely expressed on these cells (Fig. 1E).
AD-MSC engraftment protects the kidney
in vivo
To determine whether the AD-MSCs were able to
ameliorate renal function and structure following AKI, serum and
urine samples were collected from the experimental rats every day,
from which the levels of BUN, Ccr, urinary mALB and β2 mG were
analyzed. The measurements were compared among the three groups of
male rats, including the healthy controls, the rats treated with
cisplatin and the rats grafted with AD-MSCs following cisplatin
treatment.
The level of BUN markedly increased within the
four-day period following the cisplatin injection, with the levels
peaking between days 4 and 5 in the group of rats who were injected
with cisplatin (11). For the rats
treated with AD-MSCs, the level of BUN was significantly decreased
compared with the non-treatment group (P<0.01). No statistically
difference was observed in the BUN level between the AD-MSC-treated
group and the healthy control group. Similarly, the Ccr in the
AD-MSC-treated group was significantly reduced in contrast to the
non-treatment group (P<0.01). Notably, the levels of mALB and β2
mG in the AD-MSC-treated group were significantly decreased when
compared with the non-treatment AKI group; however, the levels were
similar to those observed in the healthy controls (Fig. 2A).
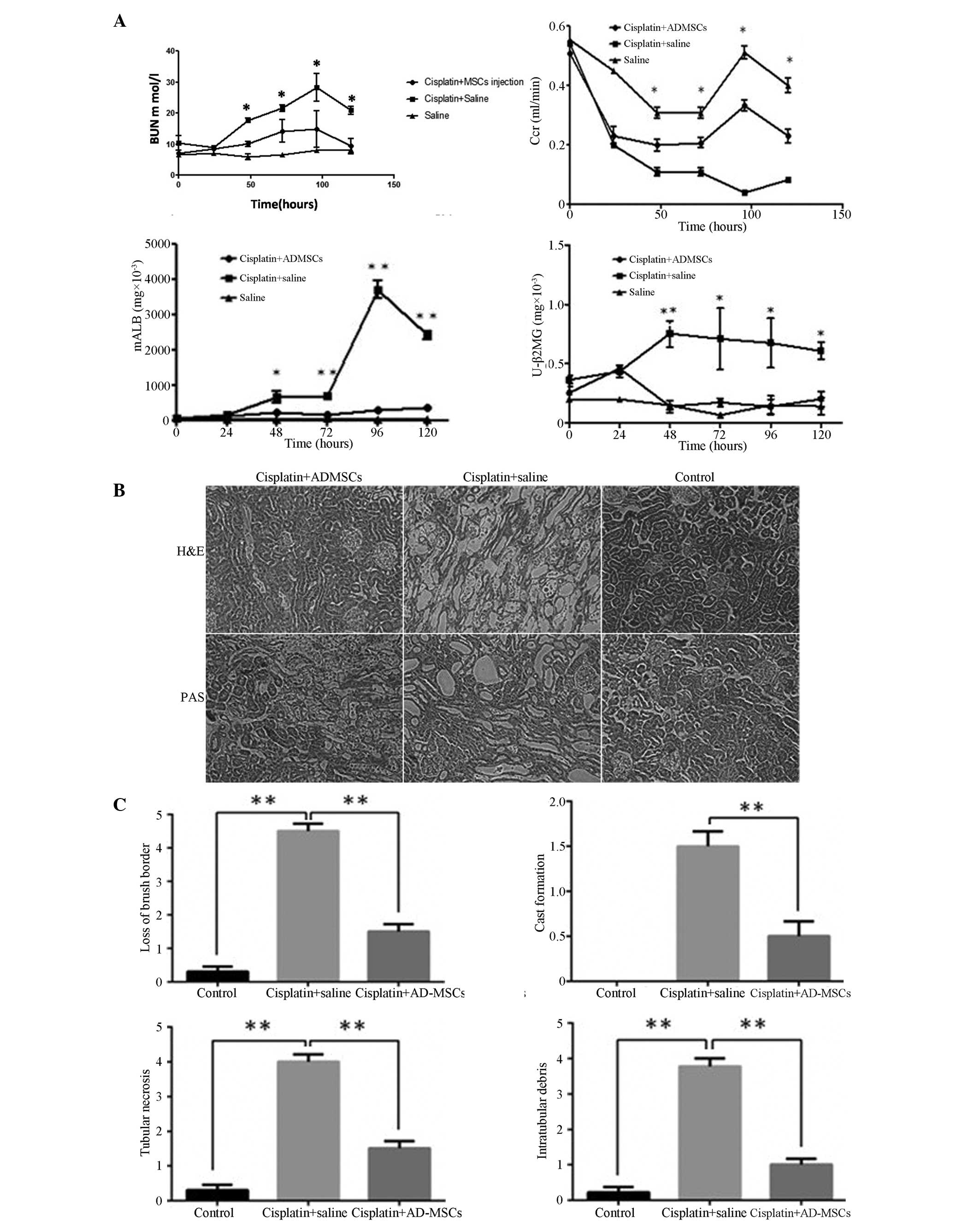 | Figure 2.Kidney functional recovery and
structural repair using human AD-MSCs in animals with acute kidney
injury (AKI). (A) Renal function was assessed daily by the levels
of BUN, serum Ccr, urinary mALB and β2 mG. Data are presented as
mean ± standard error of the mean (SEM). *P<0.01 and
**P<0.005, cisplatin + saline group vs. cisplatin + AD-MSCs
group. (B) Representative images from H&E and PAS staining of
the kidney sections from the healthy control, cisplatin + saline
and cisplatin + human AD-MSCs groups (magnification, × 200). (C)
Histopathological scoring of cisplatin-induced kidney tubular
injury, as indicated with tubular necrosis, cast formation, loss of
brush border and intratubular debris. Histopathological scoring was
based on the percentage of affected tubules in the kidney sections.
Data are presented as the mean ± SEM. **P<0.005, between
indicated groups. AD-MSCs, adipose-derived mesenchymal stem cells;
BUN, blood urea nitrogen; Ccr, creatinine clearance rate; mALB,
microalbumin; β2 mG, microglobulin; U, urinary; H&E,
hematoxylin and eosin; PAS, periodic acid-Schiff. |
To evaluate the integrity of the renal structures,
H&E or PAS staining were performed on the kidney sections. The
results clearly demonstrated that the injured kidneys were notably
recovered when treated with the AD-MSCs, with observations
morphologically similar to those of the healthy control group rats
(Fig. 2B), Furthermore, a scoring
system was introduced to quantify the injury of the kidney tubules.
In the cisplatin-induced injury group, a loss of the brush border,
flattening and a loss of epithelial cells were observed, as well as
luminal cell debris and hyaline casts in the proximal tubules.
However, when the rats were treated with AD-MSCs, the
histopathological scores were significantly reduced compared with
the injury group (P<0.005; Fig.
2C), which indicated engraftment of AD-MSCs effectively
promoted the recovery of the tubular structure.
Human AD-MSC treatment reduces
apoptosis and promotes cell proliferation
BM-MSCs have been shown to repair kidneys following
AKI through inhibiting apoptosis and increasing DNA synthesis
(unpublished data). Therefore, the present study investigated
whether AD-MSCs behave similarly in the injured kidneys. A TUNEL
assay was performed, and the results revealed that the percentage
of apoptotic cells (counted in every 1×103 cells) was
highly reduced in the AD-MSC treatment group when compared with the
cisplatin-induced injury group, exhibiting similar levels to those
in the healthy control group (Fig. 3A
and B).
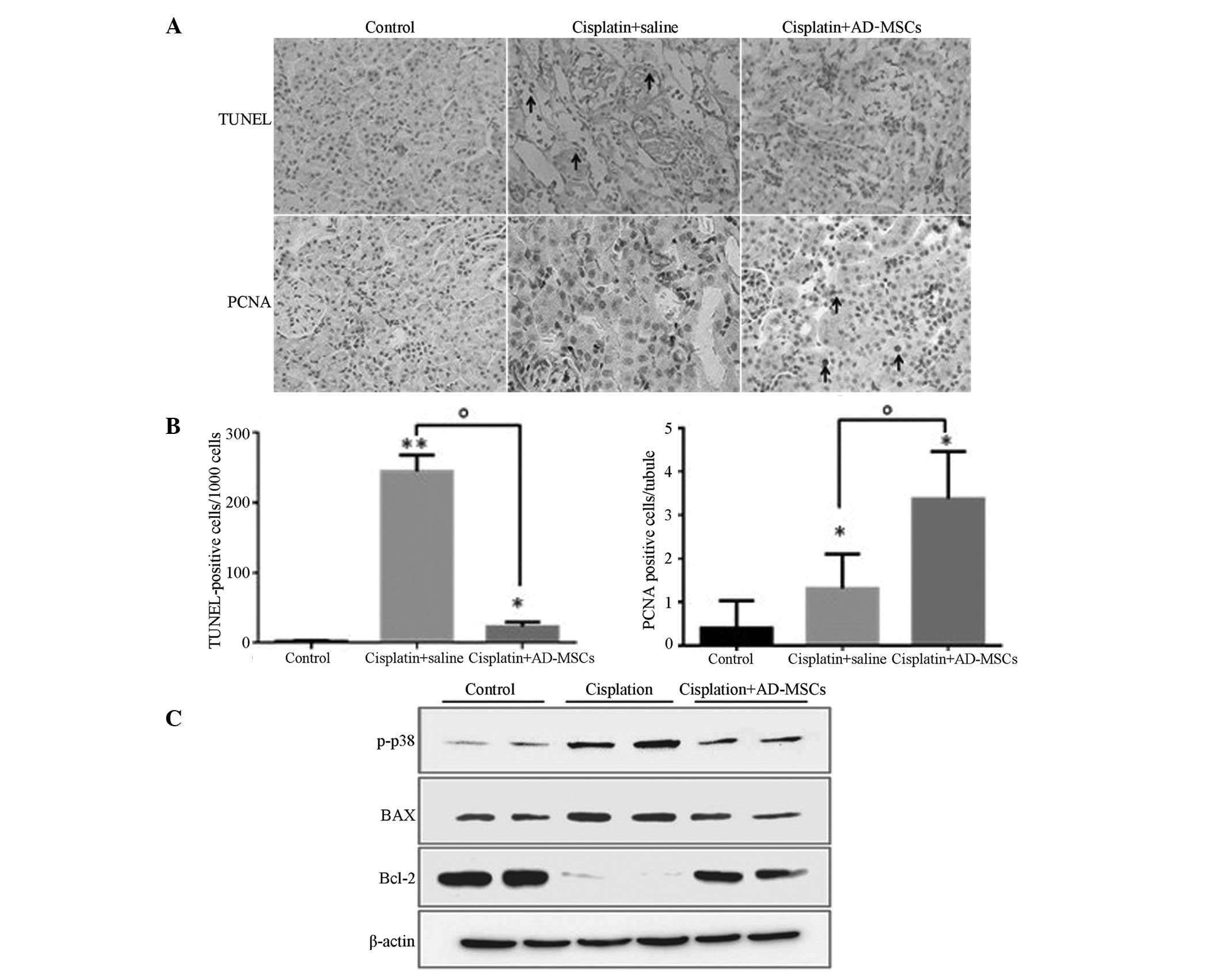 | Figure 3.AD-MSCs significantly reduced the
extent of apoptosis and promoted tubular cell proliferation in
cisplatin-induced injured kidney tissues. (A) Representative images
(magnification, ×400) from TUNEL and PCNA staining in the kidney
sections of the healthy control, cisplatin + saline and cisplatin +
AD-MSCs groups. (B) Apoptotic cells and (C) PCNA-positive tubular
cells were quantified for each kidney section. *P<0.01 and
**P<0.005, vs. control; oP<0.01, between the
indicated groups. Data are presented as the mean ± standard error
of the mean. (D) Immunoblot analysis examining the phosphorylation
of p38 and the expression levels of the apoptotic regulators, BAX
and Bcl-2. β-actin was used as a loading control. A representative
blot from three independent experiments is shown. AD-MSCs,
adipose-derived mesenchymal stem cells; TUNEL, terminal
transferase-mediated dUTP nick-end labeling; PCNA, proliferating
cell nuclear antigen, p-p38, phosphorylated p38. |
PCNA staining was also performed to evaluate the
proliferative capacity of the tubular cells in the injured kidneys
treated with AD-MSCs. The results revealed that the number of
PCNA-positive cells significantly increased in the AKI rats when
compared with the control group (P<0.01), which indicated that a
self-repair system may be stimulated in the injured kidneys
(Fig. 3A and B). As expected, the
number of proliferative cells/tubules labeled with PCNA in the
AD-MSC-treated group was significantly higher compared with the
cisplatin-induced group without treatment (P<0.01). These
results indicated that AD-MSCs were able to repair the kidneys with
AKI through inhibiting apoptosis and promoting tubular cell
proliferation (Fig. 3A and B).
Tracking of human AD-MSCs in vivo
following engraftment
To further understand the mechanisms underlying the
effects of AD-MSCs in injured kidneys, AD-MSCs were tracked in
vivo following engraftment. Prior to injection with
PKH-26-labeled AD-MSCs into the injury-induced rats, the binding
efficiency of PKH-26 and the effects on cell proliferation to the
cultured AD-MSCs were investigated. The results revealed that
PKH-26 was able to effectively label the AD-MSCs (Fig. 4A) and were experimentally feasible
(Fig. 4B). Notably, only a small
number of positive cells were found to be located around the kidney
tubules in each section (red; Fig.
4C). However, there were a greater number of PKH-26 labeled
cells located in the liver and spleen (data not shown). To further
confirm the localization of the AD-MSCs in the kidney tubules, the
human AD-MSCs were stained with the specific surface marker, CD-105
(human). Similarly, this marker was detected in a small number of
cells around the tubules (red, Fig.
4D). To further verify the localization of the AD-MSCs in the
injured kidneys, the AD-MSCs were co-stained with CD105 (mouse
anti-human) and PKH-26, avoiding discrepancy due to membrane
fluidity. PKH-26 and CD-105 double-positive cells were only
identified in the cell infusion rat sections; however, they were
not observed in all the fields (Fig. 4E
and F). These results indicated that AD-MSCs were located
around the tubules in the cortex, repairing the tubular cells;
however, the number of cells was very small.
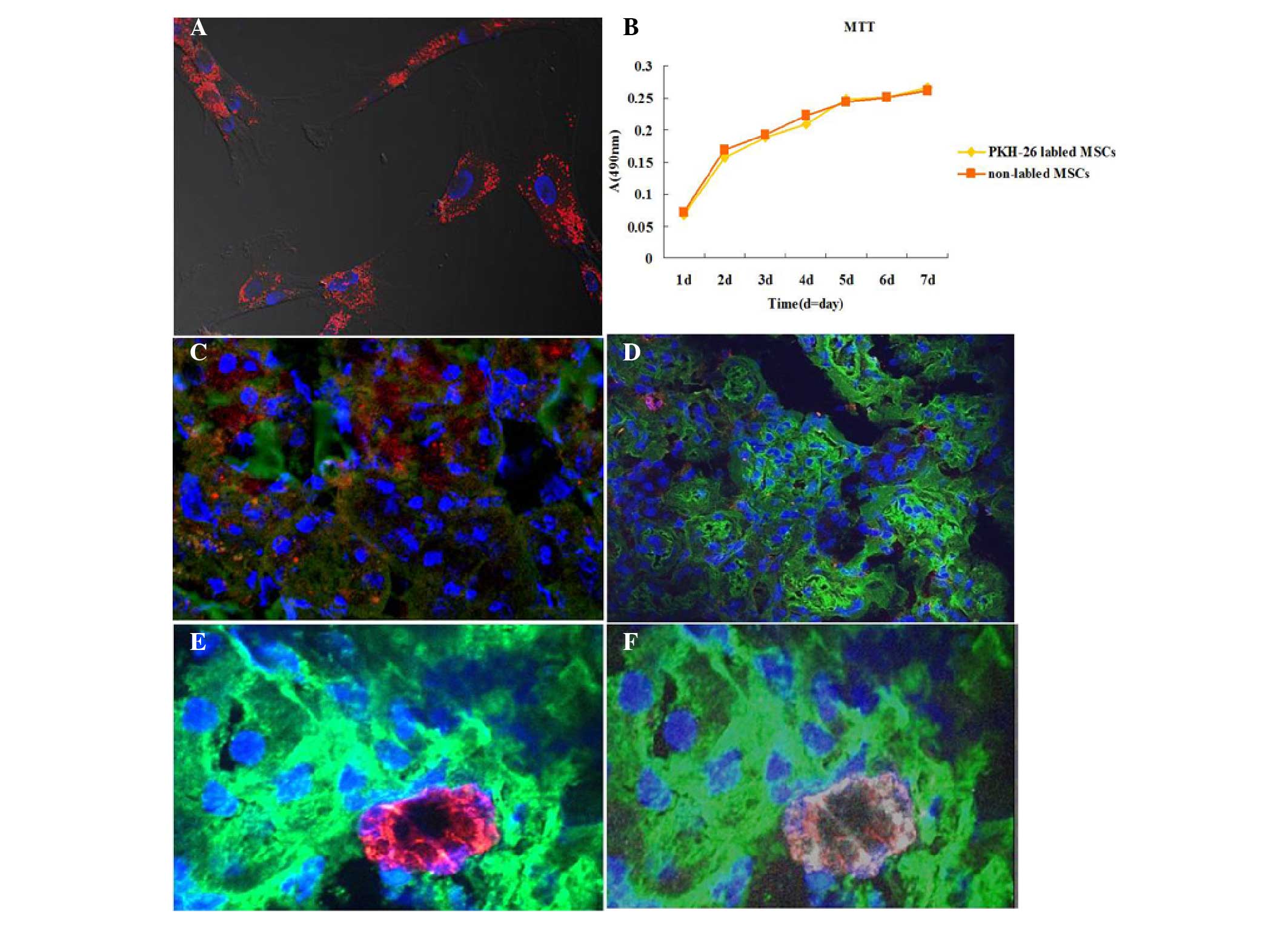 | Figure 4.Tracking of human adipose-derived
(AD)-MSCs through the injured kidneys induced with cisplatin
(magnification, ×630). (A) AD-MSCs were cultured on cover slips and
stained with PKH-26 (red); nuclei were stained with
4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI; blue).
(B) A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(MTT) assay indicated that PKH-26 labeling did not affect the
viability and proliferation of AD-MSCs. (C) AD-MSCs-treated kidney
sections were stained with fluorescein isothiocyanate
(FITC)-labeled wheat germ agglutinin (WGA; green), PKH-26 (red) and
DAPI (blue). As observed, PKH-26-labeled MSCs were located around
the tubules. (D) Following an injection of AD-MSCs without PKH-26
labeling, the kidney sections were stained with Cy5-anti-human
CD105 (red), FITC-labeled WGA and DAPI (blue). (E and F)
PKH-26-positive cells (red) were localized around the tubules, and
co-stained with Cy5-anti-CD105 (white), while nuclei were stained
with DAPI (blue). MSCs, mesenchymal stem cells; A, absorbance. |
Treatment of human AD-MSCs in
vitro
The p38 mitogen-activated protein kinase (MAPK)
signaling pathway plays a significant role in apoptosis (35), and AD-MSCs have been shown to
strongly inhibit the phosphorylation of p38 in cisplatin-induced
AKI in vivo (23).
Furthermore, Bcl-2 family members, who are crucial regulators of
apoptosis, have been demonstrated to play key roles in AKI induced
by cisplatin (36). However, whether
the apoptotic function of MSCs, particularly AD-MSCs, results from
cell fusion or the secretion of certain factors is yet to be
determined. To investigate further, NRK-52E cells and AD-MSCs were
co-cultured without direct contact, after which an immune-blot was
performed. The levels of p-p38 and BAX were shown to be
upregulated, while the expression levels of Bcl-2 were markedly
downregulated in the NRK-52E cells cultured with the
cisplatin-treated tubular cells, as compared with the cells from
the healthy control. However, co-culture of AD-MSCs with NRK-52E
cells was shown to eliminate the dysregulated effect of these
proteins in NRK-52E cells (Fig. 3C).
Therefore, these results indicated that the mechanisms underlying
the modulation of the apoptotic pathways by AD-MSCs in the repair
of injured tubular cells involved the release of a substance into
the media, which was produced following the stimulation of the
injured cells.
Discussion
The roles of human BM-MSCs in AKI repair have been
well documented (11,12,14,33,34).
However, as a new potential therapeutic source, AD-MSCs may be able
to replace BM-MSCs due to their easier method of collection,
improved proliferative capacity and easier induction into the
epithelium (24,25).
The function of injured kidneys has been shown to
recover well following MSC engraftment, as indicated by BUN levels,
Ccr values and observations of tubular structures (14,18,23,30). In
the present study, the results from a serial analysis of
biochemical indicators, including BUN, Ccr, mALB and β2 mG,
demonstrated that human AD-MSCs were able to largely ameliorate
kidney function in animals with AKI. In addition, the observations
from H&E and PAS staining revealed that a number of glomeruli
were damaged, which indicated that the rats treated with cisplatin
may suffer kidney injury at a later stage. However, damaged
glomerluli were not observed in the AD-MSC group, indicating that
AD-MSCs were able to ameliorate AKI at an early developmental stage
of renal failure, preventing glomeruli damage at the later
stage.
AD-MSCs were tracked using PKH-26 and CD105.
Notably, only a small number of positive cells were detected around
the kidney tubules, with a greater number of cells visualized in
other organs, including the liver and spleen (data not shown).
These results indicated that AD-MSCs protect the kidneys against
nephrotoxicity primarily through the release of certain types of
factors, rather than the fusion of the cells to the injured
tubules, which is similar to the behaviors of BM-MSCs and cord
blood MSCs (12,33). In addition, it was hypothesized that
the secreted factors may reach the injured tubules through the
blood circulation, instead of the paracrine system, as previously
demonstrated (unpublished data). This hypothesis was formed since
during paracrine signaling, the cells produce signals, including
cytokines, which induce changes to neighboring cells (including
damaged cells); however, only a small number of AD-MSCs were
identified around the damaged tubular cells in the kidney
sections.
Apoptosis and necrosis of tubular cells have been
considered as the major consequences of cisplatin-induced AKI
(6,10,36).
Human AD-MSCs have been shown to exhibit antiapoptotic capacity
in vivo (23,28). In the present study, the results from
the TUNEL assay further confirmed the antiapoptotic function of
AD-MSCs. In addition, p38 plays a critical role in
cisplatin-induced kidney injury. The function of injured kidneys
has been reported to improve following inhibition of the p38
signaling pathway (35).
In the present study, a co-culture system of AD-MSCs
and cisplatin-induced human tubular cells was developed to
investigate the antiapoptotic effects of human AD-MSCs. The results
from western blot analysis demonstrated that human tubule cell
injury may have triggered the AD-MSCs to secrete multiple factors
that inhibit p38 activation, without the occurrence of cell fusion.
BAX and Bcl-2 are two key regulators (proapoptotic and
antiapoptotic, respectively) of apoptosis. Previous studies have
demonstrated that MSC engraftment downregulates the expression
level of BAX, but upregulates the expression level of Bcl-2 in
kidneys with AKI, which modulates their expression back to a
control level (13,23,36). In
the current in vitro co-culture system, the expression
patterns of three key regulators (p38, BAX and Bcl-2) of apoptosis
were clearly demonstrated following AD-MSC treatment, which was an
important addition to the in vivo data demonstrating that
AD-MSCs are able to effectively regulate apoptotic proteins
(23). More importantly, these in
vitro results strongly indicate that AD-MSCs are able to
inhibit the apoptosis of NRK-52E cells using a mechanism of
producing certain cytokines that are diffused in the media to reach
NRK-52E cells, since this system was completely independent of
cell-cell contact and cell fusion.
In conclusion, the present study demonstrated that
AD-MSCs were able to repair cisplatin-induced AKI in rodents, as
evidenced by biochemical indicators and observations of the kidney
structures. According to the data from the co-culture experiments,
a possible mechanism of kidney recovery with AD-MSC treatment may
be that certain signals are released from the injured tubular cells
that trigger AD-MSCs to produce specific cytokines. These are able
to inhibit p38 MAPK activation, which results in the modulation of
the expression levels of BAX and Bcl-2 to the level of a healthy
control. As shown by the in vivo data, only a small number
of AD-MSCs were identified around the injured tubules following
engraftment. Therefore, from the in vitro and in vivo
data, it was hypothesized that the cytokines produced by AD-MSCs
reach the injured renal tubules through the blood circulation, not
through the paracrine system, as described in a previous study
(23). The results of the present
study indicate that the cytokines produced by AD-MSCs are
potentially able to replace cell therapy, which may resolve the
safety issues from cell transplantation.
References
|
1
|
Lebwohl D and Canetta R: Clinical
development of platinum complexes in cancer therapy: an historical
perspective and an update. Eur J Cancer. 34:1522–1534. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
dos Santos NA, Carvalho Rodrigues MA,
Martins NM and dos Santos AC: Cisplatin-induced nephrotoxicity and
targets of nephroprotection: an update. Arch Toxicol. 86:1233–1250.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidemann HT, Müller S, Mertins L, Stepan
G, Hoffmann K and Ohnhaus EE: Effect of aminophylline on cisplatin
nephrotoxicity in the rat. Br J Pharmacol. 97:313–318. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Francescato HD, Costa RS, Scavone C and
Coimbra TM: Parthenolide reduces cisplatin-induced renal damage.
Toxicology. 230:64–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Werner M, Costa MJ, Mitchell LG and Nayar
R: Nephrotoxicity of xenobiotics. Clin Chim Acta. 237:107–154.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang M and Dong Z: Regulation and
pathological role of p53 in cisplatin nephrotoxicity. J Pharmacol
Exp Ther. 327:300–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Clark JS, Faisal A, Baliga R, Nagamine Y
and Arany I: Cisplatin induces apoptosis through the ERK-p66shc
pathway in renal proximal tubule cells. Cancer Lett. 297:165–170.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ramesh G and Reeves WB: TNF-alpha mediates
chemokine and cytokine expression and renal injury in cisplatin
nephrotoxicity. J Clin Invest. 110:835–842. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang B, Ramesh G, Norbury CC and Reeves
WB: Cisplatin-induced nephrotoxicity is mediated by tumor necrosis
factor-alpha produced by renal parenchymal cells. Kidney Int.
72:37–44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhuang S and Schnellmann RG: A
death-promoting role for extracellular signal-regulated kinase. J
Pharmacol Exp Ther. 319:991–997. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morigi M, Imberti B, Zoja C, et al:
Mesenchymal stem cells are renotropic, helping to repair the kidney
and improve function in acute renal failure. J Am Soc Nephrol.
15:1794–1804. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morigi M, Rota C, Montemurro T, et al:
Life-sparing effect of human cord blood-mesenchymal stem cells in
experimental acute kidney injury. Stem Cells. 28:513–522.
2010.PubMed/NCBI
|
|
13
|
Peng X, Xu H, Zhou Y, et al: Human
umbilical cord mesenchymal stem cells attenuate cisplatin-induced
acute and chronic renal injury. Exp Biol Med (Maywood).
238:960–970. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Humphreys BD and Bonventre JV: Mesenchymal
stem cells in acute kidney injury. Annu Rev Med. 59:311–325. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kusaba T, Lalli M, Kramann R, Kobayashi A
and Humphreys BD: Differentiated kidney epithelial cells repair
injured proximal tubule. Proc Natl Acad Sci USA. 111:1527–1532.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maeshima A, Yamashita S and Nojima Y:
Identification of renal progenitor-like tubular cells that
participate in the regeneration processes of the kidney. J Am Soc
Nephrol. 14:3138–3146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tadagavadi RK and Reeves WB: Renal
dendritic cells ameliorate nephrotoxic acute kidney injury. J Am
Soc Nephrol. 21:53–63. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu XY, Urbieta-Caceres V, Krier JD,
Textor SC, Lerman A and Lerman LO: Mesenchymal stem cells and
endothelial progenitor cells decrease renal injury in experimental
swine renal artery stenosis through different mechanisms. Stem
Cells. 31:117–125. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altun B, Yilmaz R, Aki T, et al: Use of
mesenchymal stem cells and darbepoetin improve ischemia-induced
acute kidney injury outcomes. Am J Nephrol. 35:531–539. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bian X, Zhang B, Guo W, et al: Effects of
mesenchymal stem cells transplanted at different time points in a
rat remnant kidney model. Am J Nephrol. 39:75–84. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bussolati B, Tetta C and Camussi G:
Contribution of stem cells to kidney repair. Am J Nephrol.
28:813–822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kitamura S, Yamasaki Y, Kinomura M, Sugaya
T, Sugiyama H, Maeshima Y and Makino H: Establishment and
characterization of renal progenitor like cells from S3 segment of
nephron in rat adult kidney. FASEB J. 19:1789–1797. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim JH, Park DJ, Yun JC, et al: Human
adipose tissue-derived mesenchymal stem cells protect kidneys from
cisplatin nephrotoxicity in rats. Am J Physiol Renal Physiol.
302:F1141–F1150. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reinders ME and Rabelink TJ: Adipose
tissue-derived stem cells: can impure cell preparations give pure
results? Nephrol Dial Transplant. 25:3805–3807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brzoska M, Geiger H, Gauer S and Baer P:
Epithelial differentiation of human adipose tissue-derived adult
stem cells. Biochem Biophys Res Commun. 330:142–150. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang HC, Chang YJ, Chen WC, Harn HI, Tang
MJ and Wu CC: Enhancement of renal epithelial cell functions
through microfluidic-based coculture with adipose-derived stem
cells. Tissue Eng Part A. 19:2024–2034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Katsuno T, Ozaki T, Saka Y, et al: Low
serum cultured adipose tissue-derived stromal cells ameliorate
acute kidney injury in rats. Cell Transplant. 22:287–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shih YC, Lee PY, Cheng H, Tsai CH, Ma H
and Tarng DC: Adipose-derived stem cells exhibit antioxidative and
antiapoptotic properties to rescue ischemic acute kidney injury in
rats. Plast Reconstr Surg. 132:940e–951e. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shin S, Kim Y, Jeong S, Hong S, Kim I, Lee
W and Choi S: The therapeutic effect of human adult stem cells
derived from adipose tissue in endotoxemic rat model. Int J Med
Sci. 10:8–18. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang W, Wang W, Jiang Y, Li Z, et al:
Human adipose-derived stem cells modified by HIF-1α accelerate the
recovery of cisplatin-induced acute renal injury in vitro.
Biotechnol Lett. 36:667–676. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang L, Li K, Liu X, et al: Repeated
systemic administration of human adipose-derived stem cells
attenuates overt diabetic nephropathy in rats. Stem Cells Dev.
22:3074–3086. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuk PA, Zhu M, Ashjian P, et al: Human
adipose tissue is a source of multipotent stem cells. Mol Biol
Cell. 13:4279–4295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morigi M, Introna M, Imberti B, et al:
Human bone marrow mesenchymal stem cells accelerate recovery of
acute renal injury and prolong survival in mice. Stem Cells.
26:2075–2082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qi S and Wu D: Bone marrow-derived
mesenchymal stem cells protect against cisplatin-induced acute
kidney injury in rats by inhibiting cell apoptosis. Int J Mol Med.
32:1262–1272. 2013.PubMed/NCBI
|
|
35
|
Francescato HD, Costa RS, da Silva CG and
Coimbra TM: Treatment with a p38 MAPK inhibitor attenuates
cisplatin nephrotoxicity starting after the beginning of renal
damage. Life Sci. 84:590–597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Iwayama H and Ueda N: Role of
mitochondrial Bax, caspases, and MAPKs for ceramide-induced
apoptosis in renal proximal tubular cells. Mol Cell Biochem.
379:37–42. 2013. View Article : Google Scholar : PubMed/NCBI
|


















