Introduction
In patients with hematological malignancies, the
spleen is a common site of involvement (1,2). The
incidence of metastasis to the spleen in patients with lung cancer
has been reported to be a few percent to 21% when investigated by
autopsy (3,4). By contrast, however, reports of spleen
metastasis from lung cancer either at the time of initial diagnosis
or during the clinical course are extremely rare (5). In the present study, the case of a
patient with lung adenocarcinoma who developed isolated metastasis
to the spleen following surgery is described. A review of this rare
metastasis in patients with lung cancer was also conducted.
Case report
A 63-year-old woman presented with the incidental
detection of a nodule 30 mm in diameter in the left lung on chest
radiography, at the Mito Medical Center of the University of
Tsukuba (Mito, Japan). At the age of 57 years, the patient had
undergone a left mastectomy due to breast cancer; however the
patient was asymptomatic and had been in good health since then.
The physical examination was unremarkable. The chest computed
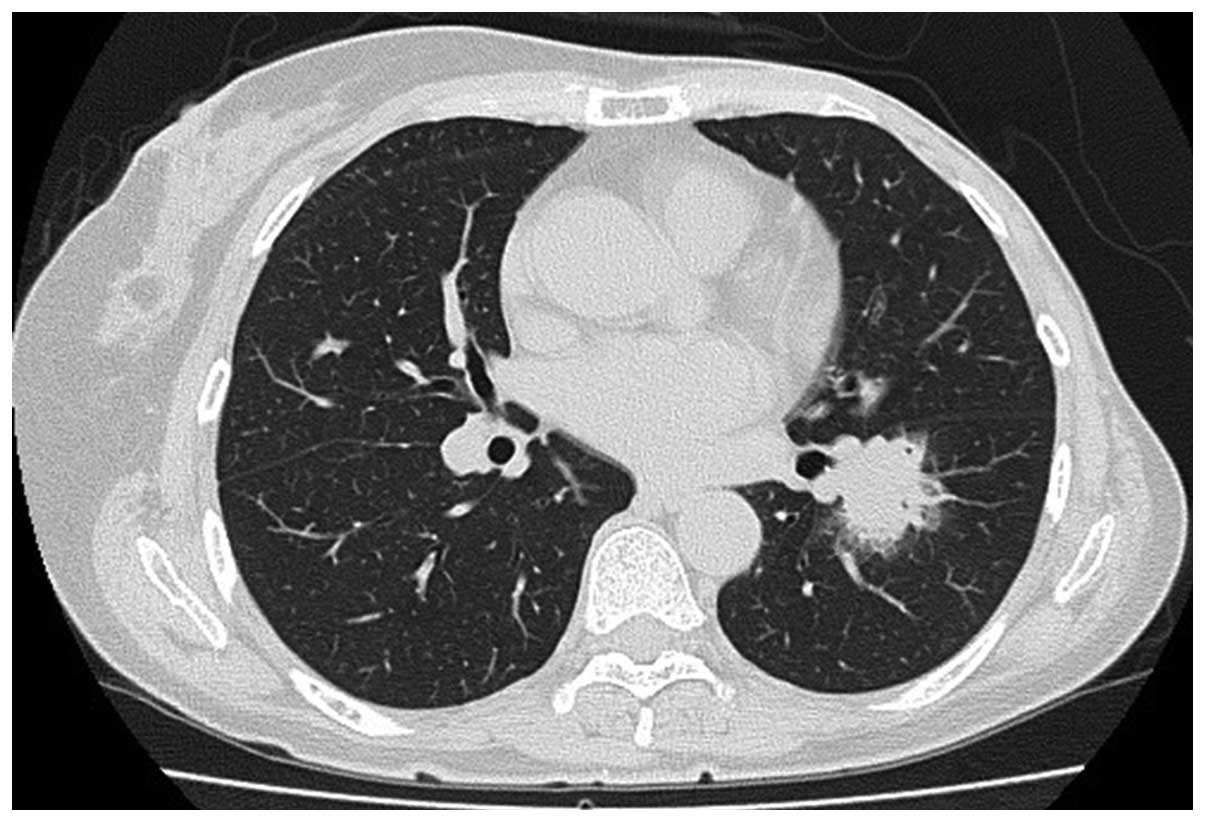
tomography (CT) scan revealed a well-circumscribed mass in the left
lower lobe of the lung that measured 30×27×18 mm with specular
appearance (Fig. 1). The routine
laboratory tests were normal, as were tumor markers including
carcinoembryonic antigen. The patient was diagnosed with
adenocarcinoma on the basis of pathological examination of
transbrochial biopsy specimens. Distant metastasis was not
detected. Informed consent was obtained, and the patient underwent
lobectomy of the left lower lung and mediastinal lymph node
dissection. The final pathological diagnosis was lung
adenocarcinoma staged as pT3N2M0, stage IIIB. An EGFR exon 19
deletion was identified. Soon after the surgical resection, the
patient received adjuvant postoperative chemotherapy. However, 12
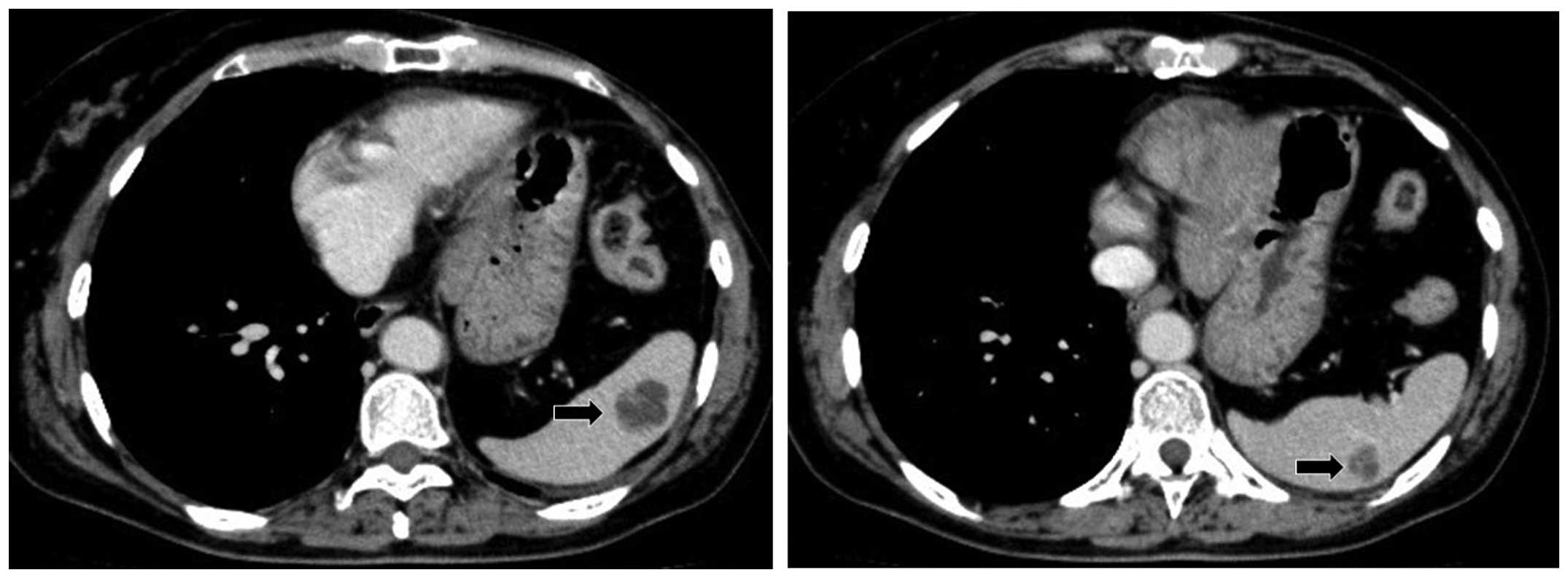
months after the surgery, a follow-up abdominal CT scan revealed
three nodular lesions of ≤10 mm in diameter in the spleen (Fig. 2). No metastatic lesions in the liver,
adrenal gland and abdominal lymph nodes were found. The splenic
nodular lesions were considered to indicate an inflammatory disease
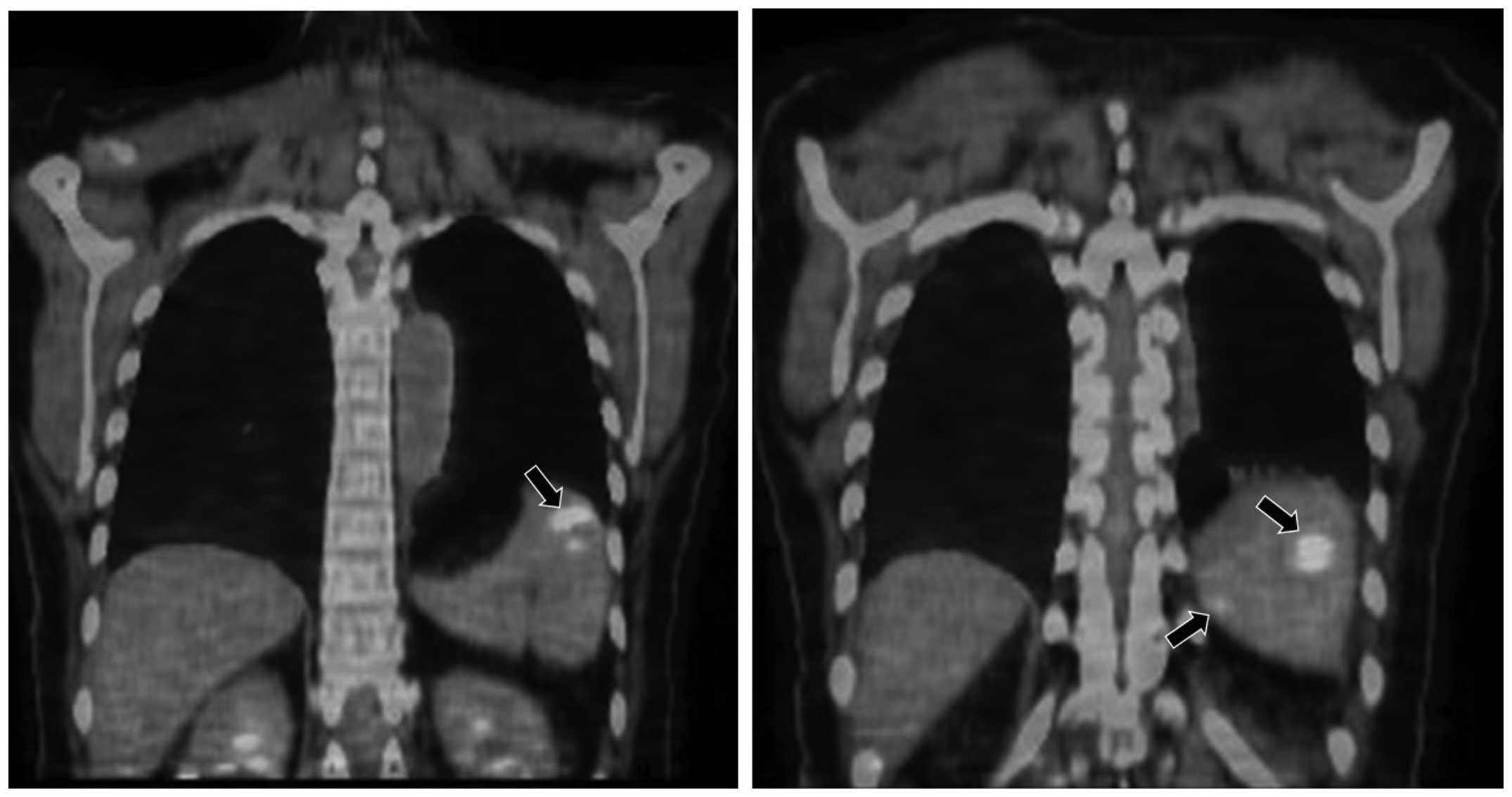
such as granuloma. However, fluorodeoxyglucose positron emission
tomography (FDG-PET)/CT confirmed uptake in these nodules in the
spleen, but there was no other metastatic sites (Fig. 3). The patient then underwent
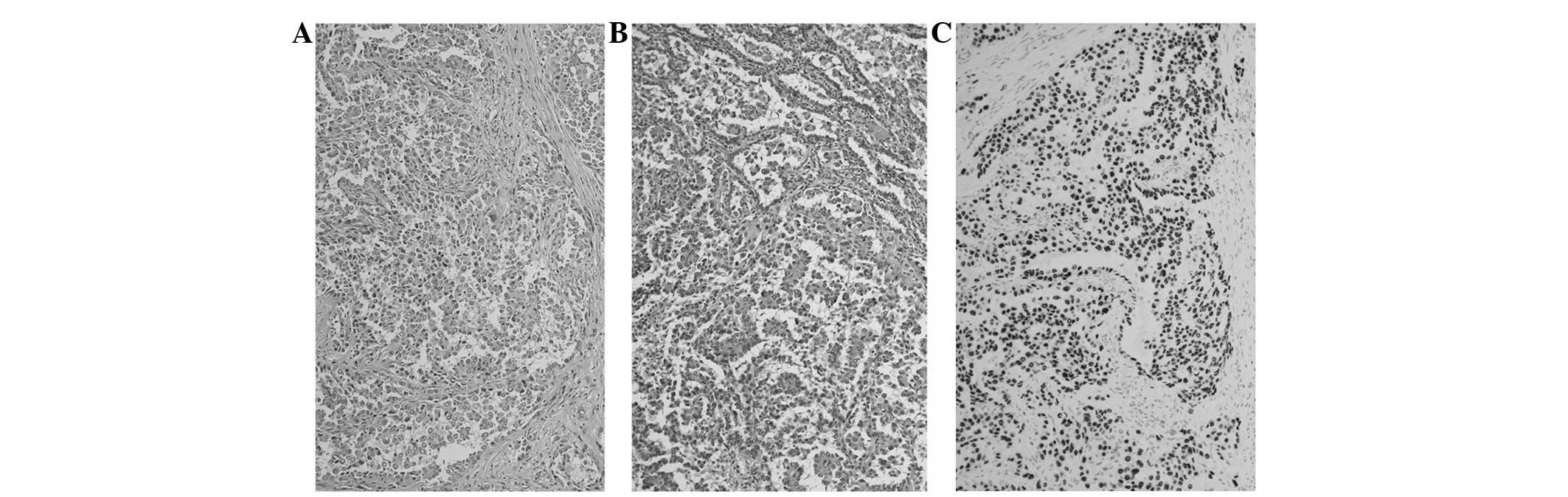
splenectomy. As the resected tumor had the same characteristics as
the specimen of the primary lung carcinoma and was positively
immunostained for thyroid transcription factor-1 and cytokeratin 7,
the tumor was pathologically diagnosed as spleen metastasis from
lung adenocarcinoma (Fig. 4).
Thereafter, the patient received treatment with four courses of
cisplatin and vinorelbine, and was well without any signs of tumor
progression.
Discussion
Although numerous cases of abdominal visceral
metastases from lung cancer have been reported (6,7),
metastasis to the spleen from lung cancer is considered to be rare
and is usually detected only at autopsy (8,9). The
early detection of metastasis to the spleen is challenging since
the majority of patients are asymptomatic in the early stage of
such metastasis. However, as the availability of CT scanning and
ultrasonography has increased, this has enabled spleen metastasis
to be diagnosed more accurately (10–12). CT
scanning and ultrasonograpy can provide a quick, direct and
non-invasive evaluation of spleen metastasis; however, some
metastasis cannot be identified by ultrasonograpy because of the
effect of air in the stomach and colon. Therefore, abdominal CT
scans should be considered for use if spleen metastasis is
suspected. Several studies have reported the clinical significance
of FDG-PET/CT scanning in the detection of spleen metastasis from
lung cancer (13–17). In the present case, FDG-PET/CT was
useful for confirming the presence of malignant lesions in the
spleen.
We previously evaluated spleen metastasis from lung
cancer (18). In the study, 12
(1.2%) of 997 consecutive patients with lung cancer had spleen
metastasis, and isolated spleen metastasis in the absence of
widespread disease was considered an extremely rare event (18). All 12 cases had accompanying
metastasis to other organs, including the paraaortic lymph nodes,
bone, lung and liver, which also suggested a poor outcome for
patients with spleen metastasis (18). Moreover, it is noteworthy that all 12
patients exhibited more than one metastatic site in the abdomen.
The most common site of metastasis was the lymph nodes in the
abdomen. Therefore, it was concluded at that time that spleen mass
accompanying metastasis to other abdominal organs in patients with
a known lung cancer should be regarded as a metastasis. We recently
conducted a review of patients with lung cancer and spleen
metastasis, and found 20 such patients (10–15,17,19–30). In
these 20 patients, it was notable that all but 4 of the 20 patients
had no abdominal metastasis other than spleen metastasis.
Therefore, it should now be recognized that spleen metastasis is
not necessarily accompanied by abdominal organ metastasis. The
difference between our previous study (18) and the recent case reports might be
due to the development of imaging modalities and the advancement of
knowledge in such rare metastasis.
The incidence of spleen metastasis is considered to
differ among histologic types of lung cancer (3,4,18). Small cell lung cancer has been
recognized to be the most common cell type likely to metastasize to
the spleen and squamous cell lung carcinoma the least likely
(3,4). In our previous study of lung cancer
metastasis, adenocarcinoma had almost the same frequency of spleen
metastasis as small cell lung cancer (1.3 vs. 2.0%, respectively),
while in squamous cell carcinoma, only 0.6% of patients developed
spleen metastasis (18). When
reviewing recent case reports, however, 5 of the 20 patients with
lung cancer and spleen metastasis had squamous cell carcinoma
(12,21,27–29).
These results indicate that the spleen is a possible site to which
disease may spread in patients with advanced or recurrent lung
cancer, including those with squamous cell carcinoma of the
lung.
With regard to the radiological classification of
spleen metastasis dependent on pathological examination, there may
be four types, known as solitary, multiple, microscopic and
diffuse. Sardenberg et al presented three main macroscopic
patterns: Macronodular, micronodular and diffuse (17). The latter two types might not be
apparent in abdominal CT scans, although FDG-PET/CT scanning may
detect the diffuse type of spleen metastasis. In our previous
study, the solitary nodular type was the most common, and in a
review of recent case reports, the solitary type was confirmed as
the most common type (10,12–17,19–21,23,26). The
differential diagnosis of a solitary nodule or nodules in the
spleen includes several diseases, either benign or malignant
(31). In differential diagnosis,
FDG-PET/CT scanning can be useful to identify whether the lesion is
malignant (13–17).
A mass in the spleen accompanied by metastasis to
other abdominal organs in a patient known to have lung cancer or
with a history of lung cancer should be regarded as a metastasis.
In addition, an isolated splenic mass in the absence of abdominal
metastatic disease elsewhere should also be considered likely to
represent a metastasis. An isolated spleen mass identified at the
initial diagnosis or during the clinical course in a patient with
lung cancer should be considered for an interventional approach and
can provide pathological material.
References
|
1
|
Kraemer BB, Osborne BM and Butler JJ:
Primary splenic presentation of malignant lymphoma and related
disorders. A study of 49 cases. Cancer. 54:1606–1619. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharpe RW, Rector JT, Rushin JM, Garvin DF
and Cotelingam JD: Splenic metastasis in hairy cell leukemia.
Cancer. 71:2222–2226. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Auerbach O, Garfinkel L and Parks VR:
Histologic type of lung cancer in relation to smoking habits, year
of diagnosis and sites of metastases. Chest. 67:382–387. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Densert O and Söderström J: Diffuse
metastases in bronchial cancer. Acta Pathol Microbiol Scand.
64:477–484. 1965.PubMed/NCBI
|
|
5
|
Morgenstern L, Rosenberg J and Geller SA:
Tumors of the spleen. World J Surg. 9:468–476. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abrams HL, Spiro R and Goldstein N:
Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer.
3:74–85. 1950. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Warren S and Gates O: Lung cancer and
metastasis. Arch Pathol. 78:467–473. 1964.PubMed/NCBI
|
|
8
|
Hirst AE Jr and Bullock WK: Metastatic
carcinoma of the spleen. Am J Med Sci. 223:414–417. 1952.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Klein B, Stein M, Kuten A, et al:
Splenomegaly and solitary spleen metastasis in solid tumors.
Cancer. 60:100–102. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Edelman AS and Rotterdam H: Solitary
splenic metastasis of an adenocarcinoma of the lung. Am J Clin
Pathol. 94:326–328. 1990.PubMed/NCBI
|
|
11
|
Imada H, Nakata H and Horie A:
Radiological diagnosis of splenic metastasis and its prevalence at
autopsy. Nihon Igaku Hoshasen Gakkai Zasshi. 51:498–503. 1991.(In
Japanese). PubMed/NCBI
|
|
12
|
Kinoshita A, Nakano M, Fukuda M, et al:
Splenic metastasis from lung cancer. Neth J Med. 47:219–223. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yen RF, Wu YW, Pan MH and Tzen KY: Early
detection of splenic metastasis of lung cancer by
18F-2-fluoro-2-deoxyglucose positron emission
tomography. J Formos Med Assoc. 104:674–676. 2005.PubMed/NCBI
|
|
14
|
Fujii M, Tanaka H, Sawazumi T, et al: A
case of solitary splenic metastasis following operation for
pulmonary pleomorphic carcinoma: Detected at an early stage by
FDG-PET. Nihon Kokyuki Gakkai Zasshi. 46:950–954. 2008.(In
Japanese). PubMed/NCBI
|
|
15
|
Tang H, Huang H, Xiu Q and Shi Z: Isolated
splenic metastasis from lung cancer: Ringleader of continuous
fever. Eur Respir Rev. 19:253–256. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Soussan M, Pop G, Ouvrier MJ, Neuman A and
Weinmann P: Diagnosis of synchronous isolated splenic metastasis
from lung adenocarcinoma: Complementary role of FDG PET/CT and
diffusion-weighted MRI. Clin Nucl Med. 36:707–709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sardenberg RA, Pinto C, Bueno CA and
Younes RN: Non-small cell lung cancer stage IV long-term survival
with isolated spleen metastasis. Ann Thorac Surg. 95:1432–1434.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoh H, Watanabe K, Ishikawa H, Yamashita
YT, Ohtsuka M and Sekizawa K: Splenic metastasis of lung cancer.
Oncol Rep. 8:1239–1241. 2001.PubMed/NCBI
|
|
19
|
Scintu F, Carta M, Frau G, Marongiu L,
Pipia G and Casula G: Splenic metastases of pulmonary carcinoma.
Apropos of a clinical case. Minerva Chir. 46:1277–1280. 1991.(In
Italian). PubMed/NCBI
|
|
20
|
Tomaszewski D, Bereza S and Sternau A:
Solitary splenic metastases from lung cancer - one-time surgical
procedure. Pneumonol Alergol Pol. 71:533–537. 2003.(In Polish).
PubMed/NCBI
|
|
21
|
Pramesh CS, Prabhudesai SG, Parasnis AS,
Mistry RC and Sharma S: Isolated splenic metastasis from non small
cell lung cancer. Ann Thorac Cardiovasc Surg. 10:247–248.
2004.PubMed/NCBI
|
|
22
|
Lachachi F, Abita T, Durand Fontanier S,
Maisonnette F and Descottes B: Spontaneous splenic rupture due to
splenic metastasis of lung cancer. Ann Chir. 129:521–522. 2004.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmidt BJ and Smith SL: Isolated splenic
metastasis from primary lung adenocarcinoma. South Med J.
97:298–300. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Assouline P, Leger-Ravet MB, Paquet JC, et
al: Splenic metastasis from a bronchial carcinoma. Rev Mal Respir.
23:265–268. 2006.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sánchez-Romero A, Oliver I, Costa D, et
al: Giant splenic metastasis due to lung adenocarcinoma. Clin
Transl Oncol. 8:294–295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Hul I, Cools P and Rutsaert R:
Solitary splenic metastasis of an adenocarcinoma of the lung 2
years postoperatively. Acta Chir Belg. 108:462–463. 2008.PubMed/NCBI
|
|
27
|
Ando K, Kaneko N, Yi L, et al: Splenic
metastasis of lung cancer. Nihon Kokyuki Gakkai Zasshi. 47:581–584.
2009.(In Japanese). PubMed/NCBI
|
|
28
|
Chloros D, Bitzikas G, Kakoura M,
Chatzikostas G, Makridis C and Tsitouridis I: Solitary splenic
metastasis of squamous lung cancer: A case report. Cases J.
2:90912009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dias AR, Pinto RA, Ravanini JN, Lupinacci
RM, Cecconello I and Ribeiro U Jr: Isolated splenic metastasis from
lung squamous cell carcinoma. World J Surg Oncol. 10:242012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oussama B, Makrem M, Neji FM, et al: Non
small cell lung cancer revealed by a solitary splenic metastasis of
lung cancer. Tunis Med. 91:484–485. 2013.(In French). PubMed/NCBI
|
|
31
|
Compérat E, Bardier-Dupas A, Camparo P,
Capron F and Charlotte F: Splenic metastases: Clinicopathologic
presentation, differential diagnosis, and pathogenesis. Arch Pathol
Lab Med. 131:965–969. 2007.PubMed/NCBI
|