Introduction
The no-reflow (NR) phenomenon, an important
manifestation of disordered myocardial microcirculation, is the
failure of the blood to reperfuse an ischemic area following the
removal of the physical obstruction by primary percutaneous
coronary intervention (PCI) or thrombolysis (1,2). The
incidence and extent of NR strongly predict adverse clinical
outcomes, including persistent contractile dysfunction of the left
ventricle, malignant arrhythmias and cardiac death (3).
Acute inferior myocardial infarction (AIMI), in
which the right coronary artery (RCA) is often the infarct-related
artery (IRA), is usually indicative of two characteristics: i) A
heavier thrombus burden, which significantly increases the risk of
NR following primary PCI (4); and
ii) unstable hemodynamic states, such as hypotension, bradycardia
and high vagus tone, which can result in circulatory collapse,
reperfusion injury and serious arrhythmias, including severe
atrioventricular block, during primary PCI (5).
Anisodamine, a drug with multiple pharmacological
effects, can maintain the stability of the hemodynamic states and
increase heart rate (HR), blood pressure (BP), coronary perfusion
pressure and heart inotropy, making it suitable for AIMI.
Anisodamine is also an M-receptor blocker and can dilate the small
vessels and improve the microcirculation (6). Our previous studies found that
intracoronary administration of anisodamine was effective in
reversing the NR following primary PCI (6–9).
Nicorandil maintains the activation of adenosine
triphosphate (ATP)-sensitive K+ channels and induces
nitric oxide production in an identical manner to that of nitrates,
resulting in the dilatation of the coronary microcirculation,
ischemic preconditioning, anti-arrhythmic effects and a reduction
in reperfusion injury. Intravenous nicorandil administration in
patients with acute MI (AMI) undergoing PCI has been shown to
effectively improve left ventricular systolic function and reduce
NR and slow flow (10–12); however, to the best of our knowledge,
no studies have investigated the effect of intracoronary nicorandil
and anisodamine administration in patients with AIMI and,
therefore, the optimal dosage, administration method and safety of
the combination therapy remain unknown. Based on this, the
objective of the present study was to assess the effect of
anisodamine and nicorandil regimens on the prevention of NR and the
amelioration of myocardial reperfusion in patients with AIMI
undergoing primary PCI.
Materials and methods
Study population inclusion
criteria
Between September 2011 and January 2014, a total of
115 consecutive patients with AIMI who were admitted within 12 h of
symptom onset and treated through primary PCI were enrolled into
this open-label, randomized, controlled study. Eleven patients were
excluded subsequently due to a lack of file information and an
emergency status requiring surgical intervention. Consequently, the
population of this study consisted of 104 patients.
Patients were eligible if they fulfilled the
following criteria: i) Ischemic chest pain lasting for ≥20 min,
which could not be relieved by oral nitrates; and ii) clear
AIMI-related changes in the electrocardiogram (ECG) during chest
pain, i.e. new ST-segment elevation with the cut-off points ≥1 mm
in ≥2 standard leads or ≥2 mm in ≥2 contiguous precordial leads
with or without the elevation of cardiac enzymes.
Study population exclusion
criteria
Patients were excluded if one of the following
characteristics was present: i) Cardiogenic shock (Killip class
IV); ii) tachycardia (HR ≥100 bpm); iii) known allergy to every
essential drug; iv) bleeding history; v) hepatic dysfunction; vi)
renal dysfunction (creatinine >12 mg/dl); vii) thrombolysis;
viii) requirement for coronary artery bypass grafting (CABG); ix)
contraindication to antiplatelet and anticoagulation therapy; and
x) previous MI. The study was approved by the Ethics Committee of
the Second Hospital of Hebei Medical University (Shijiazhuang,
China) and the Affiliated Hospital of Hebei University (Baoding,
China), and written informed consent was obtained from all
patients.
Dose and timing of anisodamine and
nicorandil administration
Subjects were immediately transferred to the cathlab
to undergo emergency PCI (door-to-balloon ≤90 mins). The study
population was divided into four groups (n=26 per group): A
(control group, treated with PCI only), B (anisodamine group,
treated with intracoronary anisodamine), C (nicorandil group,
treated with intracoronary nicorandil) and D (anisodamine and
nicorandil group, treated with intracoronary nicorandil and
anisodamine). Patients received 2 mg anisodamine (Minsheng
Pharmaceutical Group Co. Ltd., Hangzhou, China) and 2 mg nicorandil
(Sihuan Kebao Pharmaceutical Group Co. Ltd., Beijing, China) via
intracoronary administration. The optimal timing of drug
administration was as follows: i) If initial Thrombolysis In
Myocardial Infarction [TIMI (13)]
grade >0, drugs can be administered into the coronary artery
through the guide catheter; or ii) if initial TIMI grade=0, the
guidewire should be advanced through the total occlusion to the
distal site of the IRA. The anterior flow of the IRA will then
recover, which means that the TIMI grade will be >0 so drugs can
then be administered into the coronary artery through the guide
catheter.
The administration of other medications was
performed according to the current best clinical practice: Aspirin,
loading dose of 300 mg and then 100 mg once daily (qd);
clopidogrel, loading dose of 600 mg and then 75 mg qd; heparin,
40–70 U/kg (activated clotting time >200 sec);
low-molecular-weight heparin; angiotensin-converting enzyme
inhibitors/angiotensin receptor blockers [systolic BP (SBP) >120
mmHg]; β-blockers (HR >60 bpm) and statins. Tirofiban
administration was under the careful discretion of the
interventional cardiologists.
Coronary angiography and other
examinations
Patients underwent PCI via transradial artery
access. The initial TIMI, final TIMI/corrected TIMI frame count and
TIMI myocardial perfusion grade [TMPG (14)] were evaluated by two cardiologists
blinded to the clinical status of the patient and the treatment
modality.
Coronary SBP, diastolic BP (DBP) and mean BP were
measured by invasive catheterization before and 1, 5 and 10 min
after anisodamine and nicorandil administration. Infarct size was
estimated by peak levels of creatine kinase-MB (CK-MB) and troponin
I (cTnI), which were determined before and every 4 h after the
procedure using a Coulter LH 780 Hematology Analyzer (Beckman
Coulter Ireland Inc., Galway, Ireland).
A 12-lead ECG was recorded both on admission and 90
min after PCI. ST-segment elevation was recorded 20 msec after the
J point. The sum ST-segment elevation was calculated in leads II,
III and aVF for inferior infarction. A decrease in the sum
ST-segment elevation by ≥70% was categorized as complete ST-segment
resolution (STR) (15) and used as
an indirect measure of myocardial reperfusion following PCI
(16).
Primary end-point
The primary end point was a TMPG of 3 (17) following the procedure.
Major adverse cardiovascular events
(MACEs)
The MACEs included i) all-cause mortality; ii) new
MI, as indicated by the onset of recurrent ischemic chest pain
lasting ≥30 min with new ST-T segment changes of ≥24 h duration or
new pathologic Q waves (≥2 leads) and an elevation in serum CK
levels to >2-fold the normal upper limit or an elevated CK-MB
fraction value; iii) target vessel revascularization (TVR) for
recurrent ischemia or acute stent occlusion, including repeat PCI
or CABG. Composite end-points, i.e. mortality + new MI + TVR) were
evaluated during the hospital stay and 30 days after discharge.
Statistical analysis
In the PCI era, a reasonable estimate of the
proportion of patients achieving optimal myocardial reperfusion
(TMPG 3) among patients without cardiogenic shock undergoing PCI is
~50% (1). It is speculated that the
proportion of patients achieving a postprocedural TMPG of 3 can
reach 90% following anisodamine and nicorandil administration.
Accordingly, ≥26 patients per group were required for the power of
the test set at 0.8 and the statistical significance level
(two-sided) at 0.025.
Continuous variables are expressed as the mean ±
standard deviation, and categorical variables are presented as
percentages. Continuous variables were compared using analysis of
variance (ANOVA), and proportions were compared using the
χ2 or Fisher's exact tests. Multivariate logistic
regression analysis was used to explore the possible factors
associated with the optimal myocardial reperfusion (TMPG 3). For
the peak CK-MB and left ventricular ejection fraction (LVEF),
factorial design ANOVA was applied to elucidate the main effects
and interactions of the two drugs. A Student-Newman-Keuls (q) test
was used for post hoc analysis if there was a significant
difference among the groups. Two-sided P-values of <0.05 were
considered statistically significant. All calculations were
computed with the aid of SPSS statistical software (version 16.0;
SPSS, Inc., Chicago, IL, USA).
Results
Main demographic and clinical
characteristics
No significant differences in age, gender, past
medical histories (hypertension, diabetes, current smokers,
previous angina/MI/PCI) and basic medication use were found among
the four groups (Table I).
 | Table I.Main demographic/clinical
features. |
Table I.
Main demographic/clinical
features.
| Variables | Group A | Group B | Group C | Group D | F/χ2 | P-value |
|---|
| Age, years | 59.8±4.8 | 58.9±5.1 | 57.6±4.7 | 58.6±4.5 | 0.223 | 0.889 |
| Male, n (%) | 19 (73.1) | 17 (65.4) | 18 (69.2) | 21 (80.8) | 1.619 | 0.647 |
| BMI,
kg/m2 | 20.5±2.6 | 21.2±2.5 | 20.9±2.0 | 19.9±1.8 | 0.608 | 0.611 |
| Hypertension, n
(%) | 12 (46.2) | 14 (53.8) | 10 (38.5) | 11 (42.3) | 1.228 | 0.727 |
| Diabetes, n (%) | 8 (30.8) | 7 (26.9) | 5 (19.2) | 9 (34.6) | 1.409 | 0.712 |
| Hyperlipidemia, n
(%) | 6 (23.1) | 8 (30.8) | 5 (19.2) | 6 (23.1) | 0.969 | 0.805 |
| Current smokers, n
(%) | 17 (65.4) | 16 (61.5) | 15 (57.7) | 20 (76.9) | 1.782 | 0.604 |
| Family history of
CHD, n (%) | 6 (23.1) | 7 (26.9) | 4 (15.4) | 9 (34.6) | 2.156 | 0.526 |
| Preinfarction angina,
n (%) | 5 (19.2) | 4 (15.4) | 8 (30.8) | 5 (19.2) | 2.026 | 0.544 |
| Previous MI, n
(%) | 3 (11.5) | 3 (11.5) | 4 (15.4) | 3 (11.5) | 0.335 | 0.934 |
| Previous PCI, n
(%) | 2 (7.7) | 0 (0.0) | 2 (7.7) | 4 (15.4) | 2.837 | 0.412 |
| Killip class >1, n
(%) | 4 (15.4) | 5 (19.2) | 7 (26.9) | 6 (23.1) | 0.754 | 0.826 |
| SCr, µmoI/l | 82.1±12.5 | 82.9±17.1 | 83.1±12.8 | 84.5±17.6 | 0.311 | 0.749 |
| Medications, n
(%) |
|
|
|
|
|
|
|
Aspirin | 26 (100) | 26 (100) | 26 (100) | 26 (100) | – | – |
|
Clopidogrel | 26 (100) | 26 (100) | 26 (100) | 26 (100) | – | – |
|
Statin | 26 (100) | 26 (100) | 26 (100) | 26 (100) | – | – |
|
ACEI/ARB | 18 (69.2) | 20 (76.9) | 21 (80.8) | 16 (61.5) | 2.727 | 0.428 |
|
β-blockers | 7 (26.9) | 10 (38.5) | 9 (34.6) | 11 (42.3) | 0.824 | 0.821 |
|
Tirofiban | 2 (7.7) | 4 (15.4) | 2 (7.7) | 3 (11.5) | 1.594 | 0.653 |
Procedural characteristics
In the four groups, the IRA was predominantly the
RCA followed by the left circumflex; in excess of one-half of the
IRAs had a thrombus score of 3/4 (18). Following the procedure, the
proportion of TMPG 3 cases was significantly higher in group D than
that in other groups (P=0.014); furthermore, the proportions of
postprocedural TIMI 3 and complete STR cases were the highest in
group D, although no significant differences were found (P=0.067
and 0.052, respectively) (Table
II).
 | Table II.Procedural characteristics. |
Table II.
Procedural characteristics.
| Variables | Group A | Group B | Group C | Group D |
F/χ2 | P-value |
|---|
| Onset to balloon,
h | 6.8±1.6 | 7.2±1.7 | 7.5±1.6 | 6.7±2.0 | 1.952 | 0.132 |
| Door to balloon,
min | 65.8±17.5 | 73.5±20.81 | 60.1±18.2 | 65.1±20.6 | 0.975 | 0.420 |
| IRA, n (%) |
|
|
|
|
|
|
|
RCA | 20 (76.9) | 16 (61.5) | 22 (84.6) | 23 (88.5) | 3.855 | 0.290 |
|
LCX | 8 (30.8) | 11 (42.3) | 7 (26.9) | 6 (23.1) | 2.957 | 0.429 |
|
LAD | 0 (0.0) | 1 (3.85) | 0 (0.0) | 0 (0.0) | 2.935 | 0.420 |
| Thrombus scores, n
(%) |
|
|
|
|
|
|
| 0 | 5 (19.2) | 2 (7.7) | 4 (15.4) | 3 (11.5) | 0.985 | 0.872 |
|
1–2 | 10 (38.5) | 8 (30.8) | 8 (30.8) | 12 (46.2) | 1.219 | 0.772 |
|
3–4 | 17 (65.4) | 15 (57.7) | 16 (61.5) | 16 (61.5) | 1.023 | 0.795 |
| TIMI 3 pre-PCI, n
(%) | 3 (11.5) | 2 (7.7) | 2 (7.7) | 2 (11.5) | 1.013 | 1.271 |
| Mean TIMI score
pre-PCI, n (%) | 0.83±1.61 | 0.65±1.02 | 0.58±0.78 | 0.50±0.68 | 0.320 | 0.765 |
| TIMI 3 post-PCI, n
(%) | 20 (76.9) | 22 (84.6) | 21 (80.8) | 24 (92.3) | 7.657 | 0.067 |
| Mean TIMI score
post-PCI, n (%) | 2.79±0.57 | 2.82±0.19 | 2.76±0.50 | 3.00±0.00 | 2.016 | 0.122 |
| TMPG 3 post-PCI, n
(%) | 17 (65.4) | 21 (80.8) | 20 (76.9) | 23 (88.5) | 1.065 | 0.014 |
| Mean TMPG post-PCI,
n (%) | 2.58±0.78 | 2.80±0.52 | 3.05±0.49 | 3.12±0.00 | 2.535 | 0.073 |
| Stents per patient,
n (%) | 1.5±0.9 | 1.7±1.1 | 1.6±0.8 | 1.6±0.7 | 0.312 | 0.764 |
| Complete STR, n
(%) | 17 (65.4) | 21 (80.8) | 21 (80.8) | 25 (96.2) | 7.631 | 0.052 |
Main clinical index and follow up
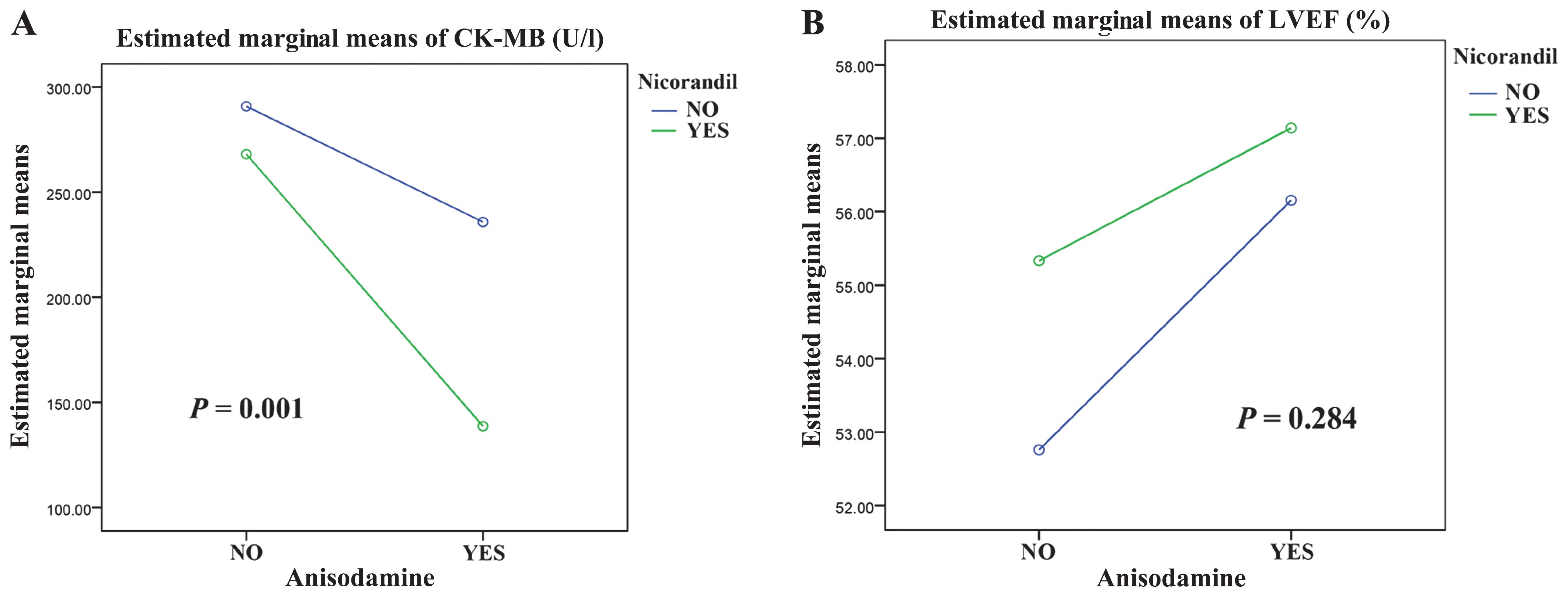
Three days after the procedure, the peak CK-MB and
cTnI levels were the lowest and the LVEF was highest in group D.
Nicorandil had no obvious effect on the vital signs during and
following the procedure. Anisodamine elevated the BP and HR. One
patient in group B developed paroxysmal atrial tachycardia;
however, no other severe tachyarrhythmias, such as ventricular
tachycardia or ventricular fibrillation, were observed during or
following the procedure. The incidence of MACEs during
hospitalization and the 30-day follow-up after discharge did not
differ among the four groups (Table
III and Fig. 1).
 | Table III.Main clinical index and follow
up. |
Table III.
Main clinical index and follow
up.
| Variables | Group A | Group B | Group C | Group D |
F/χ2 | P-value |
|---|
| Peak CK-MB,
U/l | 281.9±56.5 | 243.7±58.1 | 258.6±65.5 | 141.9±42.3 | 39.328 | <0.001 |
| Peak cTnI,
ng/ml | 64.8±19.8 | 56.9±18.9 | 61.4±21.1 | 50.2±18.1 | 4.875 | 0.005 |
| LVEF, % | 51.9±4.5 | 56.8±4.7 | 49.3±3.8 | 58.1±3.8 | 6.875 | <0.001 |
| BP and HR before
treatment |
|
|
|
|
|
|
| SBP,
mmHg | 108.1±11.7 | 106.3±13.3 | 104.3±13.6 | 105.7±12.5 | 0.039 | 0.971 |
| DBP,
mmHg | 64.1±8.7 | 61.9±8.1 | 63.7±9.8 | 62.8±7.3 | 0.835 | 0.478 |
| MBP,
mmHg | 78.8±6.0 | 76.7±7.2 | 77.2±8.2 | 77.1±2.3 | 0.393 | 0.741 |
| HR,
bpm | 51.8±9.4 | 52.6±5.5 | 52.5±6.0 | 58.3±9.2 | 0.482 | 0.701 |
| BPmax
and HRmax after treatment |
|
|
|
|
|
|
| SBP,
mmHg | 114.1±10.5 | 129.5±11.8 | 116.4±18.4 | 130.6±17.2 | 3.547 | 0.026 |
| DBP,
mmHg | 71.0±12.8 | 76.2±18.2 | 69.4±4.9 | 78.4±2.5 | 2.978 | 0.035 |
| MBP,
mmHg | 85.4±8.9 | 94.0±7.3 | 85.1±6.3 | 95.8±3.0 | 6.005 | 0.011 |
| HR,
bpm | 62.6±9.1 | 71.6±9.1 | 63.5±7.3 | 72.2±7.3 | 2.035 | 0.213 |
| In-hospital MACEs,
n (%) |
|
|
|
|
|
|
|
Total | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
|
Mortality | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
| New
MI | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
|
TVR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
| MACEs during 30-day
follow-up, n (%) |
|
|
|
| 4.371 | 0.362 |
|
Total | 3 (11.5) | 1 (3.8) | 2 (7.7) | 0 (0.0) |
|
|
|
Mortality | 1 (3.8) | 0 (0.0) | 1 (3.8) | 0 (0.0) |
|
|
| New
MI | 1 (3.8) | 1 (3.8) | 0 (0.0) | 0 (0.0) |
|
|
|
TVR | 1 (3.8) | 0 (0.0) | 1 (3.8) | 0 (0.0) |
|
|
| PAT, n (%) | 0 (0.0) | 1 (3.8) | 0 (0.0) | 0 (0.0) | 2.499 | 0.398 |
| VT/VF, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | – | – |
Predictors of TMPG 3 by multivariate
logistic analysis
A number of variables associated with the TMPG
following the procedures were enrolled into the multivariate
logistic regression model. The result showed that a thrombus score
of 3/4 and low DBP prior to anisodamine and nicorandil
administration were the independent risk factors for poor
myocardial reperfusion (expressed as TMPG <3), while 2 mg
anisodamine and 2 mg nicorandil prior to PCI were associated with
optimal myocardial reperfusion following the procedure (Table IV).
 | Table IV.Predictors of TIMI myocardial
perfusion grade 3 by multivariate logistic analysis. |
Table IV.
Predictors of TIMI myocardial
perfusion grade 3 by multivariate logistic analysis.
| Variables | P-value | OR | 95% CI |
|---|
| Age | 0.987 | 1.008 | 0.870–1.165 |
| Male | 0.959 | 0.968 | 0.249–3.671 |
| Thrombus scores
3/4 | 0.037 | 0.177 | 0.029–0.908 |
| TIMI grade 3
pre-PCI | 0.521 | 2.254 | 0.178–26.024 |
| DBP before
treatment | 0.048 | 1.075 | 1.002–1.169 |
| Anisodamine (2
mg) | 0.040 | 3.483 | 1.042–11.549 |
| Nicorandil (2
mg) | 0.047 | 2.501 | 0.739–8.346 |
Discussion
As an important manifestation of disordered
myocardial microcirculation, NR is a multifactorial phenomenon
associated with numerous pathological changes that eventually lead
to microvascular obstruction and impaired myocardial perfusion
(14,19,20). The
main factors that contribute to NR are as follows: i) Damage to the
microvascular structure by endothelial and myocardial cell edema,
which leads to microcirculatory compression and cell ischemia; ii)
ischemia-reperfusion injury-induced endothelial swelling,
interstitial and intracellular edema and intraluminal microthrombi
(14); iii) microvascular spasms
caused by a dysfunctional endothelium and potent vasoconstrictors
(serotonin, angiotensin II, endothelin-1) (21); iv) enhanced tissue damage due to
mechanical obstruction of leukocytes and their released products;
v) obstruction of the microcirculation by microemboli consisting of
thrombus fragments, blood cell aggregates, platelet plugs and
atherosclerotic debris (22); and
vi) extrinsic coagulation pathway activation (22).
TIMI grades reflect the epicardial blood perfusion,
while the TMPG reflects myocardial tissue perfusion. Patients with
NR, even those with TIMI score 3 but TMPG <2, are likely to
suffer from adverse cardiovascular complications and poor clinical
prognosis; therefore, while epicardial blood perfusion should be
monitored, more attention should be paid to myocardial tissue
perfusion. Blood perfusion without tissue perfusion is equal to no
blood. The prevention of NR is crucial due to the fact that, to
date, treatments are still lacking.
In the 1970s, anisodamine was used in the treatment
of bacteremic shock (23) which was,
in essence, a microcirculation disorder. NR is a myocardial
microcirculation disorder, which is similar to bacteremic shock. It
is therefore reasonable that anisodamine is used for the
improvement of myocardial microcirculation.
The mechanisms of anisodamine in the prevention of
NR and improvement of myocardial microcirculation are suggested to
be multifactorial. Anisodamine relieves the spasms of conductive
arteries, prearterioles and arterioles, dredges the coronary
microcirculation and restores the autonomic microvascular rhythm of
tide-like perfusion, which is destroyed by NR. Furthermore,
anisodamine acts to increase the coronary perfusion pressure by
increasing the BP (particularly the DBP and mean BP) and HR, which
is beneficial for the correction of NR and the improvement of
coronary microcirculation. In addition, anisodamine inhibits the
acetylcholine receptor and regulates the rebalance between the
sympathetic and vagus nervous systems, corrects hypotension and
bradycardia and maintains hemodynamic stabilization. To a certain
extent, anisodamine has a similar role to a calcium channel
antagonist; thus, it can prevent intracellular calcium overload and
attenuate microcirculatory spasms (6). By inhibiting lipid superoxidation and
oxygen free radical formation, anisodamine can additionally lessen
endothelial cell injury (24).
Finally, anisodamine can alleviate the oppression of myocardial
swelling on the microcirculation so as to improve the coronary
antegrade blood flow (25). The
present study showed that the intracoronary administration of 2 mg
anisodamine was safe; with the exception of one patient developing
paroxysmal atrial tachycardia, no other severe tachyarrhythmias
were observed during or following the procedure. Anisodamine
elevated BP and HR, increased the coronary perfusion pressure and
ameliorated the myocardial reperfusion, thus enhancing the clinical
prognosis.
Intravenous nicorandil acts to dilate the coronary
artery microvasculature, induces antiarrhythmic effects and reduces
reperfusion injury via ATP-sensitive K+ channels. A
previous study reported that, in AMI, intravenous nicorandil
administration reduced injury to the myocardium and improved heart
function, as shown by quantitative thallium single-photon emission
computerized tomography analysis and echocardiographic regional
wall motion scoring, respectively (26). In a different study, nicorandil
administration in patients with AMI led to reductions in NR
(nicorandil group 15% vs. control group 33%), ventricular
arrhythmia, heart failure and in-hospital mortality (27). A number of explanations have been
suggested for these effects. First, the suppression of the
Na+/Ca2+ exchange and the reduction in the
myocardial calcium levels following the activation of ATP-sensitive
K+ channels may minimize the injury to the myocardium.
Secondly, nicorandil exerts a protective effect on the heart by
improving coronary blood flow: Nicorandil reduces neutrophil
activation, which can suppress the inflammatory reactions and
thereby reduce the resistance of the microvasculature. Finally, by
dilating microvessels with a diameter of <100 µm, nicorandil can
decrease the preload of the heart and improve myocardial
microcirculation (28). A previous
study examined an intracoronary nicorandil group, a combined
intravenous and intracoronary group and a control group in a series
of AMIs; compared with the other two groups, the intracoronary
nicorandil group exhibited reductions in reperfusion arrhythmia,
NR, slow flow and chest pain (composite end-point 13% in the
intracoronary group vs. 33% in the control group), although the
differences were not significant (11); however, the dosage of intracoronary
nicorandil used in the study was low (0.5 mg per dose, maximum dose
1–2 mg). In the present study, intracoronary nicorandil
administration in AIMI led to similar results to those observed for
intravenous nicorandil administration (15). The present study showed that
intracoronary nicorandil administration (2 mg) was safe and
effective in improving post-PCI TIMI and TMPG.
The results of this study showed that anisodamine
and nicorandil could improve TIMI blood flow and TMPG, promote
complete STR following the procedure, notably decrease the
post-procedural peak levels of CK-MB and cTnI (indicating that the
size of MI was decreased) and significantly improve the cardiac
function (reflected by LVEF). This study demonstrated that
anisodamine and nicorandil exhibited cooperative interactions and
that the efficacy of anisodamine and nicorandil was superior to
that of the other regimens. The data from this study also showed
that a thrombus score of 3/4 and low DBP prior to treatment were
the independent risk factors for poor myocardial reperfusion
(expressed as TMPG <3), while anisodamine and nicorandil (2 mg
each) prior to PCI were associated with optimal myocardial
reperfusion following the procedure. In conclusion, a combinatorial
therapy of anisodamine and nicorandil is an effective means of
ameliorating myocardial reperfusion and protecting cardiac function
in patients with AIMI undergoing primary PCI.
References
|
1
|
Niccoli G, Burzotta F, Galiuto L and Crea
F: Myocardial no-reflow in humans. J Am Coll Cardiol. 54:281–292.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao YJ, Fu XH, Ma XX, Wang DY, Dong QL,
Wang YB, Li W, Xing K, Gu XS and Jiang YF: Intracoronary fixed dose
of nitroprusside via thrombus aspiration catheter for the
prevention of the no-reflow phenomenon following primary
percutaneous coronary intervention in acute myocardial infarction.
Exp Ther Med. 6:479–484. 2013.PubMed/NCBI
|
|
3
|
Chan W, Stub D, Clark DJ, Ajani AE,
Andrianopoulos N, Brennan AL, New G, Black A, Shaw JA, Reid CM, et
al: Melbourne Interventional Group Investigators: Usefulness of
transient and persistent no reflow to predict adverse clinical
outcomes following percutaneous coronary intervention. Am J
Cardiol. 109:478–485. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Svilaas T, Vlaar PJ, van der Horst IC,
Diercks GF, de Smet BJ, van den Heuvel AF, Anthonio RL, Jessurun
GA, Tan ES, Suurmeijer AJ and Zijlstra F: Thrombus aspiration
during primary percutaneous coronary intervention. N Engl J Med.
358:557–567. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Berger PB and Ryan TJ: Inferior myocardial
infarction. High-risk subgroups. Circulation. 81:401–411. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fu XH, Fan WZ, Gu XS, Wei YY, Jiang YF, Wu
WL, Li SQ, Hao GZ, Wei QM and Xue L: Effect of intracoronary
administration of anisodamine on slow reflow phenomenon following
primary percutaneous coronary intervention in patients with acute
myocardial infarction. Chin Med J (Engl). 120:1226–1231.
2007.PubMed/NCBI
|
|
7
|
Wang Y, Fu X, Wang X, Jia X, Gu X, Zhang
J, Su J, Hao G, Jiang Y, Fan W, et al: Protective effects of
anisodamine on renal function in patients with ST-segment elevation
myocardial infarction undergoing primary percutaneous coronary
intervention. Tohoku J Exp Med. 224:91–97. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Geng W, Fu XH, Gu XS, Wang YB, Wang XC, Li
W, Jiang YF, Hao GZ, Fan WZ and Xue L: Preventive effects of
anisodamine against contrast-induced nephropathy in type 2
diabetics with renal insufficiency undergoing coronary angiography
or angioplasty. Chin Med J (Engl). 125:3368–3372. 2012.PubMed/NCBI
|
|
9
|
Wang YB, Fu XH, Gu XS, Wang XC, Zhao YJ,
Hao GZ, Jiang YF, Fan WZ, Wu WL, Li SQ and Xue L: Safety and
efficacy of anisodamine on prevention of contrast induced
nephropathy in patients with acute coronary syndrome. Chin Med J
(Engl). 125:1063–1067. 2012.PubMed/NCBI
|
|
10
|
Lee HC, An SG, Choi JH, Lee TK, Kim J, Kim
JH, Chun KJ, Hong TJ, Shin YW and Lee SK: Effect of intra-coronary
nicorandil administration prior to reperfusion in acute ST segment
elevation myocardial infarction. Circ J. 72:1425–1429. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ota S, Nishikawa H, Takeuchi M, Nakajima
K, Nakamura T, Okamoto S, Setsuda M, Makino K, Yamakado T and
Nakano T: Impact of nicorandil to prevent reperfusion injury in
patients with acute myocardial infarction: Sigmart Multicenter
Angioplasty Revascularization Trial (SMART). Circ J. 70:1099–1104.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ikeda N, Yasu T, Kubo N, Hashimoto S,
Tsuruya Y, Fujii M, Kawakami M and Saito M: Nicorandil versus
isosorbide dinitrate as adjunctive treatment to direct balloon
angioplasty in acute myocardial infarction. Heart. 90:181–185.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
TIMI Study Group, . The Thrombolysis In
Myocardial Infarction(TIMI) trial. Phase I findings. TIMI Study
Group. N Engl J Med. 312:932–936. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gibson CM, Cannon CP, Murphy SA, Ryan KA,
Mesley R, Marble SJ, McCabe CH, Van De Werf F and Braunwald E:
Relationship of TIMI myocardial perfusion grade to mortality after
administration of thrombolytic drugs. Circulation. 101:125–130.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawai Y, Hisamatsu K, Matsubara H, Dan K,
Akagi S, Miyaji K, Munemasa M, Fujimoto Y, Kusano KF and Ohe T:
Intravenous administration of nicorandil immediately before
percutaneous coronary intervention can prevent slow coronary flow
phenomenon. Eur Heart J. 30:765–772. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shen J, Zhang Q, Zhang RY, Zhang JS, Hu J,
Yang ZK, Zheng AF, Zhang X and Shen WF: Clinical benefits of
adjunctive tirofiban therapy in patients with acute ST-segment
elevation myocardial infarction undergoing primary percutaneous
coronary intervention. Coron Artery Dis. 19:271–277. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huber K, Holmes DR Jr, van't Hof AW,
Montalescot G, Aylward PE, Betriu GA, Widimsky P, Westerhout CM,
Granger CB and Armstrong PW: Use of glycoprotein IIb/IIIa
inhibitors in primary percutaneous coronary intervention: Insights
from the APEX-AMI trial. Eur Heart J. 31:1708–1716. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gibson CM, de Lemos JA, Murphy SA, et al:
TIMI Study Group: Combination therapy with abciximab reduces
angiographically evident thrombus in acute myocardial infarction: A
TIMI 14 substudy. Circulation. 103:2550–2554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rezkalla SH and Kloner RA: No-reflow
phenomenon. Circulation. 105:656–662. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Reffelmann T and Kloner RA: The
‘no-reflow’ phenomenon: Basic science and clinical correlates.
Heart. 87:162–168. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galiuto L: Optimal therapeutic strategies
in the setting of post-infarct no reflow: The need for a
pathogenetic classification. Heart. 90:123–125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Niccoli G, Lanza GA, Shaw S, Romagnoli E,
Gioia D, Burzotta F, Trani C, Mazzari MA, Mongiardo R, De Vita M,
et al: Endothelin-1 and acute myocardial infarction: A no-reflow
mediator after successful percutaneous myocardial
revascularization. Eur Heart J. 27:1793–1798. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xiu RJ, Hammerschmidt DE, Coppo PA and
Jacob HS: Anisodamine inhibits thromboxane synthesis, granulocyte
aggregation, and platelet aggregation. A possible mechanism for its
efficacy in bacteremic shock. JAMA. 247:1458–1460. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao B, Li J, Luo X, Zhou Q, Chen H and
Shi H: The role of von Willebrand factor and ADAMTS13 in the
no-reflow phenomenon: After primary percutaneous coronary
intervention. Tex Heart Inst J. 38:516–522. 2011.PubMed/NCBI
|
|
25
|
Matsumoto H, Inoue N, Takaoka H, Hata K,
Shinke T, Yoshikawa R, Masai H, Watanabe S, Ozawa T and Yokoyama M:
Depletion of antioxidants is associated with no-reflow phenomenon
in acute myocardial infarction. Clin Cardiol. 27:466–470. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang A, Chen F, Xie Y, Guo Z and Yu Y:
Protective mechanism of nicorandil on rat myocardial
ischemia-reperfusion. J Cardiovasc Med (Hagerstown). 13:511–515.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Iwakura K: Modulation of individual
susceptibility to the no-reflow phenomenon after acute myocardial
infarction. Curr Pharm Des. 19:4519–4528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Akai K, Wang Y, Sato K, Sekiguchi N,
Sugimura A, Kumagai T, Komaru T, Kanatsuka H and Shirato K:
Vasodilatory effect of nicorandil on coronary arterial
microvessels: Its dependency on vessel size and the involvement of
the ATP-sensitive potassium channels. J Cardiovasc Pharmacol.
26:541–547. 1995. View Article : Google Scholar : PubMed/NCBI
|