Introduction
The oncogenic potential of Epstein-Barr virus (EBV)
is associated with its ability to infect and transform T
lymphocytes into continuously proliferating lymphoblastoid cells.
The virus has also been implicated in the development of T-cell
lymphoproliferative diseases (T-LPDs). EBV-positive T-LPD
(EBV+ T-LPD) includes the polyclonal, oligoclonal and
monoclonal proliferation of cytotoxic T cells (1). This disease is rare, with high rates of
morbidity and mortality and is more prevalent in Eastern Asian
countries (2). The disease is
associated with a poor prognosis, a progressive clinical
manifestation, diverse pathological types and cell clones and
numerous stages of development, which are different from either the
benign lesions of infectious mononucleosis or typical lymphoma
lesions. To avoid over- and under-diagnosis, the possibility of a
single disease having different stages of development, as well as
the consideration that the combination of clinical and laboratory
findings with the pathological and immunohistochemical
characteristics can be beneficial in reaching the correct
diagnosis, should be taken into account. The present study
describes a case of adult systemic EBV+ T-LPD
(ASEBV+ T-LPD).
Case report
A 21-year old female patient with a 20-day history
of high fever, fatigue and yellow sclerae was admitted to The Union
Hospital, Tongji Medical College (Wuhan, China) on September 1,
2010. The patient had no other medical history of note. Written
informed consent was obtained from the patient for the present
report.
The physical examinations that were performed on
admission showed yellowing of the skin and sclerae, a soft lymph
node without tenderness in the left of the neck and the right side
of the groin, and grade III-bilateral tonsillar enlargement with
pus emboli. The liver could not be located through touch, but the
spleen was felt 3 cm below the rib. The results of the laboratory
tests performed are shown in Table
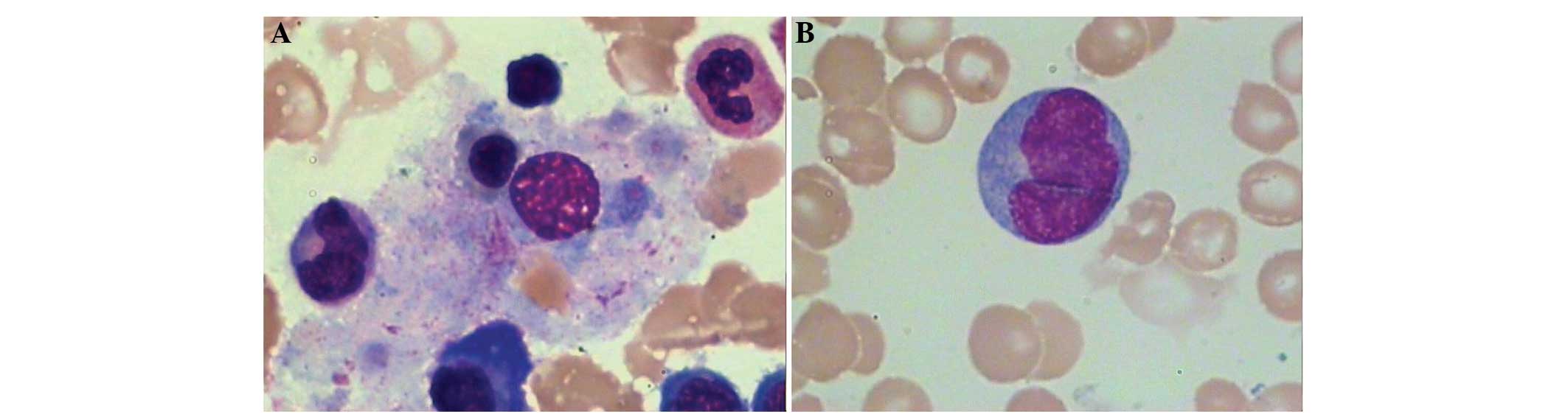
I. The cytological examination of the bone marrow exhibited
karyocytic hyperplasia (Fig. 1).
Granulocytes accounted for 43.0% of the cell population,
erythrocytes for 34.5%, lymphocytes for 19.0% and heterotypic
lymphocytes for 3.5%. Flow cytometric analysis of the bone marrow
revealed a reduction in the cluster of differentiation
CD3+CD4+/CD3+CD8+
ratio, with a value of 0.57. Lymph node immunophenotyping showed
that lymphocytes accounted for 80% of the karyocytes; of the
lymphocytes, ~40% were B lymphocytes and ~58.75% were
CD56+ cells. The CD56+ cells were
additionally found to be positive for T-antigens, such as CD2, CD3,
CD5, T cell receptor (TCR) α/β and human leukocyte antigen
(HLA)-DR, suggesting the existence of abnormal natural killer
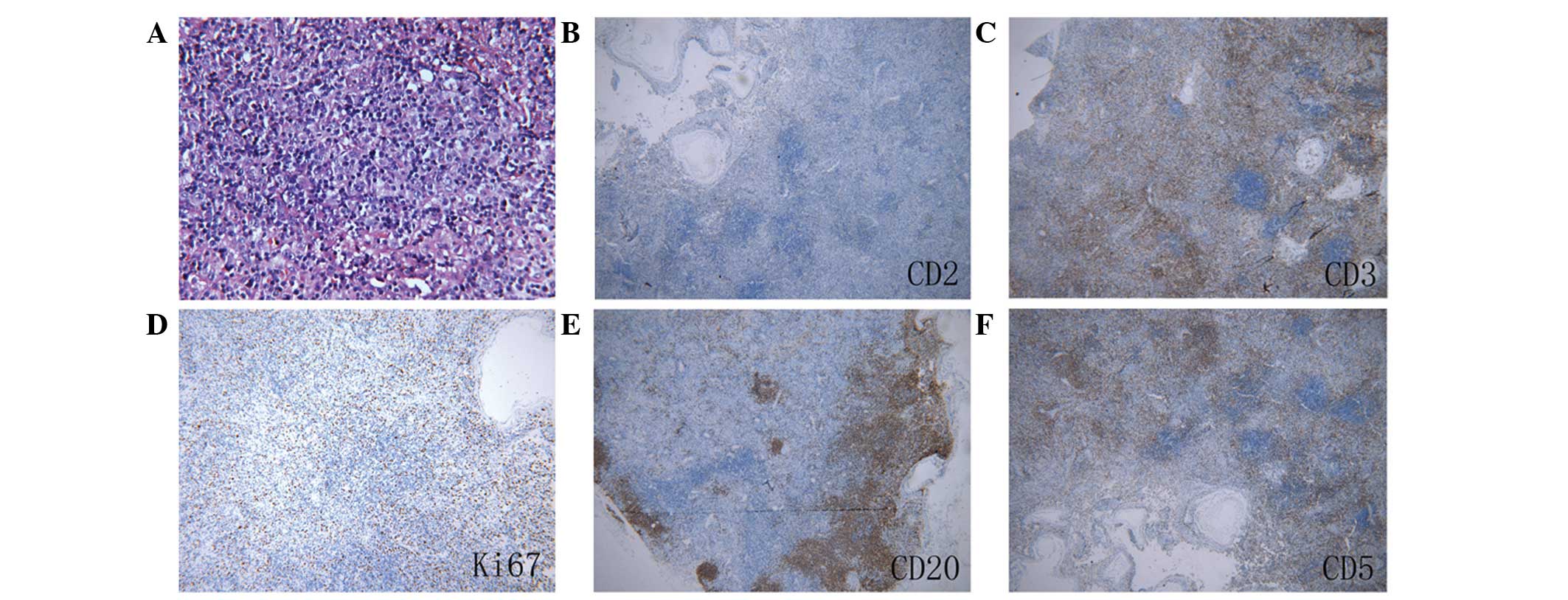
(NK)/T cells. Pathological examination of the cervical lymph nodes
revealed that the basic structural features, including lymph sinus
and reactive lymphoid follicles, were present. Immunohistochemistry
showed that infiltrated cells were T-cell-restricted intracellular
antigen 1 (TIA-1) (+), Ki67 (+) (20% only), CD4 (-), CD8 (+), CD2
(-), CD5 (+), anaplastic lymphoma kinase (ALK) (-), CD20 (+), CD3
(+), CD21 (+) (follicular dendritic cells only), CD30 (-) and
paired box protein Pax-5 (-) (Fig.
2). The pathological diagnosis was atypical T-cell hyperplasia
of the lymph node.
 | Table I.Laboratory tests. |
Table I.
Laboratory tests.
| Test | Result |
|---|
| Routine blood |
|
| WBC
(g/l) |
3.24×109 |
| Hb
(g/l) | 98 |
| PLT
(g/l) |
1.85×1011 |
| Liver/kidney
function |
|
| TBA
(µmol/l) | 417 |
| TB
(µmol/l) | 231 |
| DB
(µmol/l) | 881 |
| ALT
(U/l) | 417 |
| AST
(U/l) | 231 |
| ALP
(U/l) | 881 |
| ALB
(g/l) | 29.4 |
|
K+ (mmol/l) | 5.7 |
| Imaging | Bronchitis, bilateral
axillary lymph node enlargement, obvious hepatosplenomegaly |
| Others |
|
| Serum
EBVCA-IgM | Positive |
|
Bacterial/viral/tumor
antigens |
Negative |
|
Heterophil agglutination |
Negative |
The patient was administered liver protection
treatment and supporting therapy, which led to the gradual recovery
of liver function; however, she still presented with fever and
superficial lymphadenopathy, resulting in the suspicion of
lymphoma. On September 18, a neoplasm was found in the region of
the pharynx lying above the soft palate of the patient, and she was
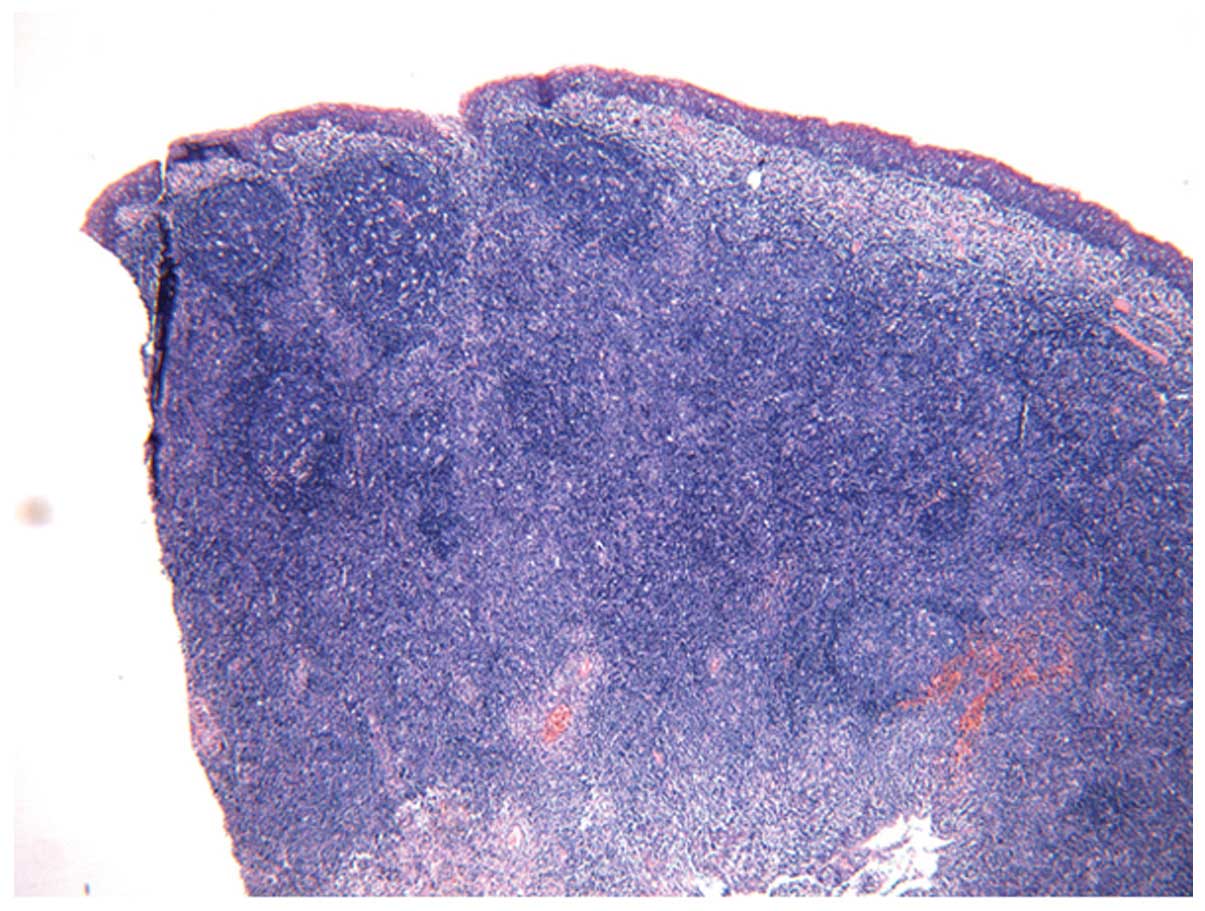
subjected to tonsillectomy and neoplasm biopsy. Pathological
examination of the nasopharynx and bilateral tonsil showed chronic
inflammatory changes (Fig. 3).
Immunohistochemistry of the nasopharynx showed that the infiltrated
cells were diffusely positive for CD20, CD3 and Ki67 (outside the
germinal center) but negative for CD15. A small number of cells
were positive for CD30.
Due to the atypical nature of the pathological
characteristics, the patient visited another hospital (Beijing
Friendship Hospital, Capital University of Medical Sciences,
Beijing, China) and was examined for other cervical lymph node
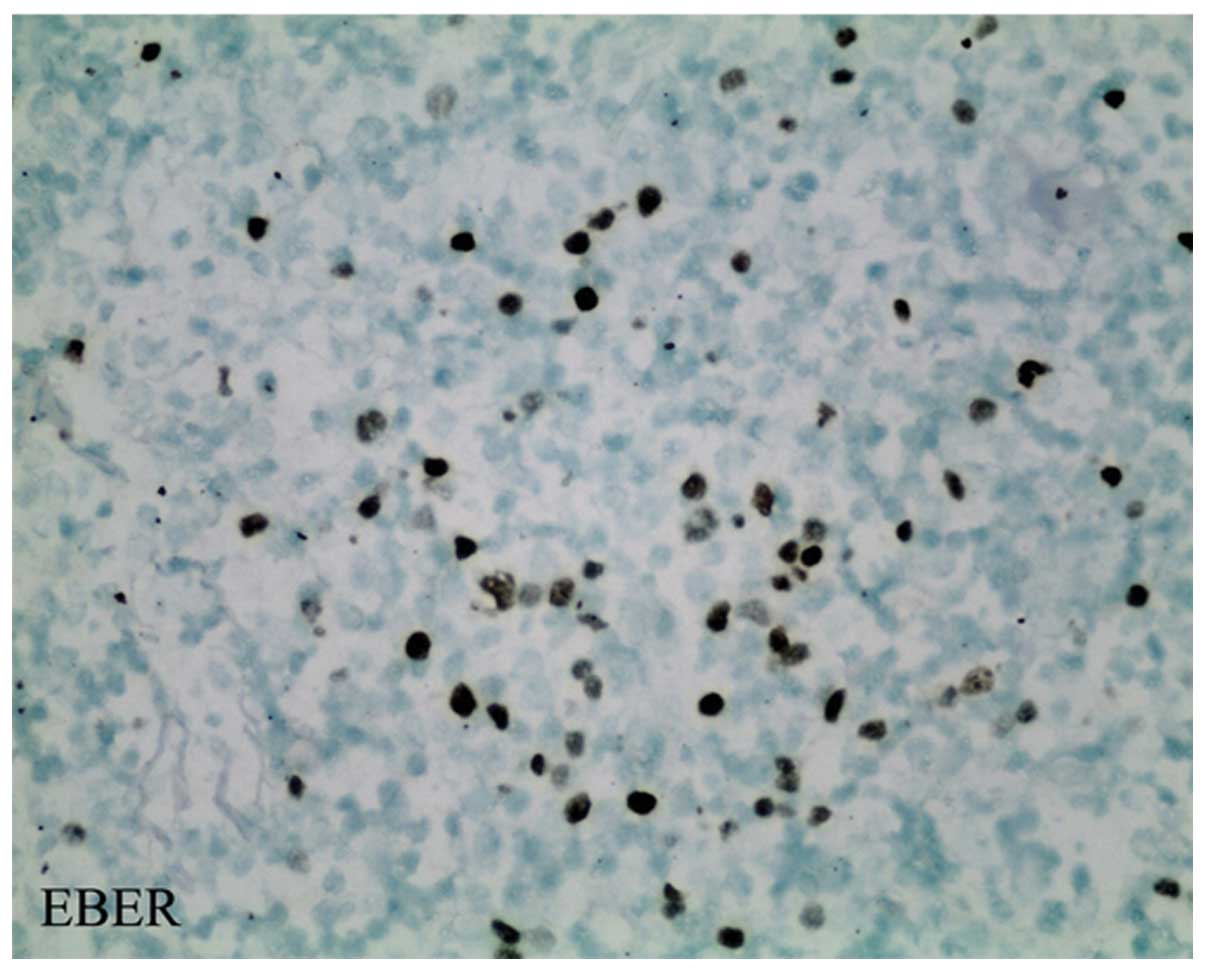
markers, giving the following results: Myeloperoxidase (MPO) (-),
CD34 (-) and EBV-encoded RNA (EBER) (+) (Fig. 4). The cells exhibited marked
proliferation and morphologically resembled lymphoma cells. T-cell
markers were expressed in the proliferating cells, with the
exception of CD2, indicating tumorigenesis; however, the absence of
tumor-related immune markers did not favor a diagnosis of lymphoma.
EBV-infected lymph node inflammation was pathologically diagnosed.
On September 28, the body temperature of the patient continued to
rise (maximum, 40°C), and numerous pus emboli had adhered to the
posterior wall of the nasopharynx. Magnetic resonance imaging (MRI)
of the nasopharynx revealed a thickened mucous membrane but no
signals indicative of an abnormal mass. The antigen receptor gene
rearrangement test and the immunoglobulin heavy- and light-chain
gene rearrangement studies by polymerase chain reaction failed to
demonstrate conclusive evidence of a clonal B- or T-cell
population. The conventional chromosomal study revealed a normal
karyotype. Given the changes in the condition of the patient,
ASEBV+ T-LPD was clinically diagnosed. During
hospitalization, the patient was given anti-infection treatment
(cefepime, fluconazole, etc.); her body temperature returned to the
normal level, the jaundice disappeared and no systemic superficial
lymphadenopathy or hepatosplenomegaly was detected. The patient was
discharged on October 15, 2010 following the improvement in her
condition. During a two-year follow-up period the patient had no
fever or enlarged superficial lymph nodes, and the peripheral blood
examination and liver and kidney function tests were normal.
Discussion
The incidence of EBV infection in the Chinese
population is 90%, and EBV is the common pathogenic factor of
numerous diseases, including infectious mononucleosis (IM), Burkitt
lymphoma and NK/T-cell lymphoma (3,4). In
addition, EBV infection is closely associated with certain LPDs
that are in a stage of development between tumor and non-cancer. In
2008, the World Health Organization classified EBV+
T-LPD (1) into childhood systemic
EBV+ T-LPD (CSEBV+ T-LPD) and
ASEBV+ T-LPD. ASEBV+ T-LPD is a rare disease
characterized by EBV-infected T-cell proliferation with a cytotoxic
phenotype. It has been suggested that, in the early stages of
CSEBV+ T-LPD, EBV-infected cells exhibit poly- or
oligoclonal proliferation, which often progresses to monoclonal
proliferation in the later disease stages (2), suggesting that CSEBV+ T-LPD
is essentially a disease spectrum that incorporates different
stages of development, ranging from benign to malignant
proliferation (5). The case reported
in the present study was clinically characterized by a subacute
onset, moderate-to-severe fever, systemic lymphadenopathy,
hepatosplenomegaly, swollen tonsils, bronchitis, jaundice,
pancytopenia, EBV infection and bone marrow proliferation.
Pathological examination of the lymph nodes revealed expansion of
the interfollicular area, which was diffusely infiltrated by a
polymorphous infiltrate of small-to-medium-sized lymphocytes,
plasma cells and immunoblasts. In terms of immunohistochemistry,
the infiltrated cells had a strong, diffuse positivity for CD3,
CD8, CD5, TIA and EBER, and CD20 staining was present in admixed
normal-appearing B-lymphocytes. The infiltrated cells were negative
for CD2, CD4, CD30, CD34, CD117, MPO, ALK and latent membrane
protein 1. Overall, the pathological and immunohistochemical
studies of the cervical lymph nodes were indicative of early-stage
disease, which is coincident with the A1 category from the
classification of EBV+ NK/T-LPD proposed by Ohshima
et al (2).
In the present case, the patient exhibited a grade
III-bilateral tonsil enlargement with pus emboli; bone marrow smear
showed heterotypic lymphocytes accounting for 3.5% of the cell
population. Immunophenotypic analysis of the lymph nodes revealed
that the CD56+ cells accounted for 58.75% of the
lymphocytes and that T-antigens, such as CD2, CD3, CD5, TCR α/β and
HLA-DR, were also simultaneously expressed in certain
CD56+ cells, which could be considered as abnormal NK/T
cells. As the disease progressed, a nasopharyngeal neoplasm was
detected, which, based on the clinical features and
immunohistochemical analysis, could have led to a misdiagnosis of
NK/T-cell lymphoma. NK/T-cell lymphoma characteristically arises in
the nasal cavity or surrounding structures and manifests as a
destructive midline facial lesion with tumor cells expressing CD2,
CD56, cytotoxic granule proteins, cytoplasmic CD3 and TIA-1, but
not CD3 (6,7). Although the immunophenotypic analysis
of the cervical lymph nodes in the present case revealed abnormal
NK/T-cell proliferation, the tonsil and nasopharyngeal neoplasm
biopsy indicated inflammatory changes, and the nasopharyngeal MRI
scan showed a thickened mucous membrane but no signals indicative
of an abnormal mass. The immunophenotyping of the cervical lymph
nodes showed negativity for CD2 and positivity for CD3. A number of
the present findings indicate the possibility of progression to
NK/T-cell lymphoma, and due caution should therefore be taken.
These findings included the fact that i) the infiltrating cells
were small-to-medium-sized lymphocytes with large cells exhibiting
different degrees of atypia scattered among them; ii) CD8
expression was present in the majority of the cells; iii) there was
diffuse CD3-positivity; and iv) the Ki67-positivity was <30%
(5).
The differential diagnosis of ASEBV+
T-LPD versus IM was raised due to the atypical pathology of
cervical lymph nodes. The clinical and pathological diagnosis of IM
was described by Chen et al (8) and Zhou (9). The characteristic pathological changes
described included expansion of the paracortical area and
morphological changes in the B-cell differentiation spectrum
(lymphoblasts, immunoblasts, plasmacytoid cells, mature plasma
cells), with CD3-positivity and varying degrees of scattered CD20
and CD30-positivity; however, in the present case the lesion did
not contain CD30 (+) cells and the pathological examination of the
lymph nodes did not demonstrate the typical pathological changes of
IM, i.e. B-cell differentiation spectrum changes. To avoid over-
and under-diagnosis, it is necessary to consider that a single
disease has different stages of development, and the combination of
clinical and laboratory findings with pathological and
immunohistochemical features is beneficial for the correct
diagnosis.
Acknowledgements
The authors would like to thank Department of
Pathology, Peking University Health Science Center for the
pathological images.
References
|
1
|
Quintanilla-Martinez L, Kimura H and Jaffe
ES: Epstein-Barr virus (EBV) positive T-cell lymphoproliferative
diseases of childhoodSwerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW: WHO Classification of
Tumours of Haematopoietic and Lymphoid Tissues. 2. 4th. IARC Press;
Lyon: pp. 278–280. 2008
|
|
2
|
Ohshima K, Kimura H, Yoshino T, Kim CW, Ko
YH, Lee SS, Peh SC and Chan JKCAEBV Study Group: Proposed
categorization of pathological states of EBV-associated T/natural
killer-cell lymphoproliferative disorder (LPD) in children and
young adults: Overlap with chronic active EBV infection and
infantile fulminant. EBV T-LPD. Pathol Int. 58:209–217. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai HH and Ma L: Research progress of EB
virus related malignancies. Zhongguo Xiao Er Yu Xue Ye Za Zhi.
18:245–249. 2013.(In Chinese).
|
|
4
|
Quintanilla-Martinez L, Kumar S, Fend F,
Reyes E, Teruya-Feldstein J, Kingma DW, Sorbara L, Raffeld M,
Straus SE and Jaffe ES: Fulminant EBV(+) T-cell lymphoproliferative
disorder following acute/chronic EBV infection: A distinct
clinicopathologic syndrome. Blood. 96:443–451. 2000.PubMed/NCBI
|
|
5
|
Jin Y, Zhou XG, He LJ, Xie JL, Zheng YY,
Zhang YN and Zhang SH: Clinicopathologic features of systemic
EBV-positive T-cell lymphoproliferative disease of childhood.
Zhonghua Bing Li Xue Za Zhi. 38:600–608. 2009.(In Chinese).
PubMed/NCBI
|
|
6
|
Yachie A, Kanegane H and Kasahara Y:
Epstein-Barr virus-associated T-/natural killer cell
lymphoproliferative diseases. Semin Hematol. 40:124–132. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li CC, Tien HF, Tang JL, Yao M, Chen YC,
Su IJ, Hsu SM and Hong RL: Treatment outcome and pattern of failure
in 77 patients with sinonasal natural killer/T-cell or T-cell
lymphoma. Cancer. 100:366–375. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YZ, Zhou XG, Jin Y, Zheng YY, Chen G
and Shi Y: Study of clinical and morphological features,
immunophenotype and Epstein-Bar virus infection in situ of
infectious mononucleosis. Zhonghua Bing Li Xue Za Zhi. 37:440–444.
2008.(In Chinese). PubMed/NCBI
|
|
9
|
Zhou XG: Increasing recognition of T zone
lymphoproliferative disorders. Zhonghua Bing Li Xue Za Zhi.
36:73–75. 2007.(In Chinese). PubMed/NCBI
|