Introduction
Shark liver oil (SLO) has long been used as a
dietary supplement with health-promoting activities, particularly
for cardiovascular health, in Japan. At present, several
SLO-containing supplements are commercially available and their
dietary consumption is increasing among the Japanese middle-aged
and elderly populations, most likely due to the fact that there has
been considerable interest in alternative therapies.
SLO is known to contain large quantities of squalene
and, thus, to be considered as the richest source of the compound.
Squalene received its name as a result of its first isolation from
liver oil of sharks (Squalus spp.) (1). Later, squalene was found in variety of
vegetable oils, including olive, palm, wheat-germ and rice bran
oils. Chemically, squalene is a polyprenyl compound, having a
structural similarity with β-carotene, coenzyme Q10 and vitamins A,
E and K (2). Among these
squalene-related compounds, vitamin E, known as the most potent
lipid-soluble antioxidant in vivo, has been most intensively
studied for its effects on cardiovascular health; there have been a
number of studies reporting that vitamin E is effective in
decreasing arterial stiffening in overweight hypertensive patients
(3,4), and others reporting that vitamin E
supplementation improves peripheral vascular diseases due to
diabetes mellitus and atherosclerosis-associated intermittent
claudication (5–7). It may be, therefore, that this
antioxidative vitamin has a beneficial effect on arterial
stiffness, which has been demonstrated to be associated with an
increased risk of cardiovascular events and mortality (8–10), as
well as on peripheral microvascular function.
These results obtained with vitamin E, a
squalene-related antioxidant compound, led to our hypothesis that
supplementation with SLO rich in squalene may also have the
potential to exert a similar central and/or peripheral vascular
modification. To the best of our knowledge, no data regarding the
vascular effects of SLO preparations or their major constituent,
squalene, in humans have been reported. The aim of the present
study is therefore to investigate the efficacy, as well as
tolerance, compliance and safety, of supplementation with a
commercially available SLO preparation in otherwise healthy
middle-aged and elderly males with marginal arterial stiffness.
Subjects and methods
Subjects
Healthy, male, non-overweight Japanese participants
without hypertension or hyperlipidaemia, aged 45–69 years, were
recruited for inclusion in this study. Participants with a higher
cardio-ankle vascular index (CAVI) were given inclusion preference.
Subjects were excluded if they were receiving any dietary
supplement, cosmetics or medicines rich in SLO or its major
constituents (squalene and alkylglycerols). Subjects were also
excluded if they currently suffered from any diagnosed diseases
requiring medical treatment or had a past history of such medical
conditions, or if they had stopped smoking within the six months
prior to the study run-in period. The other exclusion criteria were
as follows: i) Known allergies to SLO or any other major components
of the study supplement, including gelatin, glycerin and processed
starch; ii) participation in another clinical study within the
month before the initiation of the present study; and iii) the
presence of any medical condition judged by the investigator to
preclude the participant's inclusion in the study. Written informed
consent was obtained from all participants prior to their enrolment
in the study.
Study design
A randomized, double-blind, placebo-controlled study
was designed to assess the efficacy and safety of supplementation
with SLO for improving the arterial stiffness and peripheral
microvascular function in enrolled participants when compared with
placebo. The study was performed between August and November 2012.
The study protocol was approved by the Tana Orthopedic Surgery
Institutional Review Board (Yokohama, Japan), and the study was
conducted in accordance with the principles of the Declaration of
Helsinki (1995; as revised in Edinburgh, 2000) and the Ethical
Guidelines for Epidemiological Research (enacted by the Japanese
Government in 2008). The overall design of the study consisted of
an eight-week intervention period preceded by a three-week run-in
period, during which eligible subjects were screened.
Intervention and subject
assessment
The test supplement was a commercially available
product manufactured by Egao Co., Ltd. (Kumamoto, Japan) in a form
of 400-mg capsule. The supplement contained 250 mg SLO and 150 mg
vehicle (consisting of 97 mg gelatin, 34 mg glycerol and 19 mg
processed starch) and was referred to as the SLO capsule. In the
placebo capsule, SLO was substituted by the same amount of
safflower oil. Chemical analysis of the SLO preparation used in
this study revealed that it was almost free of n-3 long-chain fatty
acids and mainly composed of (w/w) squalene (38.8%) and
alkylglycerols (43.6%), indicating that a daily dose of the SLO
capsule contained squalene at 582 mg in weight. Safflower oil used
for preparing the placebo capsule was virtually free of squalene
(<0.004%). The SLO and placebo capsules were similar in colour
and packaging. Subjects were assigned to receive six SLO capsules
per day or six placebo capsules per day and were instructed to take
the allocated capsules once daily following a meal (either
breakfast, lunch or dinner) with the aid of a cup of drinking water
during the eight-week intervention period.
Each participant was instructed to maintain a study
diary, covering the time from the start of the run-in period to the
end of the intervention period, describing their allocated capsule
intake, dietary composition, any adverse effects, physical activity
and all medication and therapy received. Participants were also
requested to maintain their body weight and to continue with their
normal exercise, eating and drinking habits. At the completion of
the run-in period, the eligible subjects were sequentially assigned
to receive one of the two masked study capsules (SLO or placebo)
and instructed to take six of the allocated capsules once daily,
following a meal. Non-compliance was defined as administration of
<80% of the total course of the allocated study capsule. All
non-compliant subjects were excluded from the efficacy
assessment.
Measurement of the CAVI
To determine the extent of the arterial stiffness, a
relatively new stiffness diagnostic parameter, known as the CAVI,
was used. The CAVI was selected as it is can be measured easily and
non-invasively and is not influenced by blood pressure changes
during measurement (11,12). Furthermore, it has been reported to
be independent of blood pressure levels (13–15). In
principle, the CAVI is measured from an electrocardiogram, a
phonocardiogram and brachial artery and ankle arterial waveforms
and calculated using a specific algorithm (13). An epidemiological study on the
clinical interpretation of CAVI values in a large Japanese
population suggested that values <8.0 can be considered within
the normal range and that those ≥9.0 are indicative of suspected
atherosclerosis (16).
In the present study, measurement of the CAVI was
performed with a VaSera VS-1500 vascular screening system (Fukuda
Denshi Co., Ltd., Tokyo, Japan) using the method described by Yambe
et al (13). The subjects
were placed in a supine position in a room kept at a room
temperature and cuffs were applied to the bilateral upper arms and
ankles. Subsequent to the subject having rested for ≥10 min,
automatic measurements were performed twice for the right and left
extremities. Averages of the right and left CAVIs measured in the
run-in period were compared with each other, and the side with a
higher CAVI value was selected as the target for CAVI measurement
and data analysis.
Measurement of laser Doppler perfusion
imaging (LDPI)
Peripheral microvascular function was assessed on
the basis of hand blood flow to surface tissues measured by an LDPI
technique. The examination was performed in a room with a constant
temperature (~24°C) and humidity (~50%) and the subjects were
acclimatized for ≥20 min. The cutaneous blood flow on the dorsum of
the left hand was measured with a laser Doppler perfusion imager
(Periscan PIM-II; Perimed AB, Stockholm, Sweden) (17) and is expressed in arbitrary perfusion
units (PU) (18).
Measurement of haematochemical,
haematological, anthropometric and haemodynamic parameters
Total cholesterol, low-density
lipoprotein-cholesterol, high-density lipoprotein-cholesterol,
triglycerides (TG) and other routine haematochemical laboratory
test variables were measured in serum samples collected from
individual subjects following an overnight fast at baseline (week
0) and at eight weeks after the start of intervention (week 8).
Haematological variables, including red blood cell, white blood
cell and platelet counts, as well as haemoglobin and haematocrit,
were measured in whole blood. Several anthropometric and
haemodynamic parameters, including weight, body mass index (BMI),
blood pressure (BP) and heart rate, were also measured at weeks 0
and 8.
Safety assessment
Safety was assessed based on the incidence of
intervention-related adverse events recorded during the eight-week
intervention period, as well as on abnormal changes observed in the
haematochemical and haematological test variables and
anthropometric and haemodynamic parameters.
Statistical analyses
All analyses were performed using Microsoft Excel
[Microsoft Corporation (2003), Redmond, WA, USA] and PASW
Statistics (version 18; SPSS Inc., Chicago, IL, USA). Comparisons
between the baseline characteristics of the placebo and SLO groups
were conducted using unpaired t-tests, and paired t-tests were
utilized for intragroup comparisons for all variables. The
respective changes within the intervention groups were compared
using analysis of covariance, taking the baseline (week 0) value as
the covariate. The simple linear regression model was used to
examine the associations. P<0.05 was considered to indicate a
statistically significant difference. Values in the text are
presented as the mean ± standard error of the mean or as the change
from week 0.
Results
Enrolment and baseline
characteristics
A total of 42 subjects were enrolled in the study,
and 21 subjects were randomly assigned to each of the placebo and
the SLO supplement interventions (placebo and SLO groups,
respectively). A total of 41 subjects completed all components of
the study protocol, resulting in a retention rate of 98%. One
subject (placebo group) withdrew due to unrelated personal reasons
during the study. There was no incident of unmasking of subject
assignment.
Table I shows the
baseline characteristics of the 41 subjects who completed the
study. Their mean age at enrolment was 59.0±4.0 years (range, 45–69
years). The mean values of all the anthropometric, haemodynamic and
haematochemical parameters tested, as well as the CAVI, were within
the normal range for clinical measurements, although the mean CAVI
value was marginally high (8.63±0.10). Almost all the subjects were
judged to be free from hypertension, hyperlipidaemia or
atherosclerosis and to not be overweight.
 | Table I.Baseline characteristics of the
subjects who completed the study (n=41). |
Table I.
Baseline characteristics of the
subjects who completed the study (n=41).
| Variable | Value |
|---|
| Age (years) | 59.0±4.0 |
| Weight (kg) | 65.5±4.8 |
| BMI
(kg/m2) | 22.8±2.6 |
| Smoking habit
(smokers, n/non-smokers, n) | 10/31 |
| CAVI | 8.63±0.10 |
| LDPI (PU) | 0.73±0.05 |
| Systolic BP
(mmHg) | 132±6.7 |
| Diastolic BP
(mmHg) | 83±5.1 |
| Heart rate
(beats/min) | 70±4.8 |
| Serum total
cholesterol (mg/dl) | 222±9.1 |
| Serum
LDL-cholesterol (mg/dl) | 142±8.5 |
| Serum
HDL-cholesterol (mg/dl) | 61±6.0 |
| Serum TG
(mg/dl) | 119±12 |
| Serum glucose
(mg/dl) | 92±4.0 |
Effects on main outcome measures
Table II shows the
effects of SLO supplementation on the anthropometric, haemodynamic,
haematochemical and vascular characteristics in comparison with
those of the placebo over the eight-week intervention. No
significant group difference in the variables was observed at week
0, with the exception of the LDPI, for which the value for the
placebo group was significantly higher than that for the SLO group
(0.76±0.02 versus 0.69±0.02 PU, P<0.05). The magnitude of the
changes in the CAVI values over the eight weeks for the SLO group
was not significantly different from that for the placebo group;
however, there are a substantial number of reports demonstrating
that the CAVI as an arterial stiffness parameter is strongly
correlated with age (11,12,19–22).
This finding raised the possibility that the potential effect of
the SLO supplementation on the CAVI may be modified by the age of
the study subjects; therefore, the association between the
magnitude of the change in the CAVI and the age of subjects who
received supplementation with SLO or placebo was examined. As shown
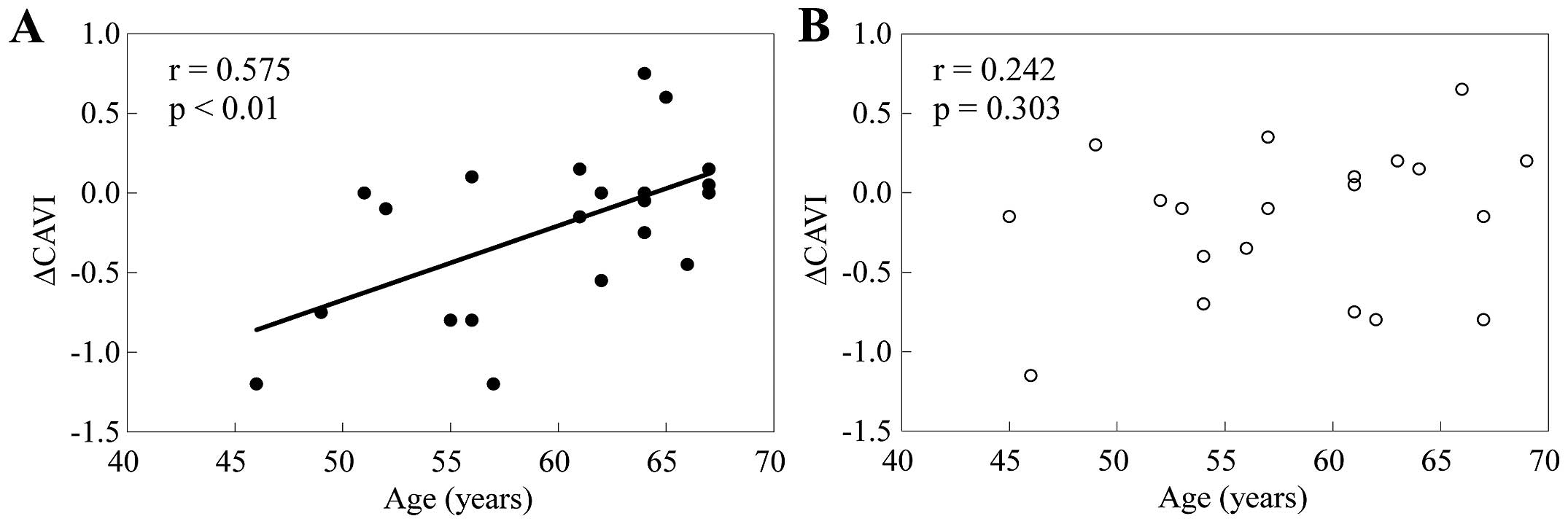
in Fig. 1, the univariate linear
regression analysis revealed that the changes (decreases) in the
CAVI after the eight-week supplementation period were significantly
correlated with the age in the SLO group (r=0.575, P<0.01),
while such a significant correlation was not noted in the placebo
group (r=0.242, P=0.303). Different from this, the baseline value
of the CAVI was not associated with the changes in the CAVI for
subjects receiving the SLO supplementation for eight weeks
(r=0.179, P=0.438) nor for those receiving the placebo for eight
weeks (r=0.099, P=0.678).
 | Table II.Values of vascular, anthropometric,
haemodynamic and haemobiochemical parameters for the placebo and
SLO groups at weeks 0 and 8. |
Table II.
Values of vascular, anthropometric,
haemodynamic and haemobiochemical parameters for the placebo and
SLO groups at weeks 0 and 8.
|
| Placebo (n=20) | SLO (n=21) |
|
|---|
|
|
|
|
|
|---|
| Variable | Week 0 | Week 8 | Change | Week 0 | Week 8 | Change |
P-valueb |
|---|
| CAVI |
8.66±0.14 |
8.49±0.17 |
−0.18±0.10 |
8.60±0.14 |
8.38±0.16 |
−0.21±0.11 | 0.800 |
| LDPI (PU) |
0.76±0.02 |
0.68±0.02a |
−0.08±0.02 |
0.69±0.02 |
0.70±0.02 |
0.00±0.01 | 0.002 |
| Weight (kg) |
65.4±1.9 |
65.3±1.8 |
−0.1±0.3 |
65.7±2.3 |
66.1±2.3 |
0.4±0.2 | 0.150 |
| BMI
(kg/m2) |
22.8±0.6 |
22.7±0.6 |
−0.02±0.09 |
22.8±0.7 |
22.9±0.7 |
0.15±0.08 | 0.165 |
| Systolic BP
(mmHg) |
132±4 |
128±4 |
−3.0±2.1 |
133±4 |
127±4 |
−5.3±3.2 | 0.563 |
| Diastolic BP
(mmHg) |
82±2 |
82±2 |
−0.2±1.4 |
83±2 |
81±3 |
−2.6±2.0 | 0.319 |
| Heart rate
(beats/min) |
69±2 |
67±3 |
−2.0±1.8 |
71±2 |
71±2 |
−0.1±1.1 | 0.369 |
| Serum total
cholesterol (mg/dl) |
217±10 |
217±10 |
−0.6±4.3 |
226±5 |
226±9 |
−0.4±
8.9 | 0.923 |
| Serum
LDL-cholesterol (mg/dl) |
138±8 |
138±8 |
0.1±3.8 |
147±5 |
148±8 |
1.5±8.3 | 0.874 |
| Serum
HDL-cholesterol (mg/dl) |
62±3 |
64±3 |
1.7±1.4 |
59±4 |
59±3 |
0.5±1.6 | 0.582 |
| Serum TG
(mg/dl) |
112±14 |
99±9 |
−13.1±13.7 |
125±13 |
121±12 |
−3.7±9.2 | 0.570 |
| Serum glucose
(mg/dl) |
92±2 |
93±2 |
1.1±1.4 |
92±1 |
93±2 |
1.2±1.5 | 0.945 |
Regarding the cutaneous blood flow or peripheral
microvascular function, the LDPI value for the placebo group at
week 8 was significantly lower than that at week 0 (0.68±0.02
versus 0.76±0.02, P<0.05). This reduction may have been due to
the seasonal drop in temperature during the eight-week intervention
period starting in August to September and ending in October to
November. It is well known that superficial blood flow is closely
associated with the skin surface temperature (23). Despite this, such a reduction in LDPI
values did not occur in the SLO group, leading to a significant
difference in the change from the baseline value between the two
groups (P=0.002, Table II). It is,
therefore, suggested that SLO supplementation is effective in
enhancing peripheral blood flow.
Effects on vascular health-related
confounding factors
Table II also shows
the changes in the mean values of weight, BMI, BP, serum lipids and
serum glucose over the eight-week intervention, with comparisons
between the placebo and SLO groups. No significant differences were
observed in any parameters within or between the groups. No subject
reported a significant change in dietary habit, physical activity
or smoking behaviour during the study period, and no subject
developed a noteworthy concurrent illness or was given any
medication.
Safety assessment
The incidence and pattern of adverse events was
virtually equivalent in the two groups. In the end-point study
diary, 19% (4/21) of subjects taking the placebo and 29% (6/21)
taking the SLO supplement reported minor adverse events (P=0.469),
the most common being gastrointestinal upset with or without
eruption. There was no intervention-related untoward side-effect
with clinical significance. No abnormality in the routine
laboratory test parameters was found in any subject in the two
groups.
Discussion
In this study, the effect of eight weeks of dietary
supplementation with 1,500 mg SLO (582 mg as squalene) daily was
compared with that of the placebo on the CAVI and LDPI values,
which are indexes of central arterial stiffness and peripheral
microvascular function, respectively. The dose of 1,500 mg SLO was
selected as it was the dose found to be well tolerated in our
preliminary toxicological studies. It is also the dose that is most
often taken by individuals using this SLO-containing
supplement.
One of main findings of this study was the presence
of a correlation between the SLO supplementation-induced changes in
arterial stiffness and the age; lower ages were associated with
greater decreases in arterial stiffness after the eight-week SLO
supplementation period. No difference, however, was found in the
mean changes of arterial stiffness between the placebo and SLO
groups. This may be due to the wide and unmatched distribution of
the age of the subjects in the two groups. To obtain conclusive
results, a similar study conducted with several different,
narrow-ranging age groups is therefore required.
Another main finding of this study was that eight
weeks of SLO supplementation significantly increased the peripheral
blood flow measured by an LDPI technique; however, the changes in
the LDPI values for the SLO group were not correlated with the
changes in the CAVI values. It is, therefore, likely that the
effects of SLO on each of these two vascular parameters may be
independent. Although the active components of SLO responsible for
the improvement of central arterial stiffness and peripheral
microvascular function by supplementation with SLO remain to be
explored, they warrant discussion.
The SLO supplement used in the present study
contained, in a daily dose, 582 mg squalene and 654 mg
alkylglycerols as the two major constituents. Information on the
biological or pharmacological activity of alkylglycerols, the
largest constituent of SLO in quantity, in animals or humans is
extremely limited. While the immunostimulatory and
haematopoiesis-stimulating effects or anti-tumor and
anti-metastasis activities of alkylglycerols have been reported
(24,25), no data are available, to the best of
our knowledge, on their central arterial or peripheral
microvascular effects. Squalene, the second largest constituent of
SLO, is a polyunsaturated triterpene containing six isoprene units
and thus has a structural similarity with various naturally
occurring polyprenyl compounds, including vitamin E (2). In vitro experimental evidence
indicates that squalene is a unique antioxidant molecule exhibiting
highly effective oxygen-scavenging activity (26,27).
Furthermore, in previous studies utilizing an isoprenaline-induced
myocardial infarction rat model it was shown that continuous oral
administration of squalene produced antioxidant and
cardioprotective effects by maintaining the levels of endogenous
antioxidant molecules, such as vitamins A, C and E, and by blocking
the induction of lipid peroxidation (28–30). It
is, therefore, likely that squalene, as a polyprenyl antioxidant
compound, has the structural and functional similarity with vitamin
E that has been reported to be effective in improving arterial
stiffness and microvascular function following continuous oral
administration (13,14,16,17).
These findings and the relatively high squalene content of SLO
suggest that squalene is the most likely contributing factor to the
beneficial vascular effects of SLO observed in the present
study.
Orally administered squalene is absorbed well
(60–85%) and is distributed to various tissues (31–33).
Although the underlying mechanisms remain to be elucidated, the
rapidity with which oral doses of SLO improve vascular structures
and/or functions indicates a direct effect on the physiological
components rather than the structural components that regulate the
elasticity of the arterial wall, as was observed for vitamin E
(34), as well as for fish oil
(35,36) and n-3 long-chain fatty acids
(37,38).
In the present study, supplementation with SLO and
thus squalene did not show clinically significant effects on
weight, BP and heart rate, or on serum levels of lipids, glucose or
any other laboratory test parameters. Among these biological
effects the major focus was on the effect on lipid profiles, as
squalene is well-known as a biochemical precursor of cholesterol,
leading to the possibility that orally administered squalene may
increase serum levels of cholesterol; however, this possibility is
challenged by a substantial number of existing in vitro and
in vivo studies demonstrating that exogenous squalene
exhibits feedback inhibition of 3-hydroxy-3-methylglutaryl coenzyme
A reductase, a key enzyme functioning in cholesterol biosynthesis
(39–41). Furthermore, Strandberg et al
(31), who conducted a human study
in which volunteers received a dietary supplement of squalene (900
mg/day for 7–30 days), reported that, while serum squalene levels
were increased 17-fold, there was no significant change in serum TG
or cholesterol levels, and that this was likely due to a
significant increase in the faecal excretion of cholesterol
synthesised from squalene, as well as of bile acids converted from
squalene. These results support the findings of the present study
showing that orally administered SLO, a dietary source of squalene,
did not increase serum levels of cholesterol nor TG.
No significant adverse events that could be
attributed to the SLO supplement were noted in this study. As
neither SLO nor squalene have been extensively studied in humans,
information on their toxicity and side-effects is limited. Kamimura
et al (42), who conducted
experiments in animals (rats and dogs), reported that no
appreciable side-effects or toxic signs were observed in animals
receiving squalene for three months. Despite this, the long-term
effects and safety of extreme squalene are not known (43). Since SLO, as a dietary source of
squalene, is of biological origin and frequently used as a dietary
supplement, it appears likely that, at reasonable supplemental
levels, such as those used in this study, SLO is safe for prolonged
administration in humans.
To the best of our knowledge, there is no previous
study that has investigated the effect of SLO on either arterial
stiffness or peripheral blood flow or both in humans; however, as
the present study has a limitation that the sample size was small,
the findings, particularly those on the apparently age-related
benefits of SLO supplementation on arterial stiffness, require
confirmation in a larger population with a narrower range of
ages.
In conclusion, SLO supplementation may have the
potential to improve central arterial elasticity and peripheral
microvascular function in otherwise healthy middle-aged and elderly
males with slightly increased arterial stiffness. Further study is
required to confirm the age-related beneficial vascular effect of
SLO supplementation on arterial stiffness.
References
|
1
|
Tsujimoto M: A highly unsaturated
hydrocarbon in shark liver oil. Ind Eng Chem. 8:889–896. 1916.
View Article : Google Scholar
|
|
2
|
Reddy LH and Couvreur P: Squalene: A
natural triterpene for for use in disease management and therapy.
Adv Drug Deliv Rev. 61:1412–1426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mottram P, Shiqe H and Nestel P: Vitamin E
improves arterial compliance in middle-aged men and women.
Atherosclerosis. 145:399–404. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Plantinga Y, Ghiadoni L, Magagna A,
Giannarelli C, Franzoni F, Taddei S and Salvetti A: Supplementation
with vitamins C and E improves arterial stiffness and endothelial
function in essential hypertensive patients. Am J Hypertens.
20:392–397. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jörneskog G, Brismar K and Fagrell B: Skin
capillary circulation severely impaired in toes of patients with
IDDM, with and without late diabetic complications. Diabetologia.
38:474–480. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nilsson L, Apelqvist J and Edvinsson L:
Effects of alpha-trinositol on peripheral circulation in diabetic
patients with critical limb ischaemia. A pilot study using laser
Doppler fluxmetry, transcutaneous oxygen tension measurements and
dynamic capillaroscopy. Eur J Vasc Endovasc Surg. 15:331–336. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collins EG, Edwin Langbein W, Orebaugh C,
Bammert C, Hanson K, Reda D, Edwards LC and Littooy FN:
PoleStriding exercise and vitamin E for management of peripheral
vascular disease. Med Sci Sports Exerc. 35:384–393. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gatzka CD, Cameron JD, Kingwell BA and
Dart AM: Relation between coronary artery disease, aortic
stiffness, and left ventricular stiffness in a population sample.
Hypertension. 32:575–578. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sutton-Tyrrell K, Najjar SS, Boudreau RM,
Venkitachalam L, Kupelian V, Simonsick EM, Havlik R, Lakatta EG,
Spurgeon H, Kritchevsky S, Pahor M, Bauer D and Newman A: Health
ABC Study: Elevated aortic pulse wave velocity, a marker of
arterial stiffness, predicts cardiovascular events in
well-functioning older adults. Circulation. 111:3384–3390. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vlachopoulos C, Aznaouridis K and
Stefanadis C: Prediction of cardiovascular events and all-cause
mortality with arterial stiffness: a systematic review and
meta-analysis. J Am Coll Cardiol. 55:1318–1327. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kubozono T, Miyata M, Ueyama K, Nagaki A,
Otsuji Y, Kusano K, Kubozono O and Tei C: Clinical significance and
reproducibility of new arterial distensibility index. Circ J.
71:89–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takaki A, Ogawa H, Wakeyama T, Iwami T,
Kimura M, Hadano Y, Matsuda S, Miyazaki Y, Matsuda T, Hiratsuka A
and Matsuzaki M: Cardio-ankle vascular index is a new noninvasive
parameter of arterial stiffness. Circ J. 71:1710–1714. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yambe T, Yoshizawa M, Saijo Y, Yamaguchi
T, Shibata M, Konno S, Nitta S and Kuwayama T: Brachio-ankle pulse
wave velocity and cardio-ankle vascular index (CAVI). Biomed
Pharmacother 58 Suppl. 1:S95–S98. 2004. View Article : Google Scholar
|
|
14
|
Shirai K, Utino J, Otsuka K and Takata M:
A novel blood pressure-independent arterial wall stiffness
parameter; cardio-ankle vascular index (CAVI). J Atheroscler
Thromb. 13:101–107. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsui Y, Kario K, Ishikawa J, Eguchi K,
Hoshide S and Shimada K: Reproducibility of arterial stiffness
indices (pulse wave velocity and augmentation index) simultaneously
assessed by automated pulse wave analysis and their associated risk
factors in essential hypertensive patients. Hypertens Res.
27:851–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki H, Ishizuka N, Makoto M, et al:
Establishment of reference values of CAVI and disease
characterisitics. From Bench to Bedside: CAVI as a Novel Indicator
of Vascular Function. Omori H and Saito Y: Nikkei Medical Custom
Publishing, Inc.; Tokyo: pp. 34–42. 2009
|
|
17
|
Wådell K, Jakobsson A and Nilsson G: Laser
Doppler perfusion imaging by dynamic light scattering. IEEE Trans
Biomed Eng. 40:309–316. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bornmyr S, Svensson H, Lilja B and
Sundkvist G: Skin temperature changes and changes in skin blood
flow monitored with laser Doppler flowmetry and imaging: a
methodological study in normal humans. Clin Physiol. 17:71–81.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okura T, Watanabe S, Kurata M, Manabe S,
Koresawa M, Irita J, Enomoto D, Miyoshi K, Fukuoka T and Higaki J:
Relationship between cardio-ankle vascular index (CAVI) and carotid
atherosclerosis in patients with essential hypertension. Hypertens
Res. 30:335–340. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kadota K, Takamura N, Aoyagi K, Yamasaki
H, Usa T, Nakazato M, Maeda T, Wada M, Nakashima K, Abe K,
Takeshima F and Ozono Y: Availability of cardio-ankle vascular
index (CAVI) as a screening tool for atherosclerosis. Circ J.
72:304–308. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura K, Tomaru T, Yamamura S,
Miyashita Y, Shirai K and Noike H: Cardio-ankle vascular index is a
candidate predictor of coronary atherosclerosis. Circ J.
72:598–604. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sakane K, Miyoshi T, Doi M, Hirohata S,
Kaji Y, Kamikawa S, Ogawa H, Hatanaka K, Kitawaki T, Kusachi S and
Yamamoto K: Association of new arterial stiffness parameter, the
cardio-ankle vascular index, with left ventricular diastolic
function. J Atheroscler Thromb. 15:261–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mirbod SM, Yoshida H, Jamail M, Miyashita
K, Takeda H, Inaba R and Iwata H: Finger skin temperature and
laser-Doppler finger blood flow in subjects exposed to hand-arm
vibration. Ind Health. 36:171–178. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hallgren B, Niklasson A, Ställberg G and
Thorin H: On the occurrence of 1-O-alkylglycerols and
1-O-(2-methoxyalkyl)glycerols in human colostrum, human milk, cow's
milk, sheep's milk, human red bone marrow, red cells, blood plasma
and a uterine carcinoma. Acta Chem Scand B. 28:1029–1034. 1974.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Deniau AL, Mosset P, Pédrono F, Mitre R,
LeBot D and Legrand AB: Multiple beneficial health effects of
natural alkylglycerols from shark liver oil. Mar Drugs.
8:2175–2184. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saint-Leger D, Bague A, Cohen E and Chivot
M: A possible role for squalene in the pathogenesis of acne. I. In
vitro study of squalene oxidation. Br J Dermatol. 114:535–542.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kohno Y, Egawa Y, Itoh S, et al: Kinetic
study of quenching reaction of singlet oxygen and scavenging
reaction of free radical by squalene in n-butanol. Biochim Biophys
Acta. 1256:52–56. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sabeena Farvin KH, Surendraraj A and
Anandan R: Protective effect of squalene on endogenous antioxidant
vitamins in experimentally induced myocardial infarction in rats.
Asian J Biochem. 4:133–139. 2009. View Article : Google Scholar
|
|
29
|
Sabeena Farvin KH, Kumar SHS, Anandan R,
Mathew S, Sankar TV and Nair PGV: Supplementation of squalene
attenuates experimentally induced myocardial infarction in rats.
Food Chem. 105:1390–1395. 2007. View Article : Google Scholar
|
|
30
|
Sabeena Farvin KH, Anandan R, Kumar SH,
Shiny KS, Sankar TV and Thankappan TK: Effect of squalene on tissue
defense system in isoproterenol-induced myocardial infarction in
rats. Pharmacol Res. 50:231–236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strandberg TE, Tilvis RS and Miettinen TA:
Metabolic variables of cholesterol during squalene feeding in
humans: comparison with cholestyramine treatment. J Lipid Res.
31:1637–1643. 1990.PubMed/NCBI
|
|
32
|
Miettinen TA and Vanhanen H: Serum
concentration and metabolism of cholesterol during rapeseed oil and
squalene feeding. Am J Clin Nutr. 59:356–363. 1994.PubMed/NCBI
|
|
33
|
Gylling H and Miettinen TA: Postabsorptive
metabolism of dietary squalene. Atherosclerosis. 106:169–178. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Motoyama T, Kawano H, Kugiyama K,
Hirashima O, Ohgushi M, Tsunoda R, Moriyama Y, Miyao Y, Yoshimura
M, Ogawa H and Yasue H: Vitamin E administration improves
impairment of endothelium-dependent vasodilation in patients with
coronary spastic angina. J Am Coll Cardiol. 32:1672–1679. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang S, Ma AQ, Song SW, Quan QH, Zhao XF
and Zheng XH: Fish oil supplementation improves large arterial
elasticity in overweight hypertensive patients. Eur J Nutr.
62:1426–1431. 2008. View Article : Google Scholar
|
|
36
|
Fahs CA, Yan H, Ranadive S, Rossow LM,
Agiovlasitis S, Wilund KR and Fernhall B: The effect of acute
fish-oil supplementation on endothelial function and arterial
stiffness following a high-fat meal. Appl Physiol Nutr Metab.
35:294–302. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mozaffarian D and Wu JH: (n-3) fatty acids
and cardiovascular health: are effects of EPA and DHA shared or
complementary? J Nutr. 142:614S–625S. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siasos G, Kioufis S, Maniatis K, Miliou A
and Siasou Z: Effects of Ω-3 fatty acids on endothelial function,
arterial wall properties, inflammatory and fibrinolytic status in
smokers: a cross-over study. Int J Cardiol. 116:340–346. 2013.
View Article : Google Scholar
|
|
39
|
Sawada M, Matsuo M, Hagihara H, Tenda N,
Nagayoshi A, Okumura H, Washizuka K, Seki J and Goto T: Effect of
FR194738, a potent inhibitor of squalene epoxidase on cholesterol
metabolism in HepG2 cells. Eur J Pharmacol. 431:11–16. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Newmark HL: Squalene, olive oil, and
cancer risk. Review and hypothesis. Ann NY Acad Sci. 889:193–203.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Smith TJ: Squalene: potential
chemopreventive agent. Expert Opin Investig Drugs. 9:1841–1848.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaminura H, Koga N, Oguri K, Yoshimura H,
Inoue H, Sato K and Ohkubo M: Studies on distribution, excretion
and subacute toxicity of squalene in dogs. Fukuoka Igaku Zasshi.
80:269–280. 1989.(In Japanese). PubMed/NCBI
|
|
43
|
Sotiroudis TG and Kyrtopoulos SA:
Anticarcinogenic compounds of olive oil and related biomarkers. Eur
J Nutr. 47(Suppl 2): 69–72. 2008. View Article : Google Scholar : PubMed/NCBI
|