Introduction
Depression is a common mental disorder with easy
relapse. The main clinical characteristics of the condition are
feeling down and cognitive dysfunction (1,2).
Although the incidence of depression increases annually, the
pathogenesis of the disease remains poorly understood (3).
Previous studies have suggested that numerous
factors are closely associated with the occurrence and outcome of
depression, including neurotrophic factors in the brain and neural
plasticity changes (4–6). These studies have indicated that
neurotrophic factors can maintain neuronal survival and promote
synaptic growth, and that the inhibition of neurotrophic factor
expression in the hippocampus can result in the suppression of
brain function and lead to depression. Currently, the main
mechanisms of action of clinical antidepressants are to increase
synaptic plasticity, increase neurotrophic factor levels in the
brain and promote the survival of neurons (7).
Tumor necrosis factor-α (TNF-α) and vascular
endothelial growth factor (VEGF) are closely associated with a
variety of neurological functions and are involved in changes in
neural plasticity and nerve regeneration; thus, TNF-α and VEGF have
received considerable focus as potential candidate genes for
depression gene therapy (8,9). TNF-α is mainly responsible for
maintenance of the immune system, homeostasis, inflammatory
reactions and the defense function; however, studies have suggested
that TNF-α can also exert a destructive effect (10). In the elderly, it has been shown that
TNF-α is involved in the pathology of certain diseases, including
chronic inflammation, autoimmune diseases and some malignant
diseases, such as depression, and it is therefore possible that
TNF-α could be considered as a target gene for depression treatment
(11–13). VEGF is involved in angiogenesis and
endothelial cell proliferation (14)
and has been confirmed to be involved in acute episodes of
depression (15,16). In addition, VEGF is involved in
angiogenesis, the protection of degenerating neurons and the
prevention and control of brain ischemia. Furthermore, VEGF can
stimulate the regeneration of neurons in the adult brain and
effectively inhibit the occurrence and worsening of depression
(17,18). There are certain similarities between
TNF-α and VEGF with regard to biological function, but it remains
unclear how they are involved in the development of depression. It
is therefore necessary to investigate whether TNF-α and VEGF act
synergistically in the occurrence of depression. The aim of the
present study was to explore the expression of TNF-α and VEGF in
the hippocampus at the mRNA and protein levels and to investigate
the interaction between TNF-α and VEGF.
Materials and methods
Establishment of depression model
rats
A total of 20 adult healthy Sprague Dawley rats
(Shanghai Silaike Experimental Animal Co., Ltd., Shanghai, China)
weighing 180–220 g were randomly divided into the control and
experimental depression groups (n=10 per group). The animals were
maintained under a 12-h light/dark cycle, and the animal room was
conditioned to a temperature of 22±2°C and a humidity of 60%. Water
and feed were supplied amply. After a week, the rat model of
depression was established using chronic, unpredictable stress for
21 days, with the following stimuli: Fasting, water deprivation,
tail clamping (5 min), foot-shock stress, noise, ice swimming,
altered circadian rhythms and heat stimulation. Identical
stimulations were separated by >2 days, and each stimulation was
applied 2–3 times according to the principles of random
arrangement. The normal animals without stimulation were used as
the control group. Measurements of weight and the sugar consumption
and open-field tests were used to determine the success of the
model construction, and the results were analyzed statistically.
For the sugar consumption experiment, rats received equivalent
water and 1% sugar solution after fasting for 24 h. The sugar
consumption rate was measured after 24 h. The rat sugar consumption
rate was calculated as: Sugar consumption/(sugar consumption +
water consumption)*100%. For the open-field tests, a 50×50×30 cm
box was used, with the bottom of box divided into 25 equilateral
squares. The rat was put into the center of the box bottom and the
horizontal and vertical activity frequency in 5 min was recorded.
The experimental methods were performed strictly in accordance with
the institutional guidelines of the Animal Care and Use Committee
of Jining Medical University (Jining, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted according to the
instructions of the RNA extraction kit manual (Invitrogen Life
Technologies, Carlsbad, CA, USA), and cDNA was then prepared
through RT. Thermoscript one-step quantitative RT-PCR with platinum
Taq kit was used to synthesize cDNA and to perform subsequent
real-time RT-qPCR (Applied Biosystems Life Technologies, Foster
City, CA, USA). The expression levels of the target genes TNF-α and
VEGF were detected using SYBR® Green-based qPCR, and the results of
the fluorescence qPCR were analyzed using an Applied Biosystems
7500 Real-Time PCR System (Applied Biosystems). The primer
sequences used were as follows: TNF-α, F 5-ATA CAC TGG CCC GAG GCA
AC-3 and R 5-CCA CAT CTC CGG ATC ATG CTT TC-3; VEGF, F 5-GTC ACT
ATG CAG ATC ATG CGG A-3 and R 5-GTC ACT ATG CAG ATC ATG CGGA-3; and
β-actin, F 5-GTCGTACCACTGGCATTGTG-3 and R 5-CTCTCAGCTGTGGTGGTGAA-3.
PCR conditions were as follows: 40 cycles of denaturation at 95°C
for 15 sec, 60 sec at 60°C annealing, and elongation with optics on
for fluorescence monitoring. β-actin was used as a reference gene
and the 2−ΔΔCT method was used to quantify the data.
Western blot analysis
The total cellular proteins in each group were
extracted. Cells were lysed in a radioimmunoprecipitation assay
buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% SDS, 1 mM EDTA and protease
inhibitor cocktail (Beyotime Institute of Biotechnology, Haimen,
China) for protein extraction. The total protein content was
determined by bicinchoninic acid assay. Following the quantitative
determination of protein content, the proteins were denatured at
100°C for 5 min and fractionated through 8% SDS-PAGE. The proteins
were electrotransferred onto polyvinylidene difluoride membranes.
Membranes were blocked with 5% skimmed milk for 1 h at room
temperature. Protein expression was subsequently detected by
incubation with rabbit polyclonal primary antibodies against TNF-α
(1:100; sc-8301), VEGF (1:100; sc-507) and β-actin (Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight.
Following incubation with the primary antibody, the membranes were
incubated with horseradish peroxidase-conjugated secondary antibody
for coloration (Santa Cruz Biotechnology, Inc.) (19). The bound antibodies were visualized
using an enhanced chemiluminescence reagent (EMD Millipore,
Billerica, MA, USA) and quantified densitometrically using an
electrophoresis image analysis system (FR980; Shanghai Furi Science
& Technology Co., Ltd., Shanghai, China). Densitometric
analyses of the bands were performed with β-actin as the loading
control. Triplicate experiments with triplicate samples were
performed.
Immunohistochemical staining
The rats were sacrificed via spinal dislocation.
Frozen slices were then prepared from brain tissue, and the slices
were rinsed with phosphate-buffered saline three times, for 10 min
each time. The endogenous peroxidase was inactivated by hydrogen
peroxide. The sections underwent 0.5% potassium citrate microwave
antigen retrieval at 100°C for 15 min. The frozen slices were then
incubated at room temperature for 10–15 min, blocked with goat
serum for 30 min and incubated with polyclonal rabbit primary
antibodies against TNF-α (1:100) and VEGF (1:100) at 37°C for 1 h.
Following the incubation with the primary antibody, the slices were
incubated with horse radish peroxidase-conjugated secondary
antibody at 37°C for 30 min and then with horseradish
peroxidase-conjugated fluorescent pigment at 37°C for 30 min. The
slices were subsequently treated with 3,3′-diaminobenzidine
chromogen and subjected to dehydration, clearing and mounting.
Statistical analysis
Data are presented as the mean ± standard deviation.
Comparisons between the control and experimental groups were
conducted using the two-tailed Student's t-test with SPSS 18.0
software (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Results of the weight measurements and
sugar water consumption and open-field tests
Prior to modeling, no significant differences were
found between the two groups. Following modeling, however, the rats
in the experimental group were observed to eat and weigh less and
to exhibit fur shedding, reduced luster, slowness of movement and
low spirits. The weight of the rats in the experimental group was
significantly lower than that of the rats in the control group
(P<0.05) (Table I). The
open-field test showed that the rats in the experimental and
control groups exhibited significant differences in the horizontal
and vertical motion scores (P<0.05), and the ability of the rats
in the experimental group to adapt to a new environment was
significantly decreased (Table II).
Significant differences were also found in the sugar
consumption-related indicators between the two groups (P<0.05;
Table III), and the sensitivity of
the rats in the experimental group to reward stimulation and
pleasure was decreased.
 | Table I.Effect of chronic stress on the food
intake and body weight of rats. |
Table I.
Effect of chronic stress on the food
intake and body weight of rats.
| Group | Net weight increment
(g) | Food intake (g) |
|---|
| Control |
131.11±2.05 |
14.24±0.11 |
| Experimental |
83.51±1.15a |
6.68±0.09a |
 | Table II.Effect of chronic stress on the
horizontal and vertical motion scores of rats. |
Table II.
Effect of chronic stress on the
horizontal and vertical motion scores of rats.
| Group | Horizontal motion
score | Vertical motion
score |
|---|
| Control |
59.77±1.08 |
13.35±0.62 |
| Experimental |
9.79±0.83a |
5.03±0.38a |
 | Table III.Effect of chronic stress on the fluid
consumption index of rats. |
Table III.
Effect of chronic stress on the fluid
consumption index of rats.
|
| Total liquid
consumption (ml/24 h) | Sugar water
consumption (ml/24 h) | Water consumption
(ml/24 h) | Percentage of sugar
water preference (%) |
|---|
|
|
|
|
|
|
|---|
| Group | Before stress | After stress | Before stress | After stress | Before stress | After stress | Before stress | After stress |
|---|
| Control |
15.4±0.1 |
14.7±0.1 |
11.4±0.1 |
11.4±0.2 |
2.9±0.1 |
2.9±0.1 |
63.7±0.2 |
62.8±0.2 |
| Experimental |
14.7±0.2 |
13.5±0.1 |
10.7±0.1 |
6.9±0.1a |
3.1±0.1 |
5.7±0.1a |
65.2±0.3 |
62.2±0.2 |
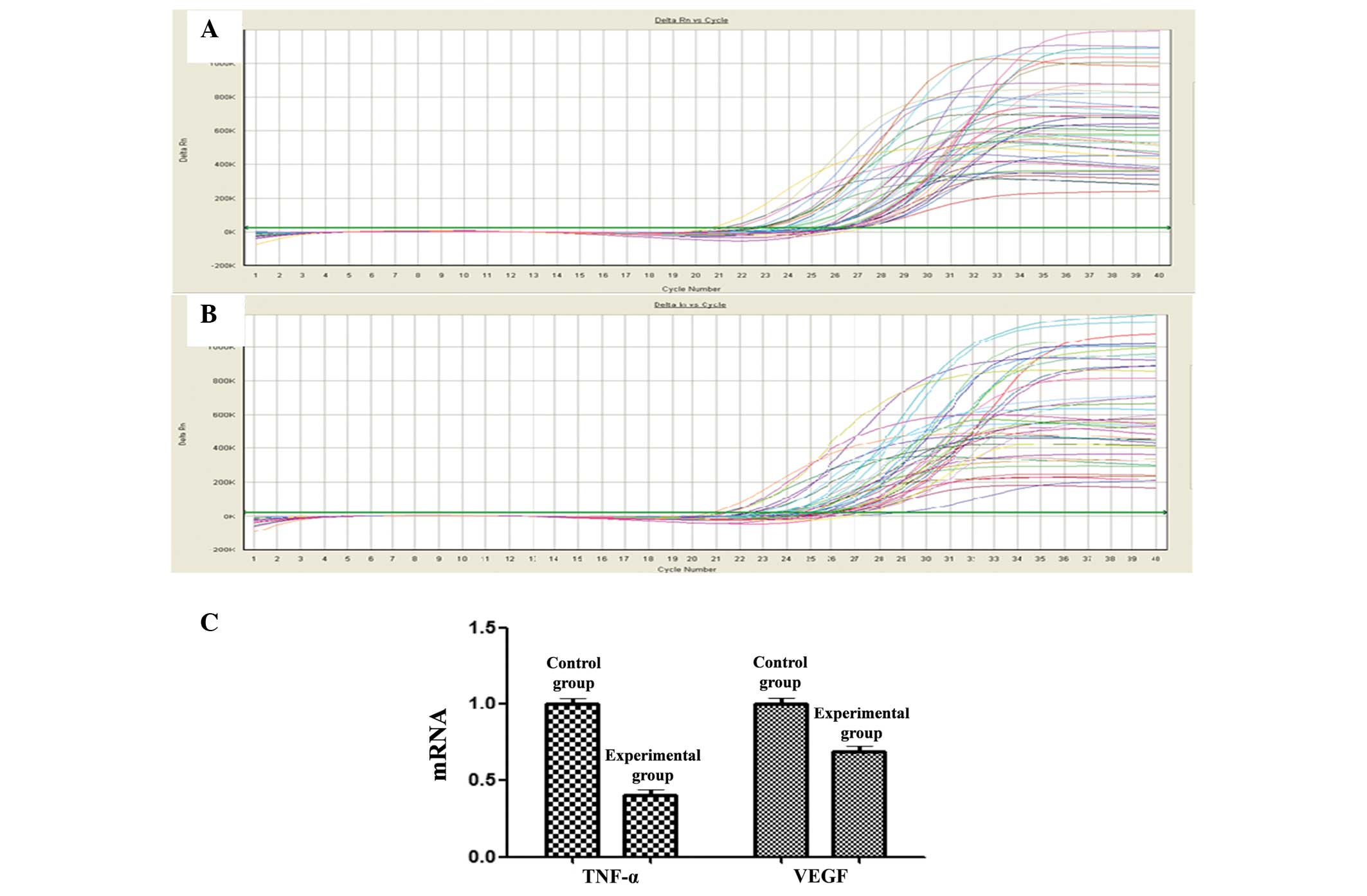
qPCR
The qPCR showed that the relative mRNA expression
levels of TNF-α and VEGF in the hippocampus of the experimental
group were lower than those in the control group. The relative VEGF
mRNA expression level of the experimental group was only half that
of the control group, while the expression level of TNF-α was only
slightly lower in the experimental group than that in the control
group. The results indicated that, compared with the control group,
the mRNA expression of TNF-α and VEGF was significantly lower in
the experimental group (Fig. 1).
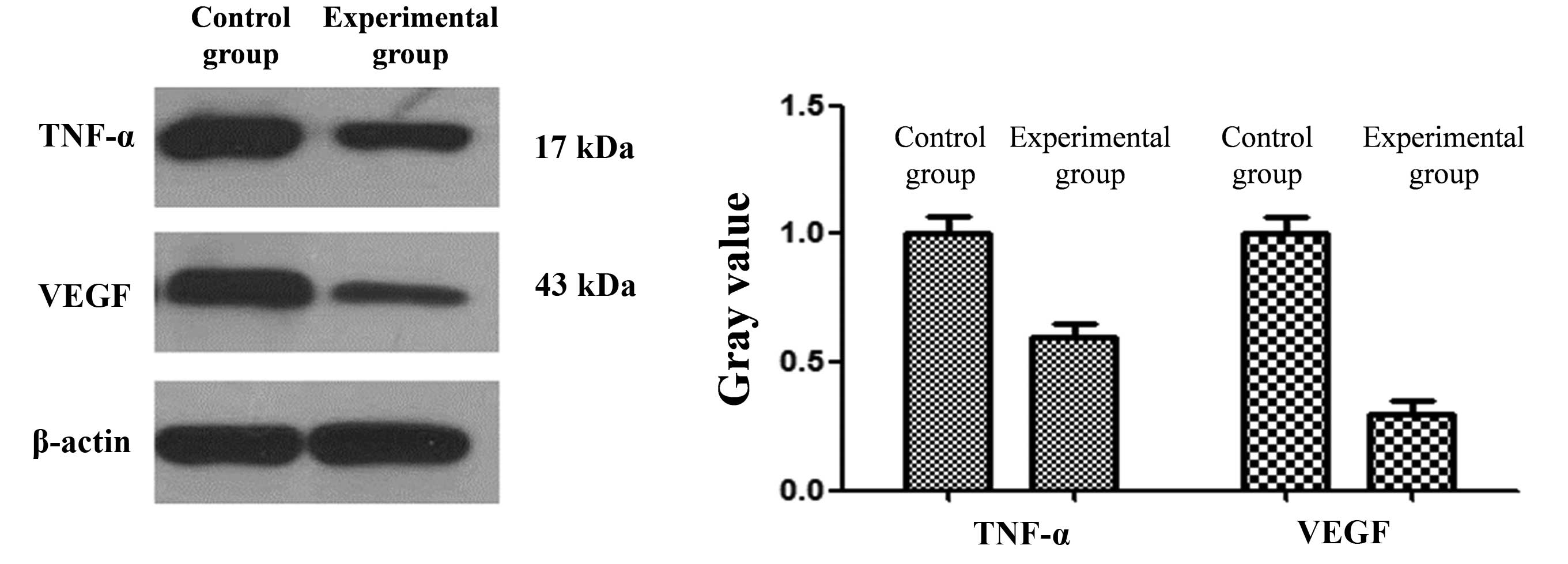
Western blotting
Compared with the expression of VEGF and TNF-α in
the hippocampus in the control group, the expression was reduced in
the hippocampus in the experimental group (Fig. 2). The differences between the two
groups were found to be statistically significant (P<0.05).
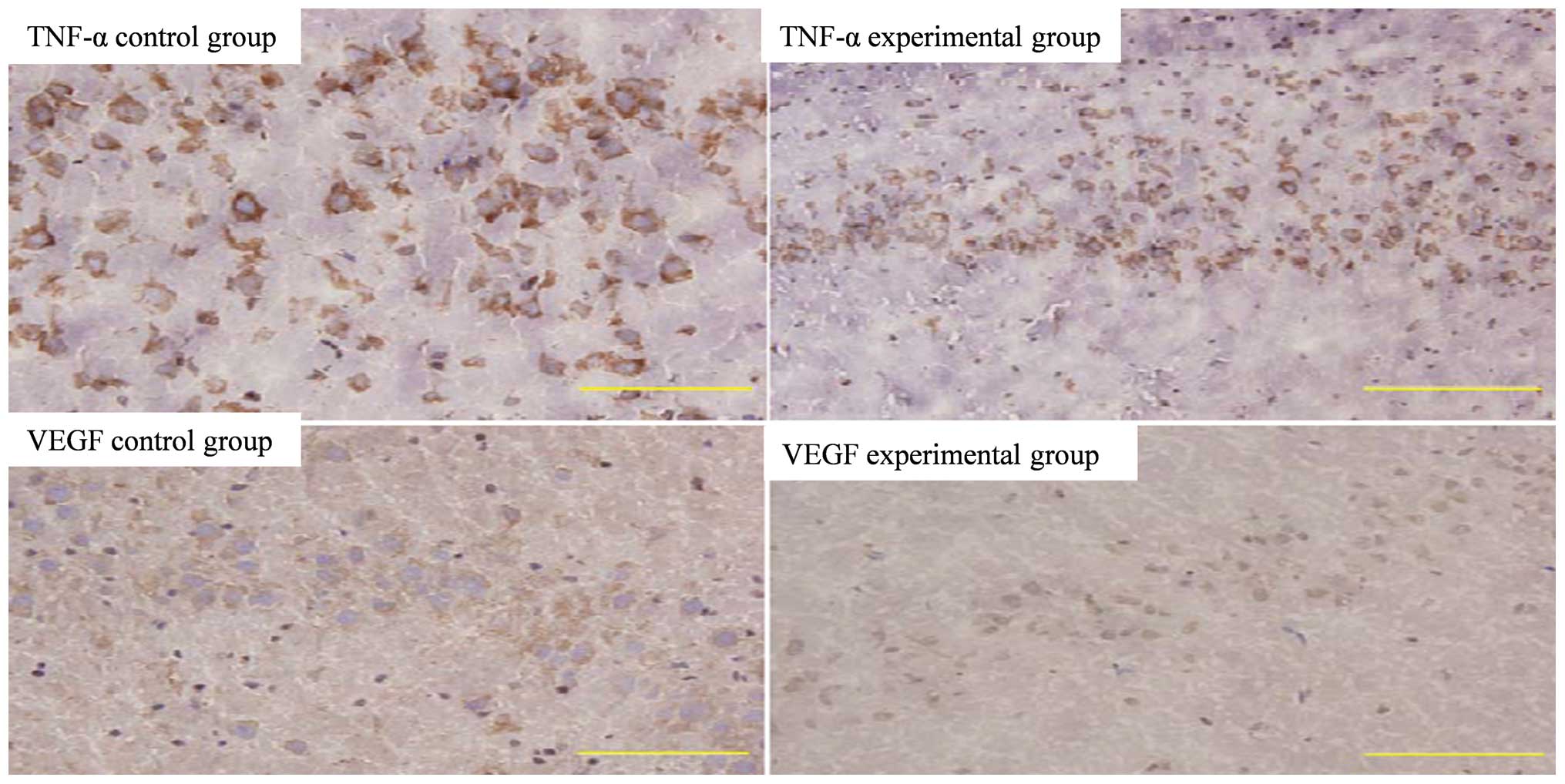
Immunohistochemistry
Compared with the expression of TNF-α and VEGF in
the normal hippocampus in the control group, the hippocampal
expression of TNF-α and VEGF in the experimental group was reduced
(Fig. 3).
Discussion
Depressive disorder, also called depression, is one
of the main emotional disorders observed in the clinic. The
condition can further develop and deteriorate, affecting overall
physiological function and leading to a series of clinical
symptoms, such as sleep disorders or loss of appetite (20). The rodent chronic, unpredictable,
mild stress (CUMS) model was first successfully established by
Willner et al in 1957 (21).
This model was demonstrated to simulate the exogenous factors for
the onset of depression, including reduced sensitivity to reward,
lack of pleasure and behavioral and spiritual malaise (22), with the main symptoms of a reduction
in the intake of sweet liquid food (21). Matthews et al (23) have proposed that weight reduction is
also a clinical symptom closely associated with depression. In
addition to the above characteristics, the CUMS model can be used
for the simulation of human physiological bradykinesia. The
open-field test can be used to verify the degree of horizontal
activity of the rat (24), a
reduction in which is a clinical sign associated with human
depression (25). Following
transferal to the new environment, the physiological activity of
healthy rats will increase, as the rats familiarize themselves with
the new environment; however, observation of the CUMS rat model
showed that the self-regulation and vertical motion ability of the
rats were markedly reduced (26).
These findings indicated that the interest in the new environment
was reduced for the CUMS rats, suggesting that the CUMS model
exhibits characteristics similar to the clinical symptoms of human
depression and therefore has significance as a biological reference
(27).
In the present study, the changes in the TNF-α and
VEGF mRNA and protein expression in the hippocampus were explored
with qPCR, western blotting and immunohistochemistry. The results
suggested that TNF-α and VEGF played a key role in the development
of depression. TNF-α is generated in the initial stages of the
inflammatory response and causes and enhances numerous reaction
effects, including increasing vascular penetration and the
activation of white blood cells at the site of infection and
injury; due to these characteristics, TNF is considered to be the
initiator of inflammation, blood vessel formation and tumor
metastasis, as well as a promoting factor of tumors (28). Studies have shown that TNF-α is
involved in the development of depression (29,30). The
mechanisms of antidepressant drugs have yet to be fully elucidated;
however, it has been proposed that they are associated with the
stimulation of growth factor signaling pathways and neurogenesis in
the hippocampus (31).
The VEGF expression in the hippocampus and the
effect of antidepressant drugs on the hippocampal expression of
VEGF have been previously studied. The proliferation of hippocampal
subgranular zone (SGZ) cells can be inhibited when the VEGF
receptor Flk-1 is blocked by pharmacological methods, such as
chronic exposure to fluoxetine or desipramine, or through
electroconvulsive shock therapy (32). SGZ cells can be induced to
proliferate following the intracerebroventricular delivery of
certain types of VEGF isoforms (33). Studies have shown that TNF-α can
promote the expression of VEGF at the mRNA and protein levels;
furthermore, synergy exists between TNF-α and TGF-β1 or VEGF, which
can promote the repair of nerve cells (34,35).
Previous studies of depression have utilized
large-scale genetic screening methods, which do not facilitate the
analysis of specific genes, and exhibit a lack of follow-up
experiments for the screened gene (36). In the present study, the occurrence
of depression was investigated at the protein and mRNA levels using
western blotting, immunohistochemistry, qPCR and animal behavior
experiments. The results of the study have provided a basis for
follow-up clinical research.
References
|
1
|
Kessler RC, Berglund P, Demler O, Jin R,
Koretz D, Merikangas KR, Rush AJ, Walters EE and Wang PS: National
Comorbidity Survey Replication: The epidemiology of major
depressive disorder: Results from the National Comorbidity Survey
Replication (NCS-R). JAMA. 289:3095–3105. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wray NR, Pergadia ML, Blackwood DH,
Penninx BW, Gordon SD, Nyholt DR, et al: Genome-wide association
study of major depressive disorder: New results, meta-analysis, and
lessons learned. Mol Psychiatry. 17:36–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
McEwen BS: Plasticity of the hippocampus:
Adaptation to chronic stress and allostatic load. Ann NY Acad Sci.
933:265–277. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Diniz BS, Teixeira AL, Machado-Vieira R,
Talib LL, Gattaz WF and Forlenza OV: Reduced serum nerve growth
factor in patients with late-life depression. Am J Geriatr
Psychiatry. 21:493–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Streeter CC, Gerbarg PL, Saper RB, Ciraulo
DA and Brown RP: Effects of yoga on the autonomic nervous system,
gamma-aminobutyric-acid, and allostasis in epilepsy, depression,
and post-traumatic stress disorder. Med Hypotheses. 78:571–579.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cao J, Wang J, Kuang L, Chen J, Ai M, Wang
W, Chen X, Lv Z and Wang H: Plasma level of brain-derived
neurotrophic factor and the related analysis in depressive patients
with suicide attempt. Zhong Guo Shen Jing Jing Shen Bing Za Zhi.
39:597–601. 2013.
|
|
7
|
Smeeding SJ, Bradshaw DH, Kumpfer K,
Trevithick S and Stoddard GJ: Outcome evaluation of the Veterans
Affairs Salt Lake City Integrative Health Clinic for chronic pain
and stress-related depression, anxiety, and post-traumatic stress
disorder. J Altern Complement Med. 16:823–835. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dean B, Tawadros N, Scarr E and Gibbons
AS: Regionally-specific changes in levels of tumour necrosis factor
in the dorsolateral prefrontal cortex obtained postmortem from
subjects with major depressive disorder. J Affect Disord.
120:245–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fournier NM and Duman RS: Role of vascular
endothelial growth factor in adult hippocampal neurogenesis:
Implications for the pathophysiology and treatment of depression.
Behav Brain Res. 227:440–449. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu B, Xu C, Wu X, Liu F, Du Y, Sun J, Tao
J and Dong J: Icariin exerts an antidepressant effect in an
unpredictable chronic mild stress model of depression in rats and
is associated with the regulation of hippocampal neuroinflammation.
Neuroscience. 294:193–205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balkwill F: TNF-alpha in promotion and
progression of cancer. Cancer Metastasis Rev. 25:409–416. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kunz M, Ceresér KM, Goi PD, Fries GR,
Teixeira AL, Fernandes BS, Belmonte-de-Abreu PS, Kauer-Sant'Anna M,
Kapczinski F and Gama CS: Serum levels of IL-6, IL-10 and TNF-α in
patients with bipolar disorder and schizophrenia: Differences in
pro-and anti-inflammatory balance. Rev Bras Psiquiat. 33:268–274.
2011. View Article : Google Scholar
|
|
13
|
Pezoa-Jares RE, Alvarez-Sekely AM,
Lopez-Bago AL, Vasquez-Medina JA, Cruz-Fuentes CS and
Lascurain-Ledesma R: 1395 - Quality of life and TNF-α levels in
Mexican patients with tuberculosis and major depressive disorder.
Eur Psychiatry. 28:12013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rapisarda A and Melillo G: Role of the
VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res.
114:237–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Perera TD, Coplan JD, Lisanby SH, Lipira
CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R,
Rosoklija G, Sackeim HA and Dwork AJ: Antidepressant-induced
neurogenesis in the hippocampus of adult nonhuman primates. J
Neurosci. 27:4894–4901. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun ST and Zhao HQ: Relationship of VEGF
and depression. Guo Ji Jing Sheng Bing Xue Za Zhi. 2:83–85.
2013.
|
|
17
|
Chauvet S, Burk K and Mann F: Navigation
rules for vessels and neurons: cooperative signaling between VEGF
and neural guidance cues. Cell Mol Life Sci. 70:1685–1703. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kiuchi T, Lee H and Mikami T: Regular
exercise cures depression-like behavior via VEGF-Flk-1 signaling in
chronically stressed mice. Neuroscience. 207:208–217. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song G, Ouyang G, Mao Y, Ming Y, Bao S and
Hu T: Osteopontin promotes gastric cancer metastasis by augmenting
cell survival and invasion through Akt-mediated HIF-1alpha
up-regulation and MMP9 activation. J Cell Mol Med. 13:1706–1718.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Frank E, Prien RF, Jarrett RB, Keller MB,
Kupfer DJ, Lavori PW, Rush AJ and Weissman MM: Conceptualization
and rationale for consensus definitions of terms in major
depressive disorder: Remission, recovery, relapse, and recurrence.
Arch Gen Psychiatry. 48:851–855. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Willner P, Towell A, Sampson D,
Sophokleous S and Muscat R: Reduction of sucrose preference by
chronic unpredictable mild stress and its restoration by a
tricyclic antidepressant. Psychopharmacology (Berl). 93:358–364.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barr AM, Brotto LA and Phillips AG:
Chronic corticosterone enhances the rewarding effect of
hypothalamic self-stimulation in rats. Brain Res. 875:196–201.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matthews K, Forbes N and Reid IC: Sucrose
consumption as an hedonic measure following chronic unpredictable
mild stress. Physiol Behav. 57:241–248. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anisman H and Matheson K: Stress,
depression, and anhedonia: Caveats concerning animal models.
Neurosci Biobehav Rev. 29:525–546. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
D'Aquila PS, Peana AT, Carboni V and Serra
G: Exploratory behaviour and grooming after repeated restraint and
chronic mild stress: Effect of desipramine. Eur J Pharmacol.
399:43–47. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Redrobe JP, Dumont Y, Fournier A, Baker GB
and Quirion R: Role of serotonin (5-HT) in the antidepressant-like
properties of neuropeptide Y (NPY) in the mouse forced swim test.
Peptides. 26:1394–1400. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cryan JF, Valentino RJ and Lucki I:
Assessing substrates underlying the behavioral effects of
antidepressants using the modified rat forced swimming test.
Neurosci Biobehav Rev. 29:547–569. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Wei, Liu Jia, Bai Jiayuan, Gu Changqin,
Zhang Wanpo, Cheng Guofu and Hu Xueying: Recent progress of tumor
necrosis factor-α. Dong Wu Yi Xue Fa Zhan. 31:108–111. 2010.
|
|
29
|
Tian XS, Hu N, Song L, Sun ZX and Cheng W:
Effects of Suanzaoren decoction on expression of TNF-α, IL-1β and
c-fos in hippocampus of depression rats model. Zhong Guo Yao Xue
Bao. 2:44–46. 2013.
|
|
30
|
Zhao SH, Kong JH, Yang CJ, Lin YH and Wang
LZ: Effect of duloxetine on the levels of cytokines in patients
with first-episode depression. Zhong Guo Xing Wei Yi Xue Yu Nao Ke
Xue. 21:158–160. 2012.
|
|
31
|
Fischer R and Maier O: Interrelation of
oxidative stress and inflammation in neurodegenerative disease:
role of TNF. Oxid Med Cell Longev. 2015:6108132015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Warner-Schmidt JL and Duman RS: VEGF is an
essential mediator of the neurogenic and behavioral actions of
antidepressants. Proc Natl Acad Sci USA. 104:4647–4652. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nowacka MM and Obuchowicz E: Vascular
endothelial growth factor (VEGF) and its role in the central
nervous system: A new element in the neurotrophic hypothesis of
antidepressant drug action. Neuropeptides. 46:1–10. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu X: The effect of Tiandan capsule on
VEGF, inflammatory cytokines and NSE of the patients with acute
cerebral infarction. Xi Nan Jun Za Zhi She. 13:24–26. 2011.
|
|
35
|
Qian XD, Luo CX and Zhu DY: Recent
progress in neural stem cells transplantation. Zhong Guo Xi Bao
Sheng Wu Xue Xue Bao. 34:212–217. 2012.
|
|
36
|
Alfonso J, Frasch AC and Flugge G: Chronic
stress, depression and antidepressants: Effects on gene
transcription in the hippocampus. Rev Neuroscience. 16:43–56. 2005.
View Article : Google Scholar
|