Introduction
The liver is a unique organ that is usually silent
under physiological conditions; however, the organ exhibits
regenerative properties following damage and/or parenchymal loss
(1). In previous years, hepatic
disease and the associated morbidity rates have increased year by
year, with the condition becoming a major global health care
problem (2). A previous study
demonstrated that reactive oxygen species (ROS) may be a cause of
liver damage, which is characterized by a progression from
steatosis to chronic hepatitis, cirrhosis and hepatocellular
carcinoma (3). Although studies have
attempted to identify efficient liver therapeutics from herbal
origins, a number of the potential candidates have not been well
characterized and require further investigation (4).
Over 50 years ago, Harman proposed the free radical
or oxidative stress theory of aging (5). The author hypothesized that free
radicals and/or ROS are produced endogenously from normal cellular
metabolic processes. In this theory, an imbalance between ROS and
antioxidants can lead to oxidative stress, which subsequently
damages various macromolecules. An increasing body of evidence has
demonstrated that an increased production of ROS plays an important
role in the development of various age-associated diseases
(6).
B cell-specific Moloney MLV insertion site-1 (Bmi-1)
belongs to the Polycomb group of genes, which are transcriptional
repressors that are essential for the maintenance of appropriate
gene expression patterns during development (7). The premature deletion of Bmi-1 produces
a typical osteoporotic phenotype, and
Bmi-1−/− mice have been shown to exhibit a
premature aging phenotype of the entire body, including the liver
(8). Furthermore, protection against
oxidative stress and apoptosis has emerged as an important
Bmi-1-downstream pathway, either through the reduction of P53
levels via Bmi-1-mediated repression of the INK4a/Arf locus or via
modulation of the oxidative stress response in an
INK4a/Arf-independent manner. In Bmi-1−/−
mice, an increase in ROS coincides with an increase in DNA damage,
and subsequently the activation of DNA damage repair pathways.
Treatment with N-acetylcysteine or the targeting of CHK2 has been
demonstrated to at least partially restore a number of the
phenotypes (9).
Pyrroloquinoline quinone (PQQ) was first identified
as a novel cofactor of ethanol and glucose dehydrogenase in
methylotrophic bacteria, whereas currently PQQ is considered as an
important nutritional growth factor (10,11).
PQQ, 4,5-dihydro-4,5-dioxo-1H-pyrrolo [2,3-f]
quinoline-2,7,9-tricarboxylic acid, is considered to be a bacterial
glucose dehydrogenase redox cofactor that is widely distributed in
plants, bacteria, animals, food and numerous biological fluids. PQQ
is soluble in water and thermally stable, and can be divided into
an oxidized and reduced form (12,13).
Previous studies have demonstrated that PQQ has
multiple physiological functions, including the promotion of growth
and reproduction (14–16), neural and cardiovascular protection
(17–19), and enhancing the learning and memory
function (20), immune function, and
antitumor effects (21). However,
the potential mechanisms underlying these effects remain poorly
understood. PQQ has also been reported to function as an
antioxidant and pro-oxidant to protect the mitochondria from
oxidative stress-induced damages (22–25). A
further study defined PQQ as an ROS scavenger in oxidative stress
(26).
In recent years, PQQ has become increasingly studied
with its role as an antioxidant in the scavenging of superoxide
radicals and the protection of the mitochondria from oxidative
stress-induced damage. However, the mechanism underlying the
effects of PQQ on Bmi-1−/− mice is yet to be
fully elucidated.
In the present study, Bmi-1 knockout (BKO) mice were
used to induce a typical liver senescent phenotype. The aim of the
study was to investigate whether PQQ was able to restore the
premature senescence induced by the deletion of Bmi-1 in the liver
through an antioxidative stress pathway.
Materials and methods
Mice and genotyping
Bmi-1 heterozygote (Bmi-1+/−)
mice (2 male and 6 female; average age, 6 weeks; average weight,
24.5 g) 129Ola/FVB/N hybrid background) were backcrossed 10–12
times to the C57BL/6 J background and mated to generate a Bmi-1
homozygote (Bmi-1−/−; 4 male and 8 female;
average age, 7 weeks; average weight, 4.5 g), and their wildtype
(WT) littermates were genotyped by polymerase chain reaction, as
described previously (8,27). One male mouse was paired with 2
female mice to produce newborn Bmi-1 knockout (BKO) and wild type
(WT) mice. Male and female mice from the same litter (littermates)
were paired with one another. All mice were obtained from the
Holland Cancer Institute. Mice were maintained in the Experimental
Animal Center of Nanjing Medical University (Nanjing, China). The
study was conducted in strict accordance with the guidelines of the
Institute for Laboratory Animal Research of Nanjing Medical
University, and the experimental protocols were approved by the
Committee on the Ethics of Animal Experiments of Nanjing Medical
University.
Animal treatment and PQQ supplementary
diet
Purified PQQ was provided by Professor Chunjun Wen
from the Academy of Life Sciences of Nanjing Normal University
(Nanjing, China). The PQQ-supplemented diet was produced by Beijing
KeAo Third Feed Co., Ltd. (Beijing, China). In vivo, the
animals were divided into three groups of six mice. The WT group
underwent 3-week weaning littermate WT mice and were fed a normal
diet for 4 weeks. Secondly, the BKO group underwent a 3-week
weaning littermate BKO mice and were fed a normal diet for 4 weeks.
Finally, the BKO + PQQ group underwent 3-week weaning littermate
BKO mice and were fed a PQQ-supplemented diet (4 mg PQQ/kg in the
normal diet) for 4 weeks (28).
After 7 weeks, the three groups of six mice were sacrificed by neck
dislocation under ether for further analysis.
Analysis of mice phenotype, percentage
survival rate and body weight
In order to investigate the effect of PQQ on the
whole body phenotype of the Bmi-1−/− mice,
the animals were divided into three groups of six mice (WT, BKO and
BKO + PQQ). Statistical analysis was conducted on the size, shape,
degree of hair smoothness, body weight and percentage survival in
the different groups of mice.
Histological examination
Liver tissues were collected and fixed in PLP
fixative (2% paraformaldehyde containing 0.075 mol/l lysine and
0.01 mol/l sodium periodate) overnight at 4°C, and processed as
described previously (9). All the
samples were dehydrated and embedded in paraffin, and sectioned at
a 5-µm thickness using an RM2235 rotary microtome (Leica
Microsystems, Inc., Buffalo Grove, IL, USA). The sections were
stained using standard hematoxylin-eosin (H&E). Stained tissue
sections were assessed for the detection of changes in the
magnitude of liver defects using an Olympus GX51 inverted light
microscope (Olympus Corporation, Tokyo, Japan).
Immunohistochemical staining
Immunohistochemical staining was conducted for
proliferating cell nuclear antigen (PCNA), γH2AX, P53 and
superoxide dismutase (SOD)2 using the avidin-biotin-peroxidase
complex technique with an affinity-purified goat anti-rabbit PCNA
antibody (1:400, ab18197; Abcam, Cambridge, UK), an
affinity-purified goat anti-mouse γH2AX antibody (1:200, sc-120804;
Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), an
affinity-purified goat anti-mouse P53 antibody (1:300, #2524; Cell
Signal Technology, Inc., Shanghai, China) and an affinity-purified
goat anti-rabbit SOD2 antibody (1:200, ab118340; Abcam), as
described previously (9). Briefly,
the dewaxed and rehydrated paraffin-embedded sections were
incubated with methanol-hydrogen peroxide (1:10) to block the
endogenous peroxidase activity and washed in Tris-buffered saline
(pH 7.6). The slides were subsequently incubated with the primary
antibodies overnight at room temperature. Following rinsing with
Tris-buffered saline for 15 min, the sections were incubated with a
biotinylated secondary antibody (1:100; Sigma-Aldrich, St. Louis,
MO, USA). Subsequently, the sections were washed and incubated with
the Vectastain Elite ABC reagent (Vector Laboratories, Inc.,
Burlington, ON, Canada) for 45 min. After washing, a brown
pigmentation was produced using 3,3-diaminobenzidine. Finally, the
stained sections were counterstained with H&E. Images were
acquired with a Leica microscope (Leica DM4000B; Leica Microsystems
GmbH, Wetzlar, Germany) using Image-Pro Plus, version 5.1 (Media
Cybernetics, Inc. Rockville, MD, USA.
Western blot analysis
Proteins were extracted from the liver and
quantitated using a kit, according to the manufacturer's
instructions (Bio-Rad Laboratories, Inc., Mississauga, ON, Canada).
Protein samples were fractionated by SDS-PAGE (1% agarose) and
transferred to nitrocellulose membranes (0.45 and 0.22 µm; Pall
Corporation, Port Washington, NY, USA). Western blot analysis was
performed as described previously (29), using antibodies against P16 (goat
anti-mouse; M-156; 1:400; sc-98520; Santa Cruz Biotechnology,
Inc.), P19 (goat anti-mouse; 1:400; sc-271566; Santa Cruz
Biotechnology, Inc.), P21 (goat anti-mouse; M19; 1:400; sc-271532;
Santa Cruz Biotechnology, Inc.), P27 (goat anti-mouse; 1:400;
#393380; Zymed Laboratories, Santa Cruz, CA, USA), P53 (goat
anti-mouse; 1:400, #2524; Cell Signaling Technology, Inc.),
glutathione (GSH; goat anti-mouse; 1:200; sc-292189; Santa Cruz
Biotechnology, Inc.), SOD1 and SOD2 (goat anti-rabbit; 1:200;
ab20926; Abcam), peroxiredoxin (PRDX) I (goat anti-rabbit; 1:400;
ab15571; Abcam), PRDX IV (goat anti-rabbit; 1:400; ab59542; Abcam)
and β-actin (goat anti-rabbit; 1:400; sc-130656; Santa Cruz
Biotechnology, Inc.). Bands were visualized using enhanced
chemiluminescence (GE Healthcare Life Sciences, Shanghai, China),
and quantitated using Scion Image Beta 4.02 software (Scion
Corporation, Bethesda, MD, USA).
Detection of ROS levels
Liver tissue samples were converted into single cell
suspensions containing 5×105 cells/ml. Subsequently,
2′,7′-dichlorofluorescein diacetate (DCFH-DA; Sigma-Aldrich) was
used for the detection of intracellular ROS. The fluorescence
intensity was proportional to the oxidant production (30). DCFH-DA was added to the liver cell
suspensions to yield a final concentration of 20 µmol/l. Next, the
cells were incubated at 37°C for 30 min in the dark, washed twice
with 0.01 mol/l phosphate-buffered saline, and centrifuged at 300 ×
g for 5 min. The ROS levels were measured according to the mean
fluorescence intensity of 10,000 cells using a flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Computer-assisted image analysis
Following H&E staining or histochemical or
immunohistochemical staining of the sections from the three groups
of six mice each, images of the interested fields were photographed
with a SONY digital camera (Sony Corporation, Tokyo, Japan). Images
of the micrographs from a single section were digitally recorded
using a rectangular template, and recordings were processed and
analyzed using Northern Eclipse image analysis software (Empix
Imaging, Inc., Mississauga, ON, Canada), as described previously
(9).
Statistical analysis
Data are expressed as the mean ± standard error of
the mean. Data were analyzed using SPSS software, version 11.5
(SPSS, Inc., Chicago, IL, USA). Statistical analyses of the
numeration data were performed using the χ2 test, while
statistical analyses of the measurement data were performed with
the Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of PQQ on the premature aging
phenotype in Bmi-1−/− mice
To investigate whether a PQQ-supplemented diet was
able to rescue the premature aging phenotype of
Bmi-1−/− mice, statistical analysis of the
phenotype, body weight and percentage survival was performed for
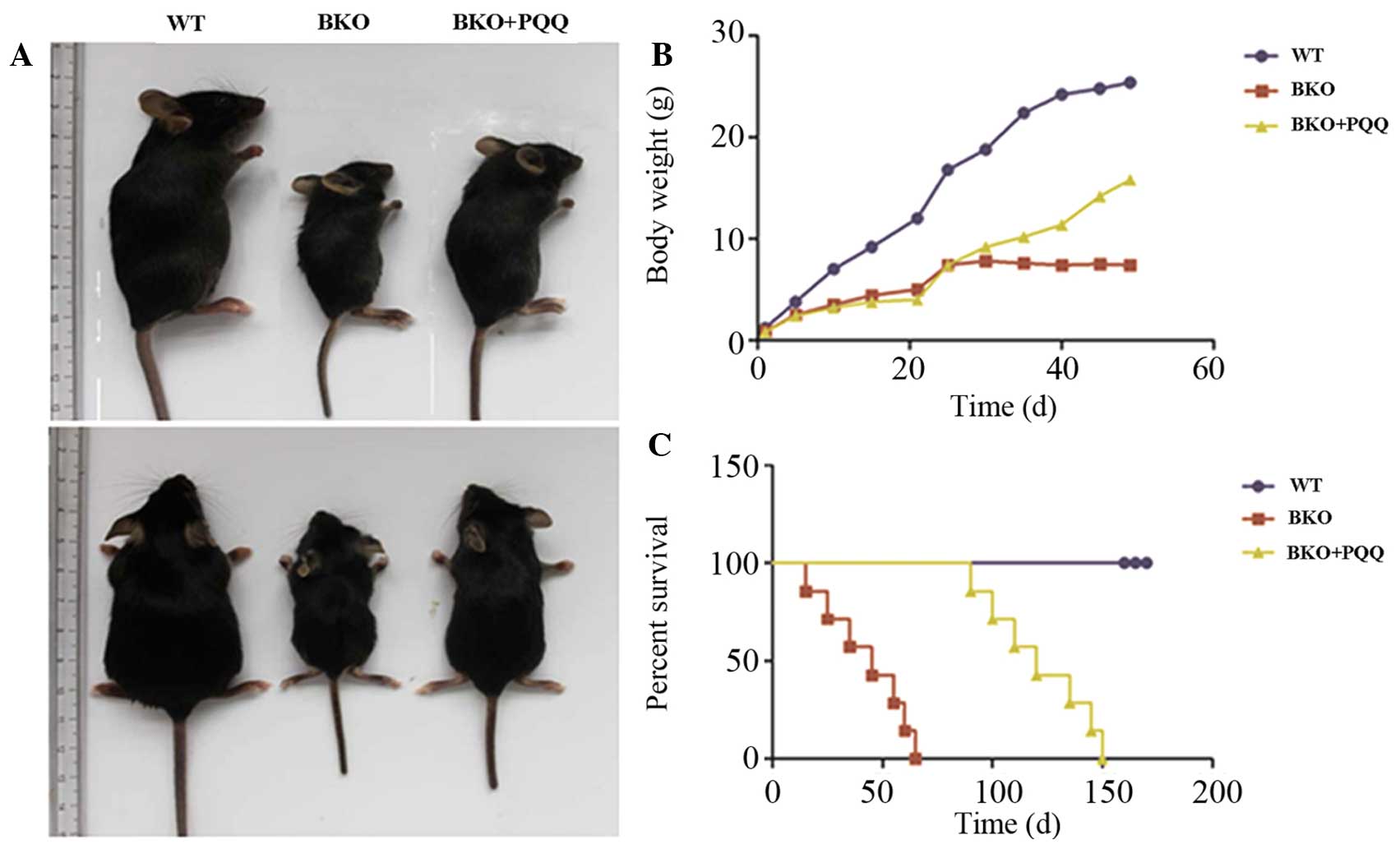
the different groups of mice. As shown in Fig. 1A–C, when compared with the normal
diet WT mice, the BKO mice who were fed a normal diet exhibited a
significant premature aging phenotype, body weight loss and a
decreased percentage survival rate. By contrast, the BKO mice
administered the PQQ-supplemented diet exhibited a partially
restored total body size, increased body weight and a prolonged
percentage survival when compared with the BKO mice fed the normal
diet. These results support the hypothesis that the
PQQ-supplemented diet partially restored the premature aging
phenotype in the Bmi-1−/− mice when compared
with the mice fed a normal diet.
Effects of PQQ on the histological
morphology of the liver in Bmi-1−/− mice
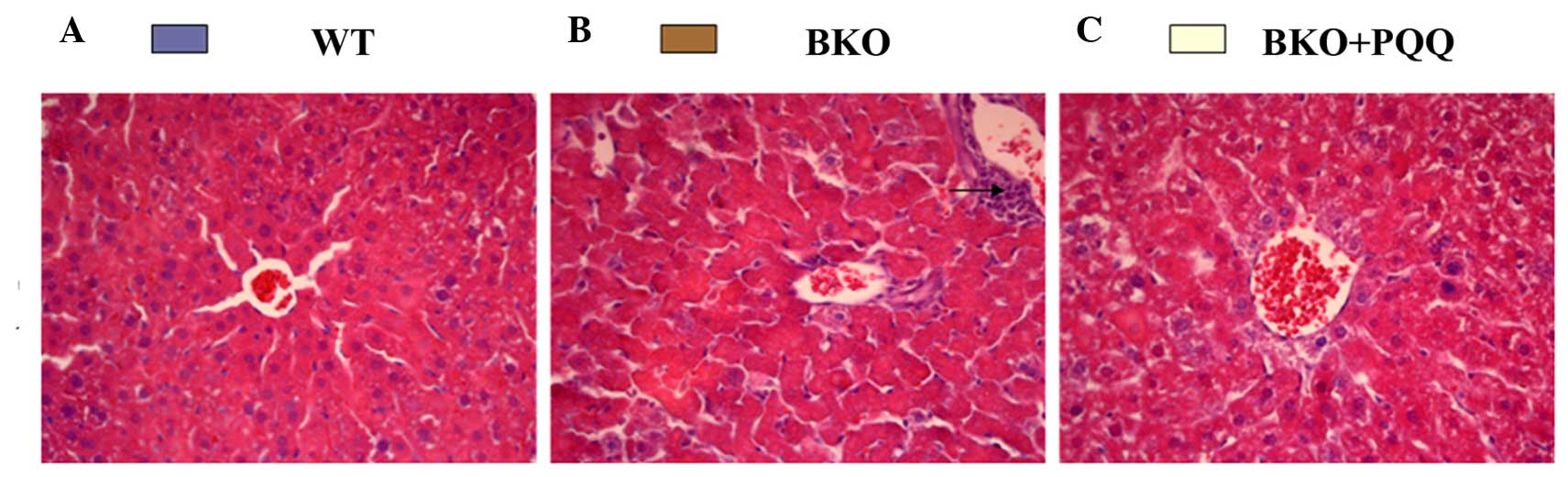
Investigations into whether
Bmi-1−/− mice exhibited an impaired liver
histological morphology were performed. As shown in Fig. 2, the histopathology of the liver in
the WT mice revealed a normal structure and a regular arrangement
of hepatocytes with clearly visible nuclei and a characteristic
pattern of hexagonal lobules (Fig.
2A). However, when compared with the WT mice, the BKO mice fed
a normal diet exhibited significant pathological alterations,
including an irregular arrangement of hepatocytes with invisible
nuclei and the presence of increased inflammatory cell infiltration
(Fig. 2B). By contrast, the BKO mice
administered the PQQ-supplemented diet exhibited good protection
against hepatocellular necrosis, with a regular arrangement of
hepatocytes (Fig. 2C). These
observations indicated that the PQQ-supplemented diet played a
protective role in the morphology of the liver damage induced by
the deletion of Bmi-1.
Effects of PQQ on cell proliferation
and DNA damage in the liver of Bmi-1−/−
mice
Since the histological morphology of the hepatocytes
was impaired in the Bmi-1−/− mice, and this
effect was partially restored by the PQQ-supplemented diet, whether
the changes in the hepatocytes were caused by the effects of the
PQQ-supplemented diet on the rate of cell proliferation was
investigated using immunohistochemical staining of PCNA (Fig. 3A). The results revealed that the
number of PCNA positively stained cells decreased in the livers of
the BKO mice. However, the PQQ-supplemented diet was shown to
significantly enhance the number of PCNA-positive cells in the
liver when compared with the BKO mice fed a normal diet, although
the overall levels remained lower than the WT mice (Fig. 3B). A deficiency in the Bmi-1 gene is
known to increase the number of DNA double strand breaks via DNA
damage repair pathways. To determine whether PQQ reduced the extent
of liver DNA damage in the BKO mice, immunohistochemical staining
of one of the most commonly used markers for double strand DNA
breaks, γH2AX, was performed (Fig.
3C). The results revealed that the BKO mice exhibited
significantly higher levels of induced cell DNA damage, as
indicated by the protein expression level of γH2AX. However, the
increased DNA damage was prevented by administration of the
PQQ-supplemented diet (Fig. 3D).
Therefore, these results indicated that BKO mice exhibited an
increased rate of cell apoptosis by enhancing the double strand DNA
damage; however, this effect was ameliorated in the BKO mice fed
the PQQ-supplemented diet.
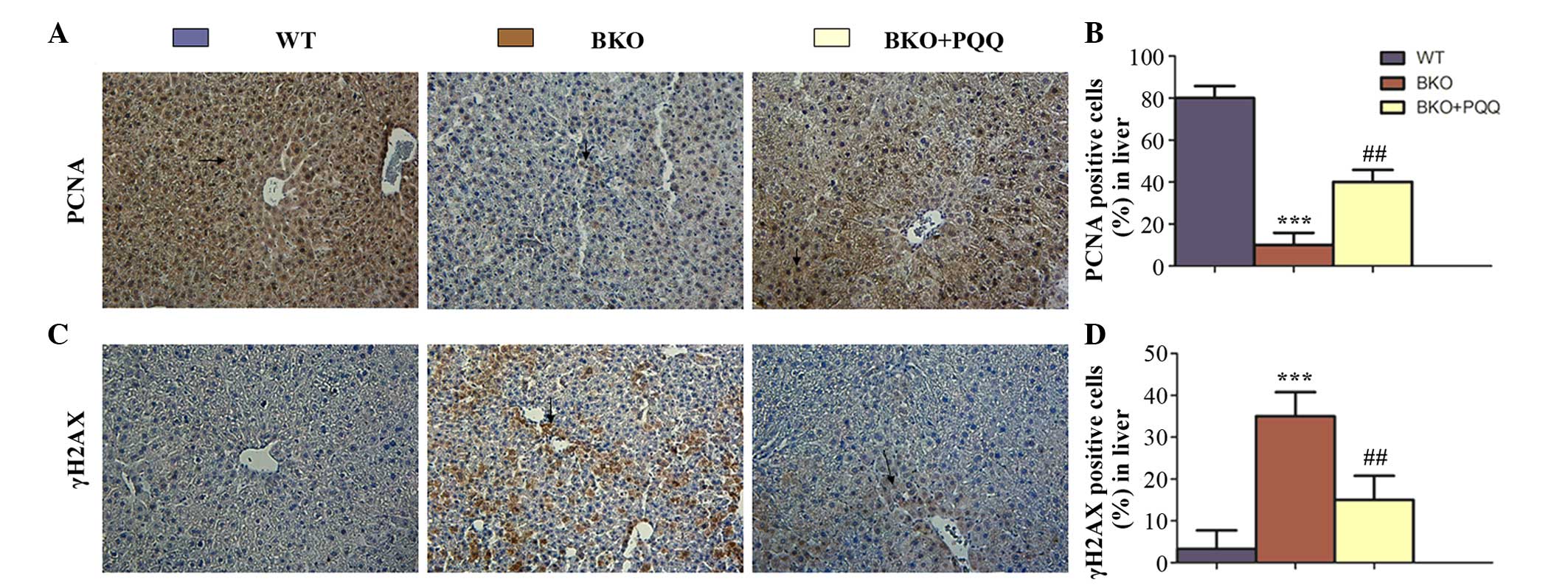 | Figure 3.Effects of PQQ on cell proliferation
and DNA damage in the liver of BKO mice. (A) Representative liver
tissues of the WT, BKO and BKO + PQQ mice stained
immunohistochemically for PCNA. (B) Percentage of liver cells
stained positive for PCNA in the WT, BKO and BKO + PQQ mice. (C)
Representative liver tissues of the WT, BKO and BKO + PQQ mice
stained immunohistochemically for γH2AX. (D) Percentage of liver
cells stained positive for γH2AX in the WT, BKO and BKO + PQQ
groups. Values are represented as the mean ± standard error of the
mean for the determination of six animals in the same group.
***P<0.001, vs. WT mice; ##P<0.01, vs. BKO mice.
Optical microscopy (magnification, x200). PQQ, pyrroloquinoline
quinone; BKO, Bmi-1−/− knockout; WT,
wildtype; PCNA, proliferating cell nuclear antigen; Bmi-1, B
cell-specific Moloney MLV insertion site-1. |
Effects of PQQ on P53 and SOD2 protein
expression in the liver of Bmi-1−/− mice
Bmi-1 regulates its downstream pathway by reducing
the expression levels of cell cycle proteins, or through
upregulating the antioxidant ability (9). To determine whether PQQ decreased the
protein expression of P53 and increased the expression of SOD2 in
the livers of the BKO mice, immunohistochemical staining of P53 and
SOD2 proteins was performed (Fig. 4A and
C). The results revealed that the number of P53-positive cells
increased in the livers of the BKO mice (Fig. 4B). In addition, the PQQ-supplemented
diet significantly reduced the number of P53-positive cells in the
BKO + PQQ mice when compared with the BKO mice. However, the number
of SOD2-positive cells decreased in the livers of the BKO mice.
Notably, the PQQ-supplemented diet was shown to ameliorate the
reduction in the expression of the antioxidant proteins in the BKO
mice (Fig. 4D). Collectively, these
results indicated that a deletion of Bmi-1 induced defects in liver
development by inhibiting cell proliferation and downregulating the
antioxidant ability; however, these effects were partially restored
following the administration of the PQQ-supplemented diet in the
BKO mice.
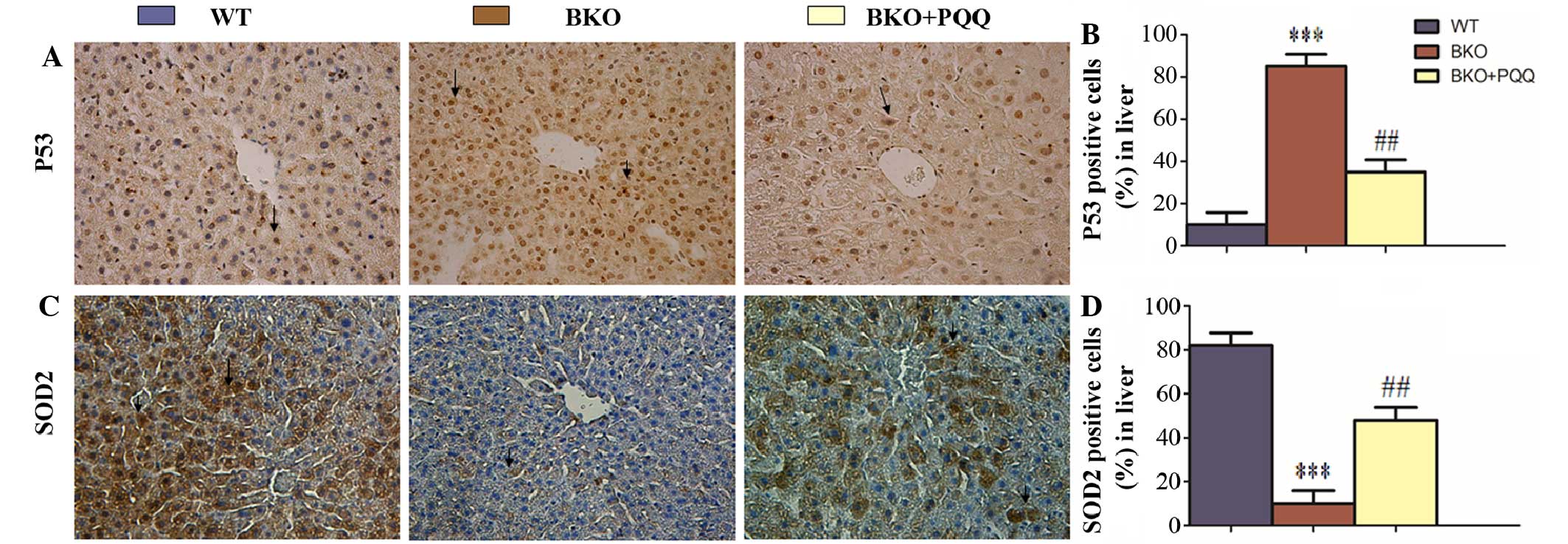 | Figure 4.Effects of PQQ on P53 and SOD2
protein expression in the liver of BKO mice. (A) Representative
liver tissues of the WT, BKO and BKO + PQQ mice stained
immunohistochemically for P53. (B) Percentage of liver cells
stained positive for P53 in the WT, BKO and BKO + PQQ mice. (C)
Representative liver tissues of the WT, BKO and BKO + PQQ mice
stained immunohistochemically for SOD2. (D) Percentage of liver
cells stained positive for SOD2 in the WT, BKO and BKO + PQQ mice.
Values are represented as the mean ± standard error of the mean for
the determination of six animals in the same groups. ***P<0.001,
vs. WT mice; ##P<0.01, vs. BKO mice. Optical
microscopy (magnification, x400). PQQ, pyrroloquinoline quinone;
BKO, Bmi-1−/− knockout; WT, wildtype; SOD2,
superoxide dismutase 2; Bmi-1, B cell-specific Moloney MLV
insertion site-1. |
Effects of PQQ on cell cycle protein
expression in the liver of Bmi-1−/− mice
Cell proliferation is known to be mediated by the
expression and activation of tumor-suppressor genes (31); thus, the expression levels of the
cell cycle proteins, P16, P19, P21, P27 and P53, were examined
(Fig. 5A). The results revealed that
the BKO mice promoted the expression of the tumor-suppressors, P16,
P19, P21, P27 and P53. However, administration of the
PQQ-supplemented diet in the BKO mice prevented the increased
expression levels of P16, P19, P21, P27 and P53 (Fig. 5B–F).
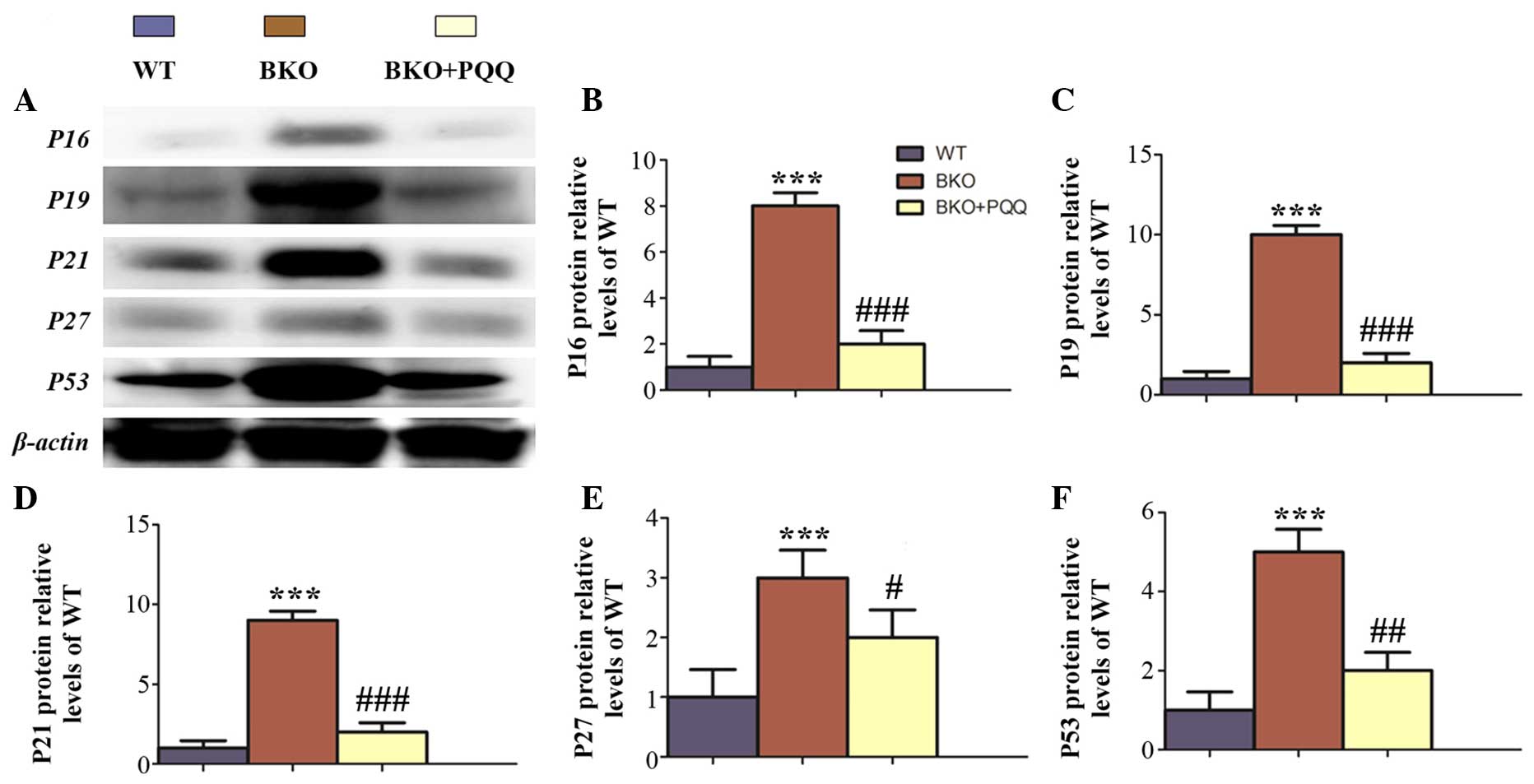 | Figure 5.Effects of PQQ on the expression of
cell cycle proteins in the liver of BKO mice. (A) Representative
western blot showing the protein expression of P16, P19, P21, P27
and P53 in the liver. β-actin was used as a loading control for the
western blots in the WT, BKO and BKO + PQQ mice. Protein expression
levels of (B) P16, (C) P19, (D) P21, (E) P27 and (F) P53, relative
to the β-actin protein levels, were assessed by densitometric
analysis and expressed relative to levels of the WT mice. Values
are represented as the mean ± standard error of the mean for the
determination of six animals in the same group. ***P<0.001, vs.
WT mice; #P<0.05, ##P<0.01 and
###P<0.001, vs. BKO mice. PQQ, pyrroloquinoline
quinone; BKO, Bmi-1−/− knockout; WT,
wildtype; Bmi-1, B cell-specific Moloney MLV insertion site-1. |
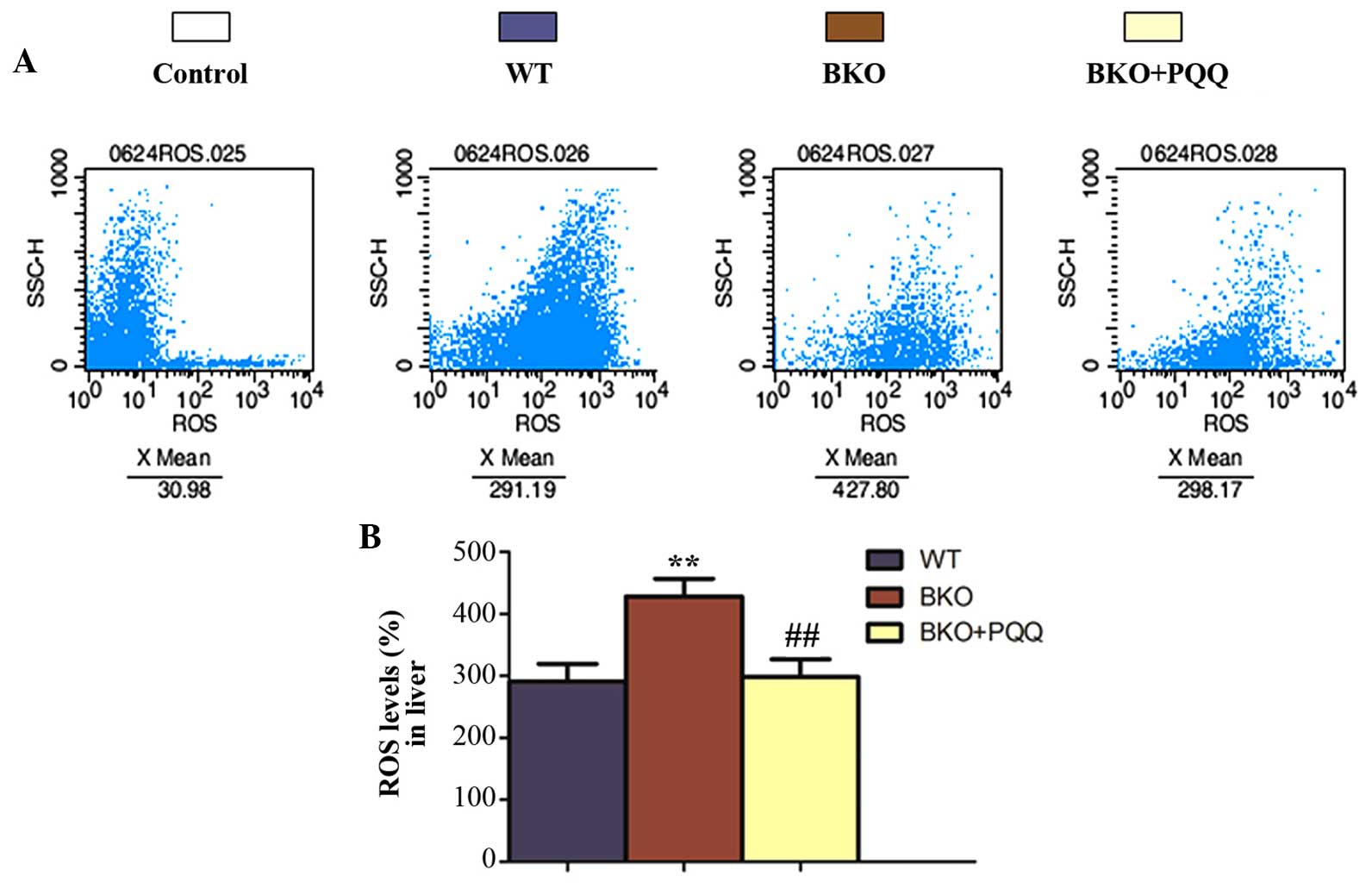
Effects of PQQ on the ROS levels in
the liver of Bmi-1−/− mice
Bmi-1−/− mice are known to
have increased levels of ROS and hydroxyl free radicals that are
produced by oxidative stress, which subsequently results in an
increase in DNA double strand breaks (32). The present results demonstrated that
the PQQ-supplemented diet decreased the number of BKO-induced
double strand DNA breaks in the liver. To determine whether PQQ
inhibited ROS formation, ROS levels were evaluated by flow
cytometry (Fig. 6A). The results
revealed that the increased ROS levels in the BKO mice were
inhibited by administration of the PQQ-supplemented diet (Fig. 6B).
Effects of PQQ on the expression
levels of antioxidant proteins in the liver of
Bmi-1−/− mice
To further investigate whether the effects of PQQ
were associated with the enhanced expression levels of various
antioxidant proteins, western blot analysis was performed to
examine the protein expression levels of PRDX I, PRDX IV, SOD1,
SOD2 and GSH (Fig. 7A). The results
demonstrated that the expression of all the antioxidant proteins
were downregulated in the BKO mice when compared with the WT mice,
which was consistent with immunohistochemical staining results.
Notably, the expression levels of all the antioxidant proteins were
increased to a viable level in the BKO + PQQ mice when compared
with the BKO mice (Fig. 7B–F). These
observations indicated that PQQ promoted an antioxidant effect to
protect against BKO-induced oxidative stress.
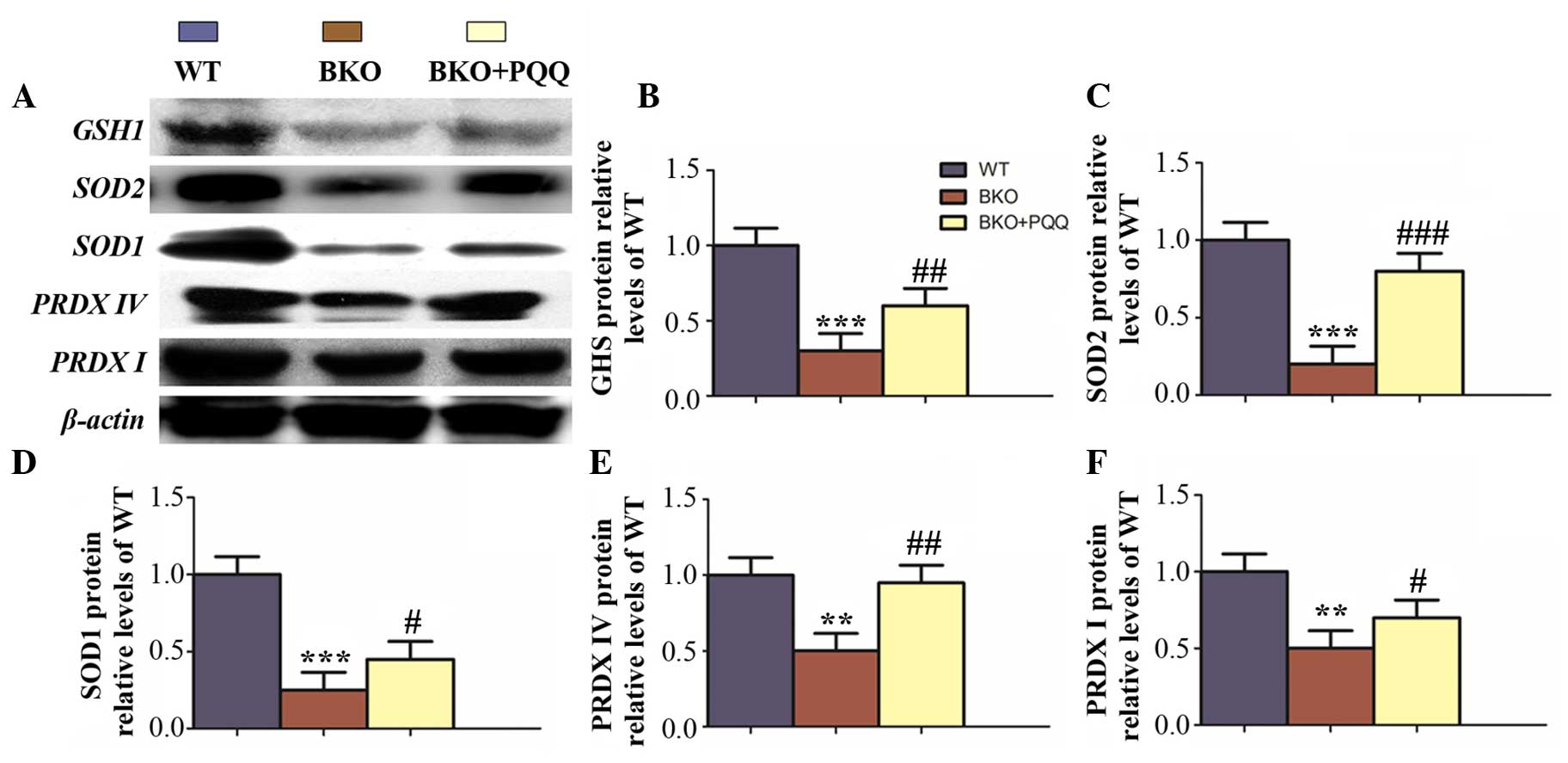 | Figure 7.Effects of PQQ on the protein
expression of antioxidants in the liver of BKO mice. (A)
Representative western blot showing the protein expression of GSH,
SOD1, SOD2, PRDXI and PRDX Ⅳ in the liver. β-actin was used as a
loading control for the western blot in the WT, BKO and BKO + PQQ
mice. Protein expression levels of (B) GSH1, (C) SOD2, (D) SOD1,
(E) PRDX IV and (F) PRDX, relative to the β-actin protein levels,
were assessed by densitometric analysis and expressed relative to
the levels of the WT mice. Values are represented as the mean ±
standard error of the mean for the determination of six animals in
the same group. **P<0.01 and ***P<0.001, vs. WT mice;
#P<0.05, ##P<0.01 and
###P<0.001, vs. BKO mice. PQQ, pyrroloquinoline
quinone; BKO, Bmi-1−/− knockout; WT,
wildtype; GSH, glutathione; SOD, superoxide dismutase; PRDX,
peroxiredoxin; Bmi-1, B cell-specific Moloney MLV insertion
site-1. |
Discussion
Bmi-1 is a member of the Polycomb family of
transcriptional repressors that mediate gene silencing by
regulating chromatin structure, and are essential for the
maintenance and self-renewal of hematopoietic and neural stem cells
(7–9). Under normal conditions, the Polycomb
protein, Bmi-1, simultaneously represses the INK4a/Arf locus,
leading to reduced expression levels of P16Ink4a and
P19Arf, as well as modulating mitochondrial function to
reduce ROS levels and suppress the activation of the DNA damage
response pathway (DDR). Activation of INK4a/Arf and the DDR have
been separately associated with tumor suppression and stem cell
aging (9). Previous studies have
proposed that oxidative stress and the associated damage may
represent a common association between different forms of diseases
(33,34). Oxidative stress has been implicated
in various liver diseases, including viral hepatitis, nonalcoholic
fatty liver disease/steatohepatitis, alcoholic liver disease and
drug-induced liver injury (35,36).
PQQ, an anionic water soluble compound, was
initially proposed to be a cofactor of certain bacterial primary
dehydrogenases (37). Subsequently,
the compound was attributed with multiple physiological functions,
including regulation of the electron transport system and
stimulation of the production of nerve growth factor (38). In addition, PQQ has been reported to
scavenge ROS and protect cells from oxidative stress-induced damage
through improving the activities of free-radical scavenging enzymes
and decreasing the levels of free radicals (39). In vitro studies have
demonstrated that PQQ protects isolated liver mitochondria from
damage following oxidative stress and scavenges superoxide radicals
(40,41). Furthermore, an in vivo study
investigating ischemia/reperfusion injury in rats demonstrated that
PQQ reduces the myocardial infarct size and improves cardiac
function (42). In addition, in
vivo and in vitro studies have shown that PQQ can
protect against several types of oxidative damage, stroke damage
and irradiation injury (19,43). However, despite all these beneficial
functions of PQQ, to the best of our knowledge, no attempt has been
made to evaluate the role of PQQ in the regulation of liver
development defects (44).
Furthermore, there are a limited number of studies that have
investigated whether PQQ can function as a potential ameliorative
agent in Bmi-1 deficiency-induced liver dysfunction. In the present
study, a liver dysfunctional animal model induced by
Bmi-1−/− was generated to determine whether
PQQ functions as an antioxidant to restore the damage of the liver.
The results of the present study, with regard to the pathological,
cellular and molecular levels of oxidative stress and antioxidant
activity, clearly demonstrate that the administration of a
PQQ-supplementary diet, at a dose of 4 mg PQQ/kg in the normal
diet, to Bmi-1−/− mice significantly reduces
the extent of damage in the liver.
However, there is no effective treatment for the
deleterious effects of a number of risk factors on the liver in
clinical practice. Therefore, studies have started to focus on
substances known as protectors that may inhibit or reduce liver
damage. The functions of these protectors are directly targeted to
reduce the free radicals formed by oxidative stress. As a powerful
antioxidant, PQQ may potentiate to prevent the loss of secretory
cells in the liver caused by BKO-induced oxidative stress (45).
In the current study, knowledge on PQQ was further
extended, and treatment with a PQQ-supplementary diet was
demonstrated to provide robust protection to the liver. As
demonstrated by the present results, PQQ is able to rescue the
damage to liver morphology, possibly by promoting cell
proliferation and inhibiting cell apoptosis and DNA damage. Our
further results indicate that PQQ not only inhibits expressions of
cell proteins (P16 and P19), cell cycle dependent kinase inhibitors
(CDKs, P21 and P27) and apoptotic genes (P53), but also upregulates
a variety of antioxidant proteins, such as GSH, SOD1, SOD2, PRDX I
and PRDX Ⅳ. Simultaneously, the present results also demonstrated
that PQQ was able to reduce the ROS levels in an impaired liver.
Collectively, the results demonstrated that PQQ, as an antioxidant,
is able to inhibit oxidative stress by decreasing the levels of
ROS, and rescue cell survival by downregulating the expression of
cell cycle proteins, including P16, P19, P21, P27 and P53.
Consequently, PQQ is able to restore the defects of liver
development induced in BKO mice.
In conclusion, the results of the present study
indicate that treatment of BKO mice with a moderate dose of PQQ
significantly protects the liver from deleterious effects by
inhibiting oxidative stress and participating in DNA damage repair.
Therefore, PQQ has a great potential as a therapeutic agent against
oxidative stress during liver damage.
Acknowledgements
The study was supported by a grant from the National
Natural Science Foundation of China (no. 81230009).
References
|
1
|
Fan L, Xu C, Wang C, et al: Bmi1 is
required for hepatic progenitor cell expansion and liver tumor
development. PLoS One. 7:e464722012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park MC, Youn HJ, Chang HK, et al: TOP1
and 2, polysaccharides from Taraxacum officinale, attenuate
CCl4-induced hepatic damage through the modulation of
NF-κB and its regulatory mediators. Food Chem Toxicol.
48:1255–1261. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Srivastava A and Shivanandappa T:
Hepatoprotective effect of the root extract of Decalepis
hamiltonii against carbon tetrachloride-induced oxidative
stress in rats. Food Chem. 118:411–417. 2010. View Article : Google Scholar
|
|
4
|
Muriel P, Alba N, Pérez-Alvarez VM, et al:
Kupffer cells inhibition prevents hepatic lipid peroxidation and
damage induced by carbon tetrachloride. Comp Biochem Physiol C
Toxicol Pharmacol. 130:219–226. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harman D: Aging: A theory based on free
radical and radiation chemistry. J Gerontol. 11:298–300. 1956.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Salmon AB, Richardson A and Pérez VI:
Update on the oxidative stress theory of aging: Does oxidative
stress play a role in aging or healthy aging. Free Radic Biol Med.
48:642–655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park IK, Qian D, Kiel M, et al: Bmi-1 is
required for maintenance of adult self-renewing haematopoietic stem
cells. Nature. 423:302–305. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang HW, Ding J, Jin JL, et al: Defects
in mesenchymal stem cell self-renewal and cell fate determination
lead to an osteopenic phenotype in Bmi-1 null mice. J Bone Miner
Res. 25:640–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu J, Cao L, Chen J, et al: Bmi1
regulates mitochondrial function and the DNA damage response
pathway. Nature. 459:387–392. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hauge JG: Glucose dehydrogenase of
bacterium anitratum: An enzyme with a novel prosthetic group. J
Biol Chem. 239:3630–3639. 1964.PubMed/NCBI
|
|
11
|
Duine JA: Cofactor diversity in biological
oxidations: Implications and applications. Chem Rec. 1:74–83. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitchell AE, Jones AD, Mercer RS and
Rucker RB: Characterization of pyrroloquinoline quinone amino acid
derivatives by electrospray ionization mass spectrometry and
detection in human milk. Anal Biochem. 269:317–325. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumazawa T, Sato K, Seno H, et al: Levels
of pyrroloquinoline quinone in various foods. Biochem J.
307:331–333. 1995.PubMed/NCBI
|
|
14
|
Stites TE, Mitchell AE and Rucker RB:
Physiological importance of quinoenzymes and the O-quinone family
of cofactors. J Nutr. 130:719–727. 2000.PubMed/NCBI
|
|
15
|
Steinberg FM, Gershwin ME and Rucker RB:
Dietary pyrroloquinoline quinone: Growth and immune response in
BALB/c mice. J Nutr. 124:744–753. 1994.PubMed/NCBI
|
|
16
|
Steinberg F, Stites TE, Anderson P, et al:
Pyrroloquinoline quinone improves growth and reproductive
performance in mice fed chemically defined diets. Exp Biol Med
(Maywood). 228:160–166. 2003.PubMed/NCBI
|
|
17
|
Zhang Y, Feustel PJ and Kimelberg HK:
Neuroprotection by pyrroloquinoline quinone (PQQ) in reversible
middle cerebral artery occlusion in the adult rat. Brain Res.
1094:200–206. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Y and Rosenberg PA: The essential
nutrient pyrroloquinoline quinone may act as a neuroprotectant by
suppressing peroxynitrite formation. Eur J Neurosci. 16:1015–1024.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu BQ, Simonis U, Cecchini G, et al:
Comparison of pyrroloquinoline quinone and/or metoprolol on
myocardial infarct size and mitochondrial damage in a rat model of
ischemia/reperfusion injury. J Cardiovasc Pharmacol Ther.
11:119–128. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohwada K, Takeda H, Yamazaki M, et al:
Pyrroloquinoline quinone (PQQ) prevents cognitive deficit caused by
oxidative stress in rats. J Clin Biochem Nutr. 42:29–34. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shankar BS, Pandey R, Amin P, et al: Role
of glutathione in augmenting the anticancer activity of
pyrroloquinoline quinone (PQQ). Redox Rep. 15:146–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ouchi A, Nakano M, Nagaoka S and Mukai K:
Kinetic study of the antioxidant activity of pyrroloquinolinequinol
(PQQH (2), a reduced form of pyrroloquinoline quinone) in micellar
solution. J Agric Food Chem. 57:450–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stites T, Storms D, Bauerly K, et al:
Pyrroloquinoline quinone modulates mitochondrial quantity and
function in mice. J Nutr. 136:390–396. 2006.PubMed/NCBI
|
|
24
|
Ishii T, Akagawa M, Naito Y, et al:
Pro-oxidant action of pyrroloquinoline quinone: Characterization of
protein oxidative modifications. Biosci Biotechnol Biochem.
74:663–666. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tao R, Karliner JS, Simonis U, et al:
Pyrroloquinoline quinone preserves mitochondrial function and
prevents oxidative injury in adult rat cardiac myocytes. Biochem
Biophys Res Commun. 363:257–262. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Misra HS, Khairnar NP, Barik A, et al:
Pyrroloquinoline-quinone: A reactive oxygen species scavenger in
bacteria. FEBS Lett. 578:26–30. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao G, Gu M, Zhu M, et al: Bmi-1 absence
causes premature brain degeneration. PLoS One. 7:e320152012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bauerly K, Harris C, Chowanadisai W, et
al: Altering pyrroloquinoline quinone nutritional status modulates
mitochondrial, lipid, and energy metabolism in rats. Plos One.
6:e217792011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xue Y, Karaplis AC, Hendy GN, et al:
Genetic models show that parathyroid hormone and
1,25-dihydroxyvitamin D3 play distinct and synergistic roles in
postnatal mineral ion homeostasis and skeletal development. Hum Mol
Genet. 14:1515–1528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zamzami N, Marchetti P, Castedo M, et al:
Sequential reduction of mitochondrial transmembrane potential and
generation of reactive oxygen species in early programmed cell
death. J Exp Med. 182:367–377. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bae I, Fan S, Bhatia K, Kohn KW, Fornace
AJ Jr and O'Connor PM: Relationships between G1 Arrest and
Stability of the p53 and p21CiP1/Waf1 Proteins following
gamma-irradiation of human lymphoma cells. Cancer Res.
55:2387–2393. 1995.PubMed/NCBI
|
|
32
|
Di Pietro C, Piro S, Tabbi G, et al:
Cellular and molecular effects of protons: Apoptosis induction and
potential implications for cancer therapy. Apoptosis. 11:57–66.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sano R and Reed JC: ER stress-induced cell
death mechanisms. Biochim Biophys Acta. 1833:3460–3470. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang W, Jiang YF, Ponnusamy M and Diallo
M: Role of Nrf2 in chronic liver disease. World J Gastroenterol.
20:13079–13087. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Weltman MD, Farrell GC, Hall P, et al:
Hepatic cytochrome P450 2E1 is increased in patients with
nonalcoholic steatohepatitis. Hepatology. 27:128–133. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Okuda M, Li K, Beard MR, et al:
Mitochondrial injury, oxidative stress and antioxidant gene
expression are induced by hepatitis C virus core protein.
Gastroenterology. 122:366–375. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Salisbury SA, Forrest HS, Cruse WB and
Kennard O: A novel coenzyme from bacterial primary alcohol
dehydrogenases. Nature. 280:843–844. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rucker R, Chowanadisai W and Nakano M:
Potential physiological importance of pyrroloquinoline quinone.
Altern Med Rev. 14:268–277. 2009.PubMed/NCBI
|
|
39
|
Killgore J, Smidt C, Duich L, et al:
Nutritional importance of pyrroloquinoline quinone. Science.
245:850–852. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bishop A, Paz MA, Gallop PM, et al:
Methoxatin PQQ in guinea-pig neutrophils. Free Radic Biol Med.
17:311–320. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
He K, Nukada H, Urakami T and Murphy MP:
Antioxidant and pro-oxidant properties of pyrroloquinoline quinone
(PQQ): Implications for its function in biological systems. Biochem
Pharmacol. 65:67–74. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhu B, Zhou H, Teerlink JR, et al:
Pyrroloquinoline quinone (PQQ) decreases myocardial infarct size
and improves cardiac function in rat models of ischemia and
ischemia/reperfusion. Cardiovasc Drugs Ther. 18:421–431. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aizenman E, Hartnett KA, Zhong C, et al:
Interaction of the putative essential nutrient pyrroloquinoline
quinone with the N-methyl-D-aspartate receptor redox modulatory
site. J Neurosci. 12:2362–2369. 1992.PubMed/NCBI
|
|
44
|
Kumar N, Kar A and Panda S:
Pyrroloquinoline quinone ameliorates l-thyroxine-induced
hyperthyroidism and associated problems in rats. Cell Biochem
Funct. 32:538–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jin J, Lv X, Chen L, Zhang W, Li J, Wang
Q, Wang R, Lu X and Miao D: Bmi-1 plays a critical role in
protection from renal tubulointerstitial injury by maintaining
redox balance. Aging Cell. 13:797–809. 2014. View Article : Google Scholar : PubMed/NCBI
|