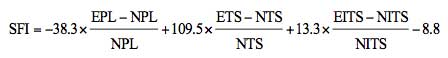Introduction
Peripheral nerve injuries are extremely common in
clinical practice. Despite a capacity for spontaneous axonal
regeneration, the regenerating axons struggle to overcome a long
gap into the distal stump to restore function without surgical
intervention. Consequently, individuals with long nerve gaps suffer
lifelong disabilities. Nerve auto graft implantation is presently
the most common repair strategy for long gaps and remains the
standard treatment in clinical settings (1). In addition, a number of
tissue-engineering nerve grafts (TENGs) have been approved for
clinical use in peripheral nerve repair (2); however, the outcomes of nerve
regeneration and functional recovery using either nerve autografts
or TENGs are generally partial and unsatisfactory (3–5).
Additional strategies are therefore necessary to favor nerve
regeneration and functional recovery across long gaps.
Pharmacotherapy is an important adjunctive treatment
for peripheral nerve injury. Several neuroprotective drugs have
been developed, which promote nerve regeneration experimentally and
clinically. Notably, numerous drugs exert neuroprotective effects
through antioxidant or anti-apoptotic mechanisms (6); therefore, substances that possess
antioxidant and anti-apoptotic properties have potential for
therapeutic applications following peripheral nerve injury.
In recent years, medical applications of hydrogen
have attracted considerable attention. Hydrogen efficiently reduces
levels of reactive oxygen (ROS) species in vitro and exerts
therapeutic antioxidant activity in vivo (7–9).
Additional studies have indicated that hydrogen exerts
neuroprotective effects through the suppression of oxidative stress
and the reduction of neuronal apoptosis in the central nervous
system (10–12). To the best of our knowledge, the
application of hydrogen for the treatment of peripheral nerve
injury has rarely been reported. The aim of the present study,
therefore, was to investigate the effectiveness of hydrogen-rich
saline on motor function recovery following the reconstruction of a
10-mm sciatic nerve gap, repaired with a nerve autograft. A
combination of behavioral analyses, electrophysiological
evaluations, Fluoro-Gold™ (FG) retrograde tracing and
histomorphological observations of the regenerated nerves and
gastrocnemius muscles were performed to assess the axonal
regeneration and functional recovery.
Materials and methods
Preparation of hydrogen-rich
saline
To prepare the hydrogen-rich saline, purified
hydrogen was dissolved into normal saline for 4 h under high
pressure (0.4 MPa), as previously described (13). Hydrogen was produced using a
hydrogen-generating apparatus (Shandong Saikesaisi Hydrogen Energy
Co., Ltd., Shandong, China). The hydrogen-saturated saline was
subsequently sterilized through exposure to 20 kGy 60Co
radiation and stored under atmospheric pressure at 4°C.
Animal groups and surgical
procedures
A total of 60 female Sprague Dawley rats (weighing
200–220 g), obtained from the Experimental Animal Center of the
Fourth Military Medical University (FMMU; Xi'an, China), were
anesthetized through an intraperitoneal injection of 2% sodium
pentobarbital solution (40 mg/kg body weight). Under aseptic
conditions, the right sciatic nerve was exposed using a gluteal
muscle-splitting incision. A 10-mm segment of the sciatic nerve was
excised and reversed under a surgical microscope to bridge the
nerve gap. The wound was closed with 6–0 stitches, and the rats
were transferred back to the animal room under standard housing
conditions. All rats were subsequently administered an
intraperitoneal injection of 5 ml/kg hydrogen saline (experimental
group) or an equal volume of normal saline (control group) daily.
All experimental procedures were approved by the Ethics Committee
of the FMMU.
Behavioral analysis
Motor function recovery of the sciatic nerve was
evaluated using the sciatic function index (SFI) on the rats at 4,
8 and 12 weeks after surgery according to the method previously
described (14,15). Briefly, the rats (n=10 in each group
at each time-point) were trained preoperatively to walk down a
narrow wooden track (80 cm long and 7 cm wide) into a darkened goal
box. Following surgery, all rats were directed to walk down the
wooden track again, and the hind paws of each animal were painted
with red dye. Pawprints were recorded on blank paper. The distance
from the heel to the top of the third toe (PL), the distance
between the second and the fourth toe (ITS) and the distance
between the first and the fifth toe (TS) were measured. E
represents the experimental foot, and N represents the
contralateral normal foot. The SFI was measured according to the
following equation:
Electrophysiological evaluation
Subsequent to behavioral analyses, the rats (n=6)
were anesthetized and subjected to electrophysiological tests
(16). The sciatic nerve was exposed
under a surgical microscope and insulated from the surrounding
muscles with a rubber dam. To record the compound muscle action
potentials (CMAPs), the bipolar stimulating electrode (Zhongshi
Dichuang Science and Technology Development Co., Ltd., Beijing,
China) was placed at the proximal portion of the sciatic nerve, and
the recording electrode was placed in the gastrocnemius belly. For
quantitative analysis, the CMAP peak amplitude, the CMAP latency of
onset and the nerve conduction velocity (NCV) were calculated.
Histomorphological analysis
At 4, 8 and 12 weeks after surgery, the regenerated
nerve of the affected leg was harvested under anesthesia. The nerve
samples (n=6) were fixed with 3% glutaraldehyde, postfixed in 1%
osmium tetroxide in 0.1 M sodium cacodylate buffer and subsequently
dehydrated and embedded in resin according to a standard protocol.
From the distal portion of the sample, 1-µm transverse semi-thin
sections were cut, stained with a 1% toluidine blue/1% borax
solution and analyzed with a light microscope. In addition, 50-nm
ultrathin sections, stained with uranyl acetate/lead citrate, were
prepared and analyzed with a transmission electron microscope. The
following parameters were measured: Total number of myelinated
axons, mean diameter of nerve fibers and degree of myelination
(G-ratio).
The gastrocnemius muscles of the operated and
contralateral control sides (n=6) were harvested at 12 weeks after
surgery, fixed in buffered 4% paraformaldehyde and subjected to
hematoxylin and eosin staining. Images were subsequently captured
under a light microscope (AH3; Olympus Corp., Tokyo, Japan). For
each sample, the cross-sectional area of the muscle fibers was
measured with images captured of 4 random fields and analyzed with
the Leica software package (Leica Microsystems GmbH, Wetzlar,
Germany) (17). The average
percentage of muscle fiber area (Pm) was
determined according to the following equation:
Pm=Am/At×100%,
where Am represents the muscle fiber area in each
field (magnification, ×200), and At represents
the total area in the same field.
Retrograde tracing with FG
Retrograde tracing was performed as previously
described (18). Briefly, the
sciatic nerve of the operated leg (n=4) was exposed at 4, 8 and 12
weeks after surgery, and 5 µl 3% FG dye (Biotium, Hayward, CA, USA)
solution was intraneurally injected into the nerve trunk at a site
2 mm proximal to the bifurcation. The incision was sutured and all
rats were returned to their cages. After 5 days, the rats were
anesthetized and perfused intracardially with paraformaldehyde. The
L4, L5 and L6 segments of the lumbar spinal cord, together with the
dorsal root ganglia (DRG), were harvested. The samples were
subsequently prepared according to a standard protocol and
sectioned on a cryostat. Transverse sections of 25-µm thickness
were obtained from the spinal cord samples, and 16-µm longitudinal
sections of the DRG were mounted on glass slides and analyzed with
a fluorescence microscope (BX-60; Olympus Corp.). The numbers of
back-labeled motoneurons and sensory neurons were counted.
Data analysis
Data are expressed as the mean ± standard error of
the mean and analyzed using one-way analysis of variance with the
SPSS 13.0 software package (SPSS Inc., Chicago, IL, USA).
Statistically significant overall differences among the groups were
further analyzed through pair-wise comparisons with Tukey's post
hoc test. The differences were considered statistically significant
at P<0.05.
Results
Hydrogen-rich saline enhances axonal
regeneration
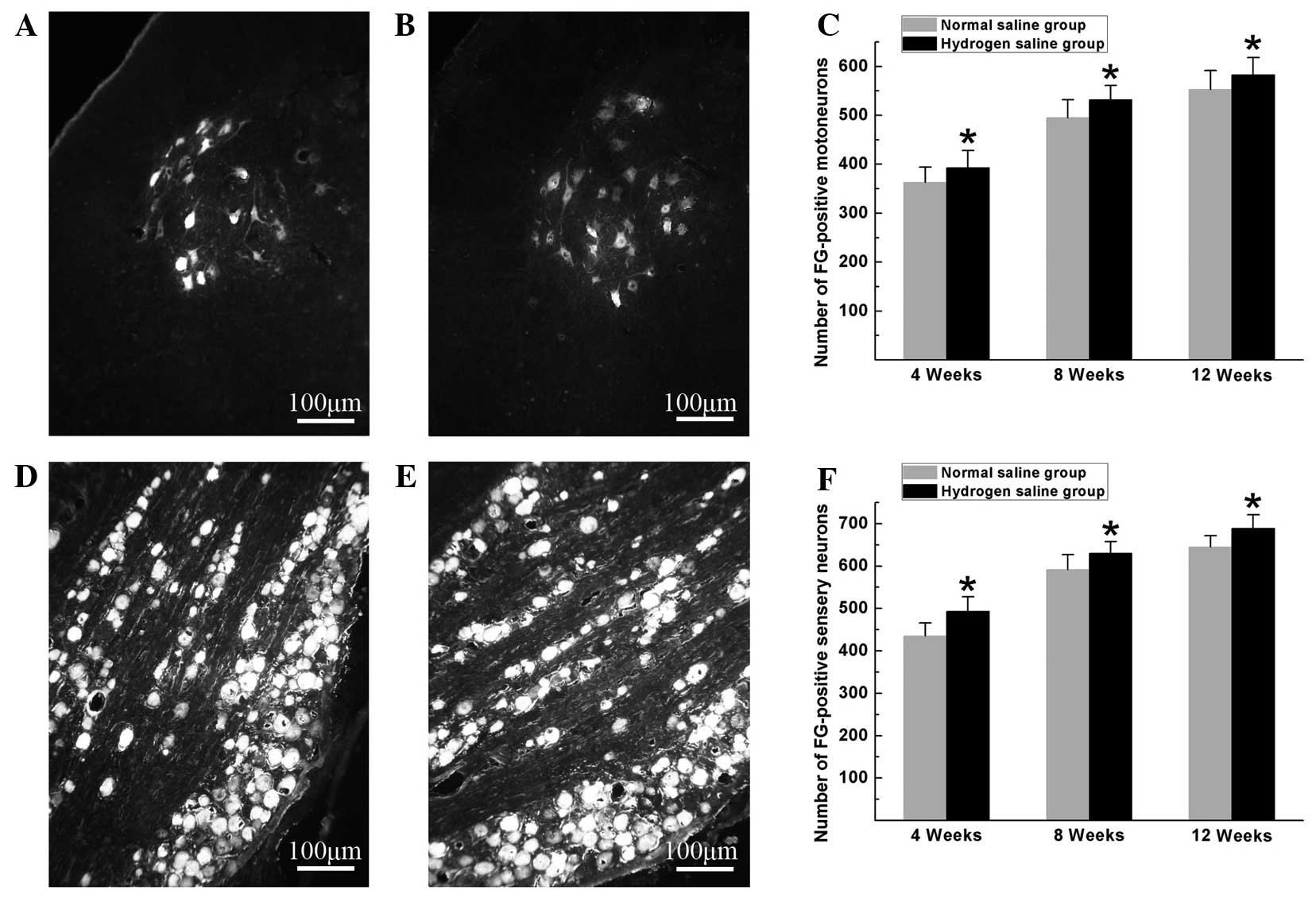
Hydrogen-rich saline exerted beneficial effects on
neuron survival and axonal outgrowth. At the pre-indicated
time-points after surgery, FG-positive motoneurons and sensory
neurons were counted to identify the number of surviving neurons
regenerated through the graft into the distal stump. As shown in
Fig. 1, the numbers of FG-positive
motoneurons and sensory neurons in the hydrogen saline group were
significantly higher than those in the normal saline group
(P<0.05), indicating that more neurons were maintained under
viable conditions and more nerve fibers were generated and
successfully regenerated through the graft into the distal stump in
the hydrogen saline group.
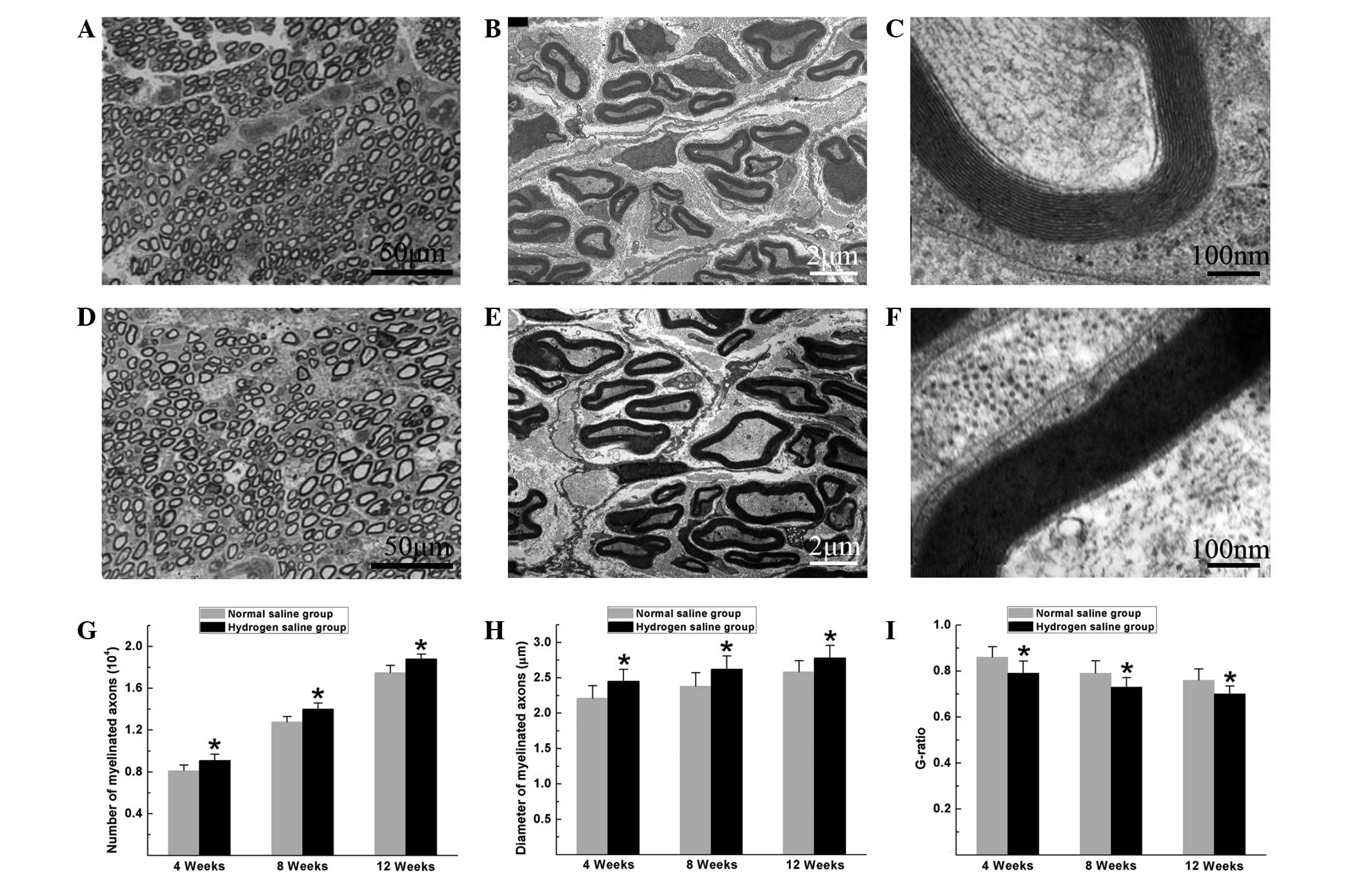
Histomorphological observation of the regenerated
nerves showed that large myelinated axons were present with an even
distribution in both groups. In the hydrogen saline group, the
total number of myelinated axons, the diameter of the regenerated
axons and the degree of myelination were significantly higher than
those in the normal saline group at the pre-indicated time-points
after surgery (P<0.05) (Fig. 2),
suggesting that hydrogen saline enhanced axonal regeneration and
remyelination.
Hydrogen-rich saline promotes the
recovery of motor function
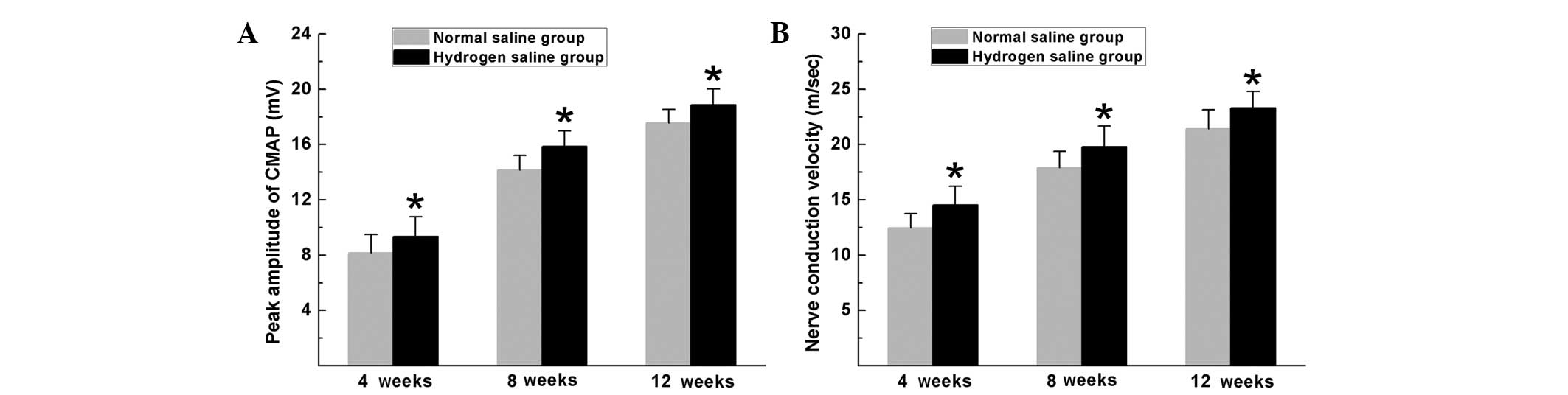
Electrophysiological tests were performed to
evaluate the effects of hydrogen saline on functional recovery. The
rats in the hydrogen saline group achieved improved functional
recovery, with a higher CMAP peak amplitude and NCV than those in
the normal saline group at 4, 8 and 12 weeks after surgery
(P<0.05, Fig. 3).
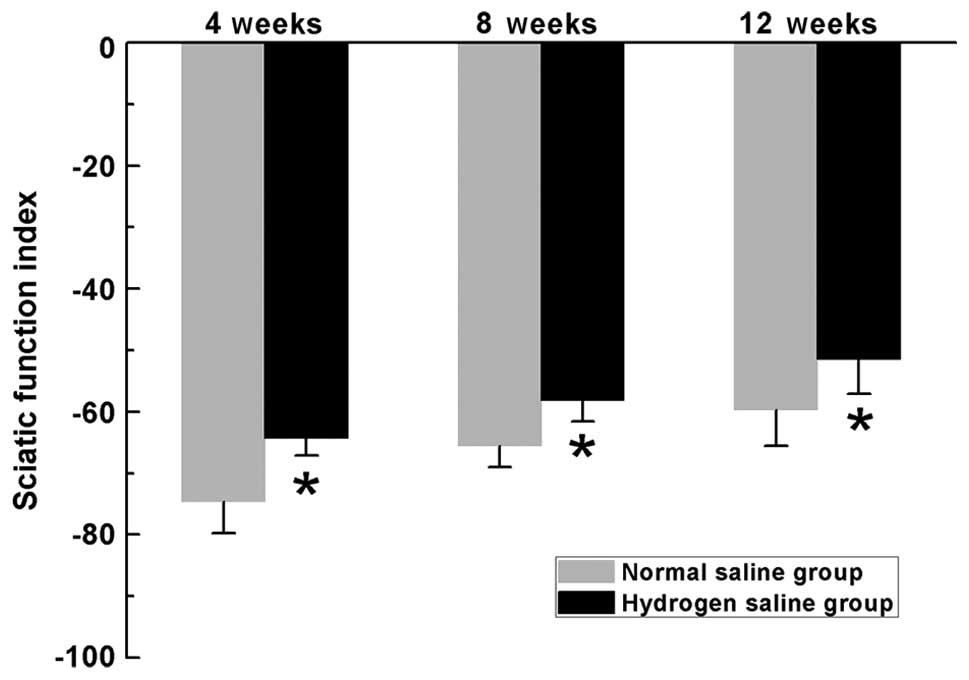
SFI is commonly used to evaluate the recovery of
motor function in rat models of sciatic nerve injury. In the
current study, the SFI measurements exhibited progressive increases
in both groups over time, indicating motor function improvement
following surgery. In the hydrogen saline group, the SFI scores
were significantly higher than those in the normal saline group at
the pre-indicated time-points (P<0.05) (Fig. 4), indicating improved recovery of
motor function in rats receiving hydrogen saline compared with rats
receiving normal saline during all evaluated periods.
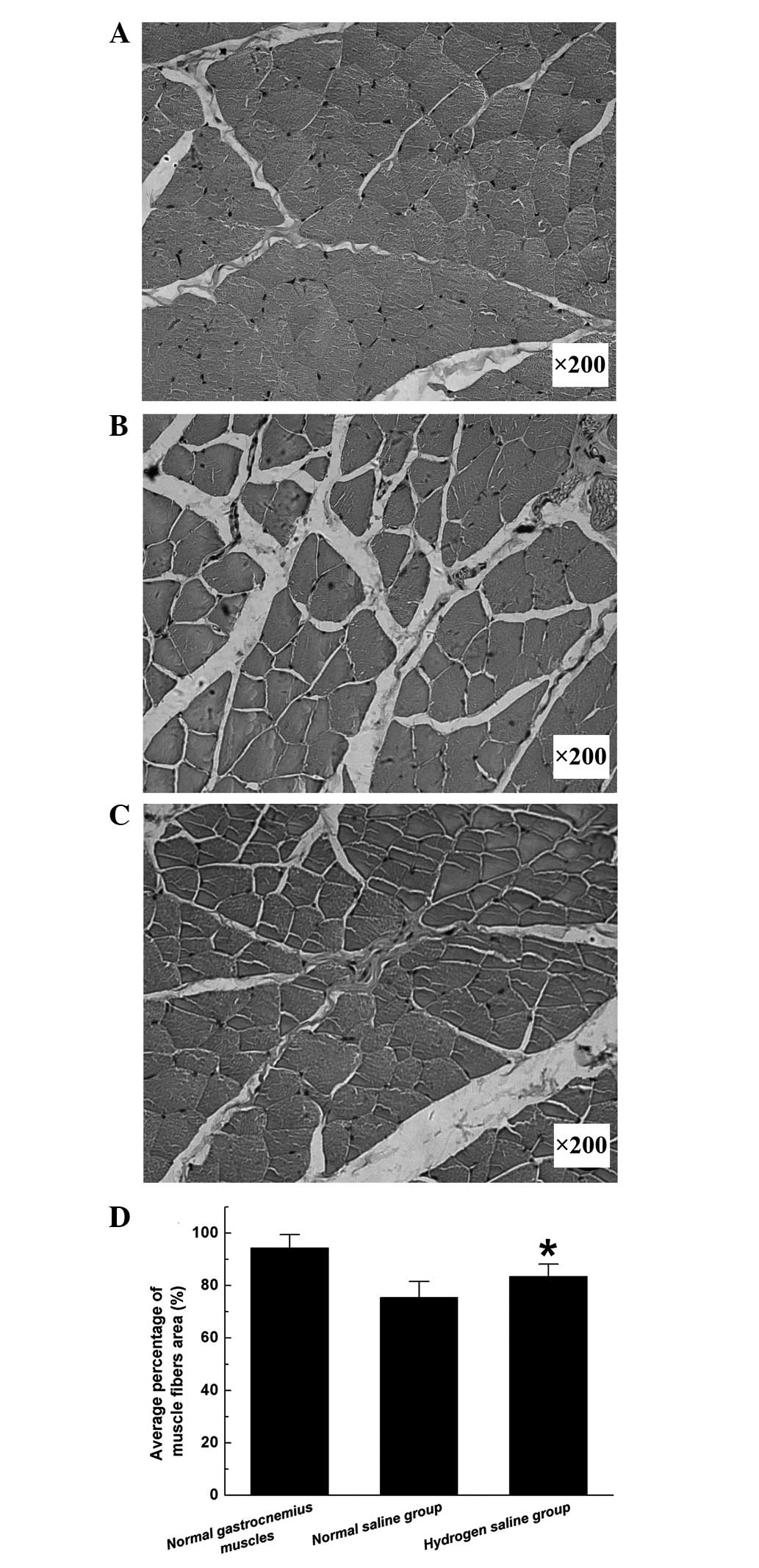
Pm reflects the extent of
gastrocnemius muscle atrophy as an index of motor function
recovery. Morphometric analysis revealed that the
Pm in the hydrogen saline group was significantly
higher than that in the normal saline group (Fig. 5), indicating that the atrophy of the
gastrocnemius muscles was partially alleviated in the hydrogen
saline group.
Discussion
In the current study, the effect of hydrogen saline
on axonal regeneration and functional recovery following
reconstructive surgery to repair a 10-mm nerve gap in rats was
investigated. The motor function evaluation, including the SFI
scores, target muscle atrophy changes and CMAP parameters, and the
histomorphological observations of the regenerated nerves and FG
retrograde tracing revealed that hydrogen saline improved
peripheral nerve regeneration with significant functional recovery,
suggesting that hydrogen-rich saline could be used for peripheral
nerve injury therapy.
In contrast with the central nervous system, the
peripheral nervous system has the potential to spontaneously
regenerate following injury. Regeneration requires
well-orchestrated interactions between neurons and Schwann cells.
In large nerve gaps, however, regenerating axons can take
considerable time to penetrate across grafts and reach distal nerve
stumps. During this process, numerous apoptotic signaling pathways
are activated within the axotomized neurons, resulting in a loss of
neurons and limiting the efficiency of nerve repair (19–21).
Oxidative stress is commonly generated following peripheral nerve
injury and is reported to be a major activator of caspase activity,
thereby promoting apoptosis (22–24).
Thus, the use of antioxidants to alleviate oxidative stress may
protect axotomized neurons from apoptosis and improve nerve
regeneration. Hydrogen efficiently reduces the levels of ROS in
vitro and exerts therapeutic antioxidant activity in
vivo (7–9). Previous studies have indicated that
hydrogen has the potential to exert neuroprotective effects through
the suppression of oxidative stress and the reduction of neuronal
apoptosis in the central nervous system (10–12). In
the present study, an increased number of FG-positive neurons was
observed within the spinal cord and DRG in the hydrogen saline
group, suggesting that hydrogen exerted anti-apoptotic actions
through the suppression of oxidative stress, thereby decreasing the
number of apoptotic cells and maintaining more viable neurons. In
addition, more nerve fibers were observed in the hydrogen saline
group, suggesting that more nerve fibers were generated by the
surviving neurons and regenerated into distal stumps. These
observations indicated that hydrogen saline is beneficial to
neuronal survival and axonal outgrowth following nerve injury;
however, the exact mechanism underlying the neuroprotection of
hydrogen-rich saline on peripheral nerve regeneration remains
largely unknown and requires investigation in further studies.
Electrophysiological properties provide objective
and reliable indices for the evaluation of nerve regeneration and
motor function recovery, and histomorphological evidence of
regenerated nerves provides the structural basis for
electrophysiological performance. The NCV depends on the diameter
of the axons and the thickness of the myelin sheath, while the
amplitude of the CMAP is associated with the number of axons that
achieve and reinnervate target muscles (25–27). In
the present study, the larger mean diameter and thicker myelin
sheath of the myelinated axons may have contributed to the
increased NCV in rats receiving hydrogen-rich saline. In addition,
the higher number of myelinated axons may have contributed to the
higher amplitude of the CMAP, while the latter was concomitant with
the improved histological appearance of gastrocnemius muscles in
rats treated with hydrogen saline. These results suggest that
hydrogen saline benefits axon remyelination and the reinnervation
of target muscles. In peripheral nerves, the myelin sheath is
formed by Schwann cells, and its thickness is regulated through
neuregulin-ErbB signaling within these cells (28). It is likely that the administration
of hydrogen-rich saline positively affected neuregulin-ErbB2
signaling within Schwann cells and contributed to the formation of
a thicker myelin sheath in the hydrogen-rich saline group; however,
additional studies are required to confirm this idea.
In combination, the results of the present study
have shown the alleviation of peripheral nerve injury by
hydrogen-rich saline. Axonal regeneration and functional recovery
were assessed though a combination of behavioral and
electrophysiological analyses, FG retrograde tracing and
morphometric analyses of the regenerated nerves and target muscles.
The encouraging outcomes indicated the potential application of
hydrogen-rich saline for nerve regeneration therapies; however, it
should be pointed out that further studies are required to
investigate the beneficial effects of hydrogen-rich saline on
peripheral nerve regeneration. In addition, it is unclear whether
the protective effect of hydrogen-rich saline on nerve injury is
dose-dependent. Furthermore, the optimal dose of hydrogen-rich
saline and the duration of use for promoting nerve regeneration and
functional recovery have yet to be identified. Additional studies
are required to address these issues.
Acknowledgements
This work was supported by the Nature Science
Foundation of China (No. 81360408) and the Nature Science
Foundation of Fujian province (No. 2015J05166). The authors would
like to thank Ms. Ming-Ya Zhu and Dr Yang Li for their excellent
technical assistance.
References
|
1
|
Alluin O, Wittmann C, Marqueste T, et al:
Functional recovery after peripheral nerve injury and implantation
of a collagen guide. Biomaterials. 30:363–373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kehoe S, Zhang XF and Boyd D: FDA approved
guidance conduits and wraps for peripheral nerve injury: A review
of materials and efficacy. Injury. 43:553–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang X, Xue C, Wang Y, Ding F, Yang Y and
Gu X: Bridging peripheral nerve defects with a tissue engineered
nerve graft composed of an in vitro cultured nerve equivalent and a
silk fibroin-based scaffold. Biomaterials. 33:3860–3867. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cui Q, Zhang J, Zhang L, Li R and Liu H:
Angelica injection improves functional recovery and motoneuron
maintenance with increased expression of brain derived neurotrophic
factor and nerve growth factor. Curr Neurovasc Res. 6:117–123.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nie X, Zhang YJ, Tian WD, et al:
Improvement of peripheral nerve regeneration by a tissue-engineered
nerve filled with ectomesenchymal stem cells. Int J Oral Maxillofac
Surg. 36:32–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hart AM, Terenghi G and Wiberg M: Neuronal
death after peripheral nerve injury and experimental strategies for
neuroprotection. Neurol Res. 30:999–1011. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ohsawa I, Ishikawa M, Takahashi K, et al:
Hydrogen acts as a therapeutic antioxidant by selectively reducing
cytotoxic oxygen radicals. Nat Med. 13:688–694. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sato Y, Kajiyama S, Amano A, et al:
Hydrogen-rich pure water prevents superoxide formation in brain
slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem
Biophys Res Commun. 375:346–350. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai J, Kang Z, Liu WW, et al: Hydrogen
therapy reduces apoptosis in neonatal hypoxia-ischemia rat model.
Neurosci Lett. 441:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen C, Chen Q, Mao Y, et al:
Hydrogen-rich saline protects against spinal cord injury in rats.
Neurochem Res. 35:1111–1118. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yonamine R, Satoh Y, Kodama M, Araki Y and
Kazama T: Coadministration of hydrogen gas as part of the carrier
gas mixture suppresses neuronal apoptosis and subsequent behavioral
deficits caused by neonatal exposure to sevoflurane in mice.
Anesthesiology. 118:105–113. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Q, Kang Z, Cai J, et al: Hydrogen-rich
saline protects myocardium against ischemia/reperfusion injury in
rats. Exp Biol Med (Maywood). 234:1212–1219. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de Medinaceli L, Freed WJ and Wyatt RJ: An
index of the functional condition of rat sciatic nerve based on
measurements made from walking tracks. Exp Neurol. 77:634–643.
1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hare GM, Evans PJ, Mackinnon SE, et al:
Walking track analysis: A long-term assessment of peripheral nerve
recovery. Plast Reconstr Surg. 89:251–258. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki Y, Tanihara M, Ohnishi K, Suzuki K,
Endo K and Nishimura Y: Cat peripheral nerve regeneration across 50
mm gap repaired with a novel nerve guide composed of freeze-dried
alginate gel. Neurosci Lett. 259:75–78. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang J, Lu L, Hu X, et al: Electrical
stimulation accelerates motor functional recovery in the rat model
of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally
oriented microchannels. Neurorehabil Neural Repair. 24:736–745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Novikova L, Novikov L and Kellerth JO:
Persistent neuronal labeling by retrograde fluorescent tracers: A
comparison between Fast Blue, Fluoro-Gold and various dextran
conjugates. J Neurosci Methods. 74:9–15. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu SY and Gordon T: The cellular and
molecular basis of peripheral nerve regeneration. Mol Neurobiol.
14:67–116. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nicholson DW and Thornberry NA: Caspases:
Killer proteases. Trends Biochem Sci. 22:299–306. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vukosavic S, Dubois-Dauphin M, Romero N
and Przedborski S: Bax and Bcl-2 interaction in a transgenic mouse
model of familial amyotrophic lateral sclerosis. J Neurochem.
73:2460–2468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Naik AK, Tandan SK, Dudhgaonkar SP, et al:
Role of oxidative stress in pathophysiology of peripheral
neuropathy and modulation by N-acetyl-L-cysteine in rats. Eur J
Pain. 10:573–579. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Senoglu M, Nacitarhan V, Kurutas EB,
Senoglu N, Altun I, Atli Y and Ozbag D: Intraperitoneal
alpha-lipoic acid to prevent neural damage after crush injury to
the rat sciatic nerve. J Brachial Plex Peripher Nerve Inj.
4:222009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Formichi P, Battisti C, Radi E, Di Maio G
and Federico A: Apoptosis, oxidative stress and neurological
disease. J Siena Acad Sci. 1:40–45. 2009. View
Article : Google Scholar
|
|
25
|
Bromberg MB: Quantitative
ElectromyographyElectrodiagnosis in Clinical Neurology. Aminoff MJ:
4th. Churchill Livingstone; New York, NY: pp. 257–263. 1999
|
|
26
|
Rosen JM, Pham HN and Hentz VR: Fascicular
tubulization: A comparison of experimental nerve repair techniques
in the cat. Ann Plast Surg. 22:467–478. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsumoto K, Ohnishi K, Kiyotani T, et al:
Peripheral nerve regeneration across an 80-mm gap bridged by a
polyglycolic acid (PGA)-collagen tube filled with laminin-coated
collagen fibers: A histological and electrophysiological evaluation
of regenerated nerves. Brain Res. 868:315–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sherman DL and Brophy PJ: Mechanisms of
axon ensheathment and myelin growth. Nat Rev Neurosci. 6:683–690.
2005. View
Article : Google Scholar : PubMed/NCBI
|