Introduction
Human ischemic heart disease is the cause of 13.2%
of all mortality worldwide (1). The
upregulation of apoptosis and cardiac systolic dysfunction in
diseased heart tissue are considered to contribute significantly to
disease development and ultimate heart failure (2). Furthermore, a previous study suggested
that the maintenance of intracellular calcium concentration
[Ca2+]i is critical for normal myocardial
function (3). The major proteins
responsible for maintaining intracellular Ca2+
homeostasis throughout excitation-contraction cycling in
cardiomyocytes include sarco/endoplasmic reticulum
Ca2+-ATPase (SERCA) and the SERCA regulatory protein
phospholamban (PLB) (4). In its
dephosphorylated state, PLB inhibits the uptake of Ca2+
by SERCA. By contrast, PLB phosphorylation by cAMP-dependent
protein kinase A (PKA) at Ser16 and by
Ca2+-calmodulin-dependent protein kinase II at Thr17
relieves this inhibitory influence on SERCA and thereby increases
Ca2+ uptake by the sarcoplasmic reticulum (SR) (5). The phosphorylation of PLB, primarily by
PKA at Ser16, is known to be associated with enhanced critical left
ventricular functions, such as contractility and relaxation
(6). The results of our previous
studies showed that the induction of acute myocardial infarction in
rats resulted in a significant downregulation of SERCA mRNA and
protein expression and, conversely, caused a significant
upregulation of PLB mRNA and protein expression (7,8). We
therefore hypothesize that aberrant cardiac contractile function
partially contributes to these observed effects, but it would be
useful to determine the mechanism used by myocardial cells to
stabilize the [Ca2+]i. In addition, it would
be beneficial to investigate whether the regulation of PLB
phosphorylation is sufficient to reduce cell apoptosis and improve
cardiac systolic function. Performing these investigations would
likely provide crucial insight into methods for improving the
treatment of coronary heart disease.
Shenmai injection (SM), derived from the shendong
drink described in the Zheng Yin Mai Zhi (which translates as
‘Pattern, Cause, Pulse and Treatment’) by Qin Changyu of the Ming
dynasty (9), has been widely applied
in recent years to treat qi-yin (10–14). The
term qi-yin derives from the word ‘qi’, which, according to
Traditional Chinese Medicine theory, is defined as the basic energy
that maintains life activities. ‘Yin’ and ‘yang’ are considered to
be the two opposing principles in nature, with ‘yin’ representing
the feminine and negative and ‘yang’ representing the masculine and
positive. A ‘qi-yin’ deficiency is associated with coronary heart
disease, chronic pulmonary heart disease and viral myocarditis, in
addition to heart and respiratory failure (15). According to Traditional Chinese
Medicine theory, SM benefits qi, prevents exhaustion, nourishes yin
and replenishes bodily fluids, while demonstrating protective
effects against adverse drug reactions (16). SM is extracted from red ginseng and
the ophiopogon root. Ginsenosides, the primary bioactive components
of ginseng, are known to scavenge oxygen free radicals (17,18),
block Ca2+ channels (17,19) and
reduce the ischemia/reperfusion (I/R) injury associated with
cardiovascular and cerebrovascular diseases (20–24).
Previous studies have indicated that ginsenoside Rg1 protects
cardiomyocytes under hypoxic conditions by reducing intracellular
Ca2+ overload (24–26).
Treatment with the total saponins of Panax ginseng (TSPG),
i.e. the total quantity of ginsenosides extracted from ginseng, can
increase cardiac SR Ca2+ levels, reduce mitochondrial
Ca2+ levels and increase mitochondrial calcium pump
activity (22,23). Furthermore, SM is able to mitigate
apoptosis and Ca2+ influx in neurocytes following
hypoxia-reoxygenation (21). It
remains unclear, however, whether the myocardial protective effect
of SM injection during I/R functions by maintaining the
[Ca2+]i via the regulation of PLB
phosphorylation. An aim of the present study was to elucidate the
mechanism underlying PLB involvement in each of these
processes.
This study aimed to determine whether the myocardial
protection from SM injection during I/R is associated with the
maintenance of the [Ca2+]i through the relief
of PLB inhibition. In addition, a further aim was to determine
whether the pharmacodynamic activity of SM injection is superior to
that of the pure ginseng saponins compounds.
Materials and methods
Chemicals and reagents
SM was purchased from Chia Tai Qingchunbao
Pharmaceutical Co., Ltd. (Hangzhou, China). Ginsenoside Rg1
(purity, >99%) was purchased from the Chinese National Institute
for the Control of Pharmaceutical and Biological Products (Beijing,
China). TSPG (purity, >80%) was purchased from Shanghai Winherb
Medical Technology Co., Ltd. (Shanghai, China). Dulbecco's modified
Eagle's medium (DMEM), fetal bovine serum (FBS) and TRIzol® reagent
were obtained from Invitrogen Life Technologies (Carlsbad, CA,
USA). Rabbit anti-PLB antibodies (#05-205) and ECL-Plus
chemiluminescent substrate were purchased from EMD Millipore
(Billerica, MA, USA). Mouse anti-phosphorylated PLB (p-PLB;
phosphorylated at Thr 17 and Ser 16; #8496) and rabbit anti-SERCA
antibodies (#9580) were purchased from Cell Signaling Technology,
Inc. (Danvers, MA, USA). Mouse anti-β-actin (#CW0096) and
horseradish peroxidase-labeled IgG secondary antibody were obtained
from Beijing ComWin BioTech Co., Ltd. (Beijing, China). The
PrimeScript™ RT reagent kit and SYBR® Premix Ex Taq™ II were
from Takara Bio, Inc. (Otsu, Japan). Primers for the reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) were
purchased from Sangon Biotech Co., Ltd. (Shanghai, China). The
Annexin-V/propidium iodide (PI) Assay kit was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). Fluo-4-AM was purchased from
Life Technologies.
Animals
The study was designed and all protocols involving
animals were conducted according to the Guide for the Care and Use
of Laboratory Animals published by the U.S. National Institutes of
Health. In addition, the animal protocol utilized in this study was
approved by the Ethics Committee of the Medical College of Zhejiang
University (Hangzhou, China).
Primary cardiomyocyte culture
Cardiomyocytes were isolated from the hearts of
neonatal Sprague Dawley rats that were ≤3 days old (SLAC Laboratory
Animal Co., Ltd., Shanghai, China), and the isolated cells were
cultured as described previously with minor modifications (27). To obtain the cardiomyocytes, heart
tissue was digested with 0.1% collagenase type II (Invitrogen Life
Technologies) and 0.125% pancreatin (Sigma-Aldrich, St. Louis, MO,
USA) for 8 min at 37°C. Following centrifugation, the supernatant
was discarded and the cell pellets were resuspended in culture
media containing 10% FBS. These steps were repeated until the
hearts were completely digested. Cells were pre-plated for 90 min
to allow fibroblasts to attach and to yield a purer cardiomyocyte
population. The cardiomyocytes were maintained in DMEM containing
10% FBS, 0.1 mM 5-bromo-2′-deoxyuridine (Invitrogen Life
Technologies) and 100 IU/ml 0.3% penicillin-streptomycin to inhibit
the growth of other cell types. Following 3 days in culture, the
cardiomyocytes were subjected to subsequent experimentation.
The cardiomyocytes were divided into the following
eight groups: Normoxia (N), TSPG-treated normoxia (N + TSPG),
Rg1-treated normoxia (N + Rg1), SM-treated normoxia (N + SM), I/R,
TSPG-treated I/R (I/R + TSPG), Rg1-treated I/R (I/R + Rg1) and
SM-treated I/R (I/R + SM).
Hypoxia/reoxygenation treatment
protocol
Cardiomyocytes were pretreated with TSPG (1.5 mg/l),
Rg1 (0.325 mg/l) or SM (5 ml/l) for 24 h. Cultured cardiomyocytes
were subsequently washed with Hank's balanced salt solution (HBSS),
containing 5 mM HEPES, 137 mM NaCl, 4 mM KCl, 1 mM MgCl2
and 1.5 mM CaCl2 (pH 7.2), and then incubated in
glucose-free DMEM. Hypoxia was used to mimic the in vivo
condition of myocardial ischemia. To induce hypoxia, the cells were
placed in an incubator at 37°C, and then N2 (95%) and 5%
CO2 were flushed into the incubator to lower the oxygen
concentration to 1%. Following 4 h of exposure to the hypoxic
conditions, the cells were subjected to reoxygenation by exchanging
the media with DMEM supplemented with 4.5 g/l glucose. Cells were
then incubated under normoxic conditions for 1 h.
RNA analysis
Total RNA from the cardiomyocytes was isolated using
TRIzol reagent according to the manufacturer's instructions. Total
RNA concentration was determined photometrically using a wavelength
of 260 nm. RNA samples were stored at −80°C. For the RT-qPCR, total
RNA was transcribed using the PrimeScript RT reagent kit. The PCR
reaction conditions were as follows: 95°C for 30 sec, then 40
cycles of 5 sec at 95°C and 34 sec at 60°C. Individual samples of
100 ng cDNA were amplified using SYBR Premix Ex Taq II,
utilizing gene-specific primers in an ABI PRISM® 7500 Fast Sequence
Detection System (Applied Biosystems, Foster City, CA, USA).
Standard curves were performed in duplicate with serially diluted
cDNA synthesized from neonatal rat heart tissue (1.5–50 ng) to
determine the PCR efficiency, which yielded similar results in all
groups. SERCA and PLB mRNA expression levels were evaluated as a
ratio of SERCA/PLB. Quantification was performed using the standard
curve and 2−ΔΔCt methods.
The primers used for PCR analysis were as follows:
SERCA2a forward, 5′-AAG CAG TTC ATC CGC TAC CT-3′ and reverse,
5′-AGA CCA TCC GTC ACC AGA TT-3′; PLB forward, 5′-TAC CTT ACT CGC
TCG GCT ATC-3′ and reverse, 5′-TAC CTT ACT CGC TCG GCT ATC-3′; and
β-actin forward, 5′-GGA GAT TAC TGC CCT GGC TCC TA-3′ and reverse,
5′-GAC TCA TCG TAC TCC TGC TTG CTG-3′.
Western blot analysis
Isolated cardiomyoctes were lysed in
radioimmunoprecipitation assay buffer (50 mM Tris-HCl pH 7.4, 150
mM NaCl, 1% NP-40, 10.5% sodium deoxycholate, 5% sodium dodecyl
sulfate and 1 mM phenylmethyl sulfonyl-fluoride). The total protein
concentration was quantified using a bicinchoninic protein quantity
assay kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
equal amounts of protein were loaded onto 12% sodium dodecyl
sulfate-polyacrylamide gels. Following separation by
electrophoresis, the proteins were electrotransferred onto
polyvinylidene difluoride membranes. Non-specific binding sites
were blocked by incubating with 5% skimmed milk in Tris-buffered
saline containing 0.05% Tween-20 at room temperature for 1 h. The
membranes were probed using rabbit anti-PLB (1:1,000), mouse
anti-p-PLB (1:1,000), rabbit anti-SERCA2a (1:1,000) and mouse
anti-β-actin (1:1,000) primary antibodies overnight at 4°C. The
membranes were subsequently probed with horseradish
peroxidase-labeled anti-rabbit IgG secondary antibody (1:10,000) at
room temperature for 1 h. The immunoreactive bands were visualized
using ECL-Plus reagent. Signal intensities of each band were
analyzed using Quantity One® software, version 4.6.2 (Bio-Rad
Laboratories, Inc., Berkeley, CA, USA), and the relative protein
levels were calculated by comparing with the β-actin loading
control.
Annexin V/PI assay
Briefly, cardiomyocytes were collected, washed with
Ca2+-free phosphate-buffered saline, resuspended in
binding buffer and incubated with 5 µl Annexin V and PI at room
temperature in the dark for 15 min. The cardiomyocytes were then
analyzed using a flow cytometer (FC500MCL; Beckman Coulter, Brea,
CA, USA).
Measurement of
[Ca2+]i
After 1 h of reoxygenation,
[Ca2+]i measurements were conducted using the
Ca2+-sensitive fluorescent probe fluo-4 AM. Cardiomyocytes
were incubated in six-well plates with 5 µM fluo-4 AM for 30 min at
37°C. The cells were washed with HBSS three times. Fluorescence
levels were measured using flow cytometry, with excitation at 484 nm
and emission at 516 nm.
Statistical analysis
Statistical analyses were performed using SPSS
software, version 19.0 (IBM SPSS, Armonk, NY, USA). Statistical
significance was detected using one-way analysis of variance and
Student's t-tests. P<0.05 was considered to indicate a
statistically significant difference.
Results
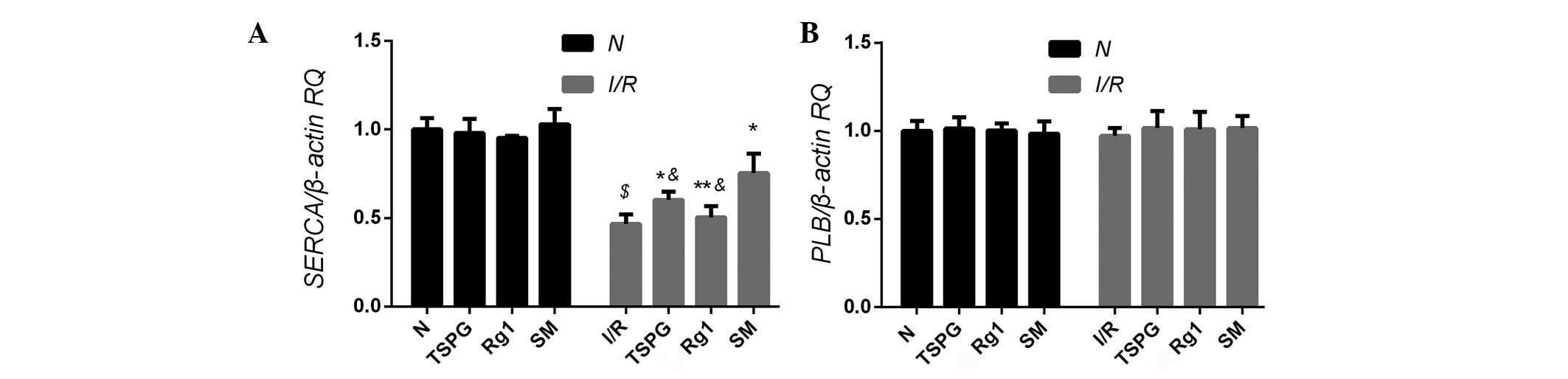
Effects of TSPG, Rg1 and SM on SERCA
and PLB mRNA expression levels
In order to determine whether TSPG, Rg1 or SM
treatment was able to affect SERCA and PLB mRNA expression, rat
myocardial cells were subjected to I/R, and the resulting mRNA
levels were compared with those of the normoxic cells. No
significant difference in the PLB mRNA levels was detected between
the I/R group and the untreated controls (P>0.05); however, the
SERCA mRNA levels were found to be significantly decreased in the
I/R group (P<0.01). Treatment of the I/R cells with TSPG (I/R +
TPSG) or SM (I/R + SM) resulted in a significant upregulation of
SERCA mRNA levels, with SM treatment showing the most marked effect
(P<0.01). Notably, this difference in mRNA expression was not
evident in the I/R + Rg1 group (P>0.05) (Fig. 1).
Effects of TSPG, Rg1 and SM on SERCA,
PLB and p-PLB protein levels
In addition to the mRNA analysis, this study aimed
to determine whether TSPG, Rg1 or SM treatment was able to affect
SERCA, PLB and/or p-PLB protein expression. Following I/R
treatment, the PLB protein levels in the I/R treatment group were
compared with those in the N group, and no statistically
significant difference was detected (P>0.05). Conversely, the
protein levels of p-PLB and the p-PLB/PLB ratio were significantly
reduced in the I/R group compared with those in the N group (both
P<0.01); however, the I/R + SM group exhibited a significant
increase in p-PLB protein levels (P<0.01), in addition to an
increase in the p-PLB/PLB expression ratio, compared with the I/R
group (P<0.01). Notably, the two other drug administration
groups (TSPG and Rg1) showed no significant alterations in p-PLB
protein expression (P>0.05) or p-PLB/PLB ratio (P>0.05).
The comparison of SERCA protein levels between the N
and I/R treatment groups revealed a significant reduction following
I/R treatment (P<0.01). Conversely, the SERCA protein expression
levels were significantly elevated in the I/R + SM and I/R + TSPG
groups compared with the levels in the I/R group (P<0.01),
whereas the SERCA protein expression in the I/R and I/R + Rg1
groups showed no significant difference (P>0.05). The PLB/SERCA
ratio was significantly higher in the I/R group compared with that
in the N group (P<0.01), while this ratio was significantly
decreased in the I/R + SM (P<0.01) and I/R + TSPG (P<0.05)
groups. The I/R + Rg1 group exhibited a modest but insignificant
reduction in the PLB/SERCA ratio (P>0.05). Notably, the I/R + SM
group showed the most marked increase in SERCA protein levels and
the most marked reduction in the PLB/SERCA ratio compared with the
I/R + TSPG group (P<0.05/0.01) (Fig.
2).
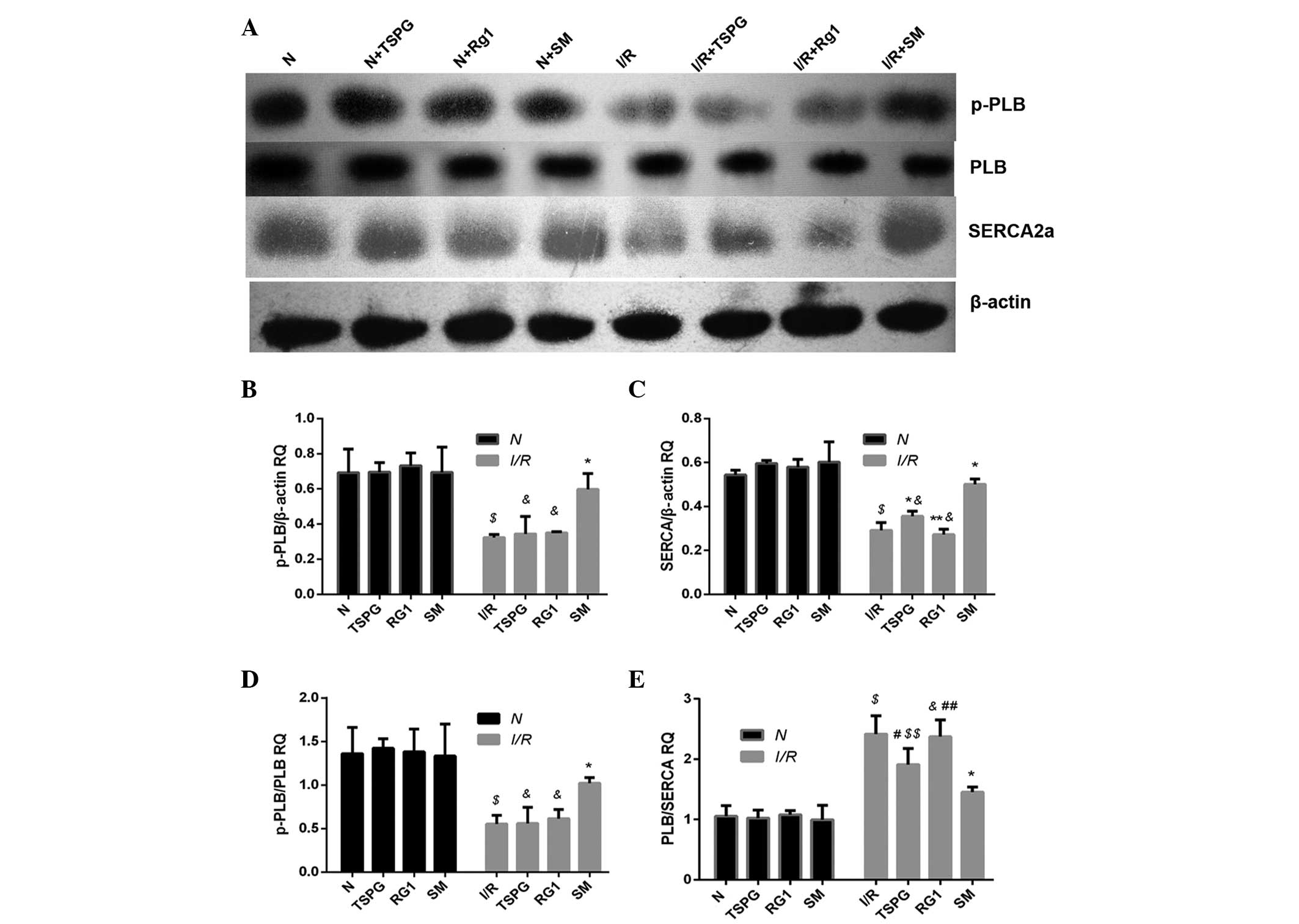 | Figure 2.Effects of TSPG, Rg1 and SM on SERCA,
p-PLB, p-PLB/PLB and PLB/SERCA levels following I/R in
cardiomyocytes. (A) Western blot analysis of SERCA, p-PLB, PLB and
β-actin levels. (B-E) Quantification of (B) p-PLB levels, (C) SERCA
levels, (D) p-PLB/PLB ratio and (E) PLB/SERCA ratio. Data are
expressed as the mean ± standard deviation (n=4).
$P<0.01 vs. N; &P<0.01 and
$$P<0.05 vs. I/R + SM; *P<0.01 and
#P<0.05 vs. I/R; **P<0.01 and
##P<0.05 vs. I/R + TSPG. N, normoxia; TSPG, total
saponins of Panax ginseng; Rg1, ginsenoside Rg1; SM, Shenmai
injection; I/R, ischemia/reperfusion; PLB, phospholamban; p-PLB,
phosphorylated-PLB; SERCA, sarco/endoplasmic reticulum
Ca2+ ATPase; RQ, relative quantification. |
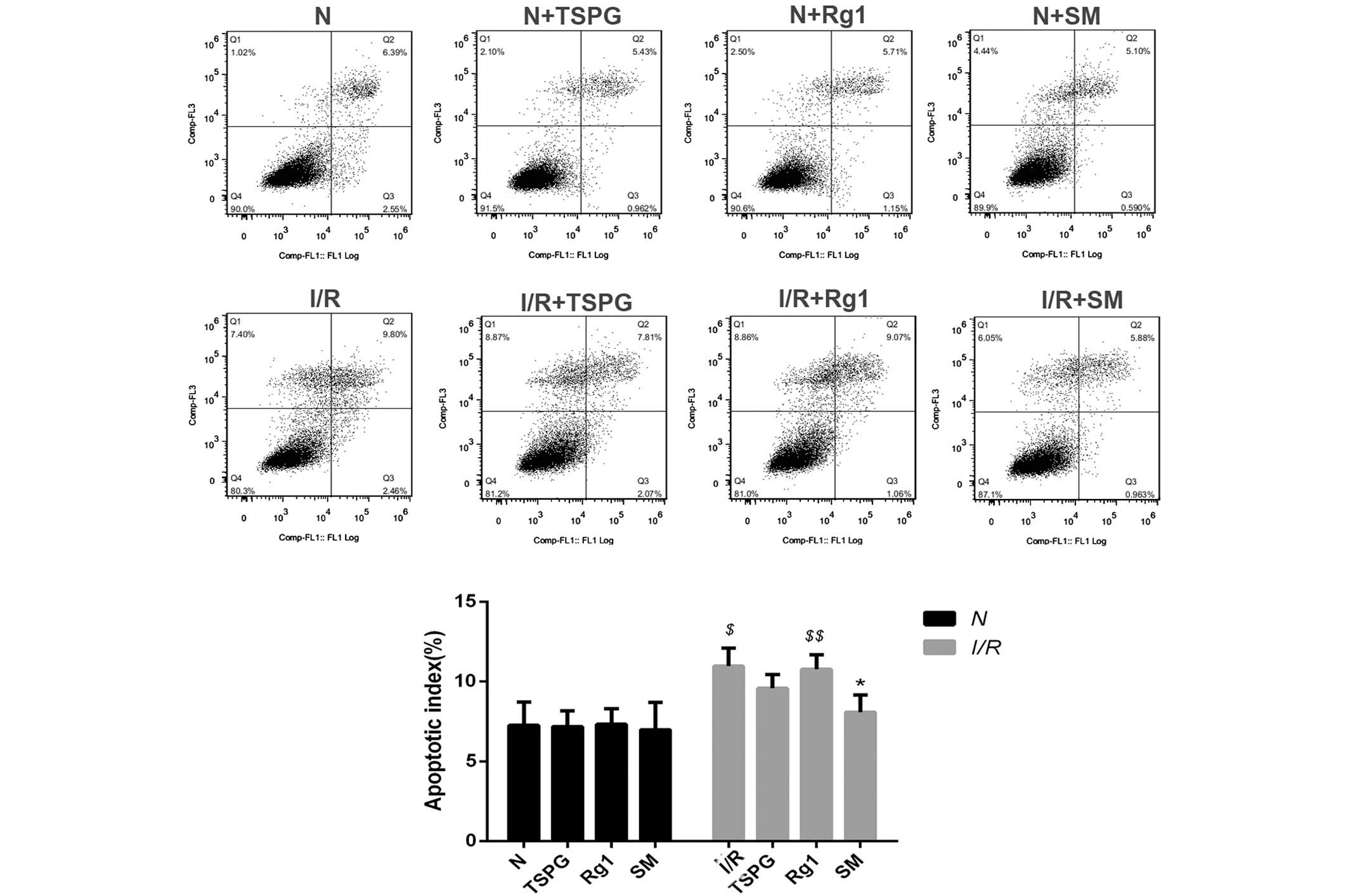
Effects of TSPG, Rg1 and SM on
cardiomyocyte apoptosis following I/R injury
In order to examine the effects of TSPG, Rg1 and SM
treatment on I/R-induced apoptosis, the apoptosis rates were
determined using flow cytometry. As shown in Fig. 3, I/R treatment markedly increased the
apoptosis rates (P<0.01); however, this effect was attenuated
with SM treatment (P<0.05). In addition, the I/R + Rg1 group
showed a modest, insignificant reduction in apoptosis (P>0.05),
and the I/R + TSPG group exhibited a greater but also insignificant
reduction in apoptosis (P>0.05) (Fig.
3).
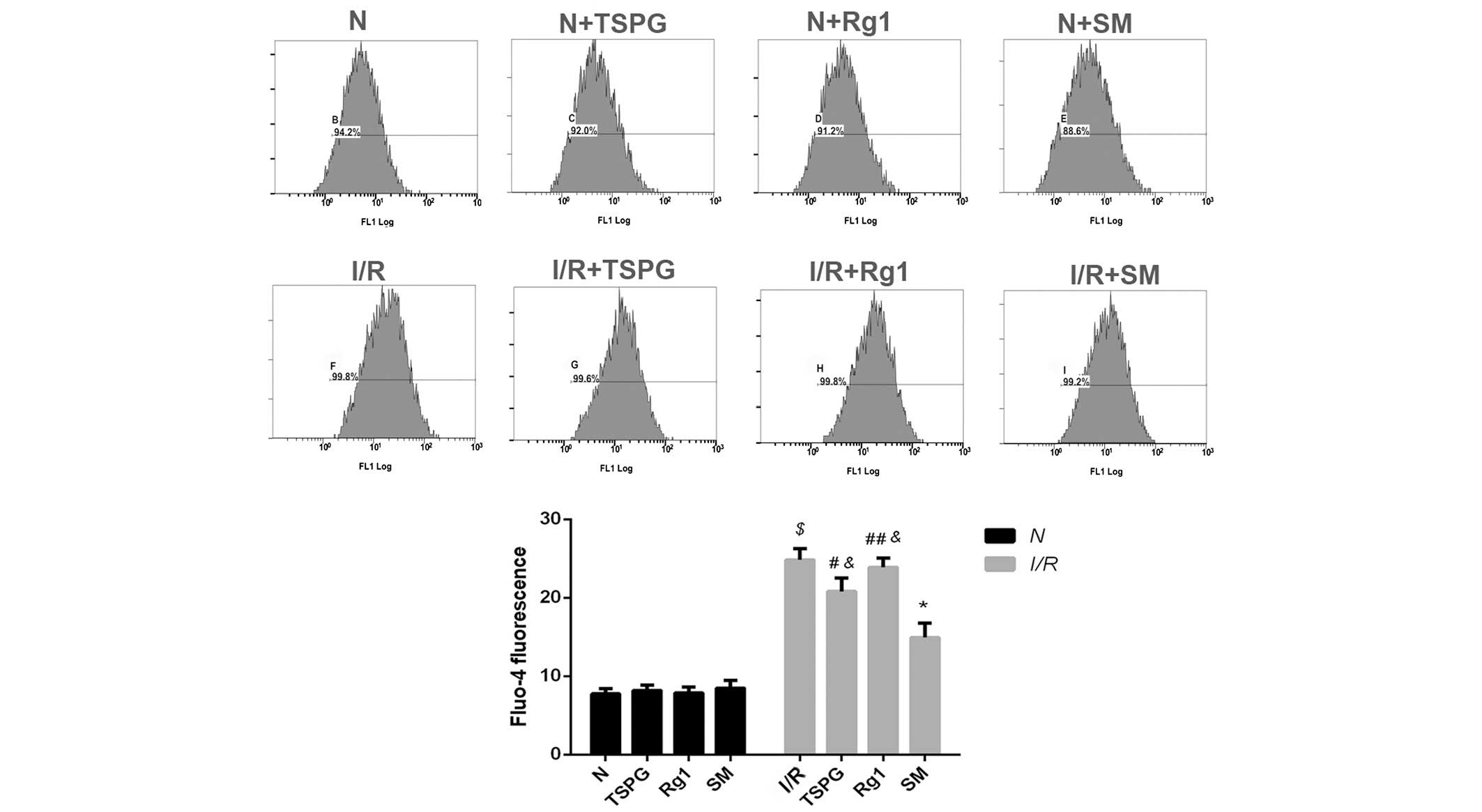
Effects of TSPG, Rg1 and SM on
[Ca2+]i in cardiomyocytes following I/R
injury
As shown in Fig. 4,
I/R markedly increased the [Ca2+]i
(P<0.01); however, this increase was suppressed by the SM
(P<0.01) and TSPG (P<0.05) treatments. The I/R + Rg1 group
exhibited a modest but insignificant decrease in
[Ca2+]i compared with the I/R group
(P>0.05). The I/R + SM group showed the greatest decrease in
[Ca2+]i following I/R treatment (P<0.01)
(Fig. 4).
Discussion
In the present study, the mechanism underlying the
myocardial protective effect of SM following I/R was investigated.
It was demonstrated that exposure to I/R resulted in significant
alterations in a variety of functional indices for cardiac
function, including a disruption in Ca2+ transport
within heart myocardial cells. In accordance with a previous study,
the apoptosis rate and [Ca2+]i of
cardiomyocytes increased significantly following I/R, indicating
that I/R injury successfully occurred (28).
In order to further clarify the effects of SM on
myocardial cells, three different drug treatments were employed to
determine their comparative efficacies in abating I/R-related
complications. Ginsenoside Rg1 is a major bioactive component of
TSPG, while TSPG is the primary bioactive component of SM. It has
been established that 1 ml SM contains 65±6.68 µg Rg1 and
293.38±40.54 µg TSPG (29). In
accordance with similar studies concerning drug treatment of
cardiomyocytes (30,31), 5 ml/l SM was administered in the
present treatment regimen, which is equivalent to ~1.5 mg/l TSPG
and ~0.325 mg/l Rg1. PLB, the primary regulator of SERCA, is a
small transmembrane SR protein present in the ventricles of the
heart and, to a lesser extent, in the atria (32). Significant alterations in the
stoichiometry between SERCA and PLB have been associated with
chronic heart failure. PLB therefore functions as a physiological
brake on excitation-contraction coupling, therein modulating
cardiac function. The present results indicated that the reduced
p-PLB expression following I/R was restored to normal levels
following SM treatment, but not following treatment with TSPG or
Rg1. This observation supports the conclusion that the ability of
SM to counteract the negative effects of I/R treatment is partially
attributable to increased PLB phosphorylation, but not to TSPG or
Rg1 when administered at similar concentrations.
The SR Ca2+-ATPase is responsible for
restoring the SR Ca2+ load per excitation-contraction
cycle. A reduction in SERCA content is associated with reduced SR
Ca2+ loading and elevated [Ca2+]i
(33,34). The restoration of SERCA levels is
potentially a critical factor in normalizing Ca2+
uptake, as observed in line-scan and frequency-dependent
experiments (35). It is therefore
plausible that the observed reduction in SERCA expression may
partially explain the observed [Ca2+]i
overload in cardiomyocytes following I/R treatment. The present
study has demonstrated that SM and TSPG are able to increase SERCA
expression and that this improves intracellular Ca2+
cycling. Notably, the numerous changes observed following I/R
treatment were not reversed by Rg1 treatment, which is inconsistent
with the results of previous studies (25,26).
This discrepancy may be attributable to the different doses of Rg1
used, as our study utilized a dose far below proven efficacy. To
the best of our knowledge, the present study is the first to
conduct a comparison between the effects of SM and its purified
constituent compounds on the expression of p-PLB. Notably, the
ratio of p-PLB/PLB levels was significantly reduced in the I/R
group relative to that in the control N group (P<0.01);
therefore, despite PLB expression remaining unaltered following I/R
treatment, p-PLB expression decreased significantly.
In conclusion, the myocardial protective effects of
SM following I/R treatment may be partially attributable to the
effects of SM on intracellular Ca2+ homeostasis,
specifically the relief of PLB inhibition. Notably, the present
results indicate that the pharmacodynamic activity of TPSG is
significantly superior to that of Rg1; however, the effects of SM
are significantly superior to those of TSPG and Rg1.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 30801213 and 81170167),
the International Collaboration Projects of Science and Technology
Department of Zhejiang Province (no. 2011c14027) and the Foundation
from Zhejiang Provincial Administration of Traditional Chinese
Medicine (no. 2011ZQ013). The corresponding author is sponsored by
the Zhejiang Provincial Program for the Cultivation of High-level
Innovative Health talents.
References
|
1
|
World Health Oganization, . Fact Sheet No.
310: The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/Accessed.
November 19–2014.
|
|
2
|
Braunwald E: Heart failure. JACC Heart
Fail. 1:1–20. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marks AR: Cardiac intracellular calcium
release channels: Role in heart failure. Circ Res. 87:8–11. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ramón de Berrazueta J: The role of calcium
in the regulation of normal vascular tone and in arterial
hypertension. Rev Esp Cardiol. (52 Suppl):3:25–33. 1999.(In
Spanish).
|
|
5
|
Spinale FG, de Gasparo M, Whitebread S,
Hebbar L, Clair MJ, Melton DM, Krombach RS, Mukherjee R, Iannini JP
and O SJ: Modulation of the renin-angiotensin pathway through
enzyme inhibition and specific receptor blockade in pacing-induced
heart failure: I. Effects on left ventricular performance and
neurohormonal systems. Circulation. 96:2385–2396. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kranias EG and Hajjar RJ: Modulation of
cardiac contractility by the phospholamban/SERCA2a regulatome. Circ
Res. 110:1646–1660. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Hu S, Wang L, Hu Y and Zhou J:
Comparison of low and high doses of carvedilol on restoration of
cardiac function and calcium-handling proteins in rat failing
heart. Clin Exp Pharmacol Physiol. 32:553–560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun Y, Hu S, Wang L, Hu Y and Zhou J:
Changes of calcium handling protein after acute myocardial
infarction in rats and effect of carvedilol. Zhong Guo Bing Li
Sheng Li Za Zhi. 21:1085–1089. 2005.(In Chinese).
|
|
9
|
Changyu Qin and Zhizhen Qin: Zheng Yin Mai
Zhi. First edition. Shanghai wei sheng chu ban she; Shanghai:
1958
|
|
10
|
Hou YZ, Mao JY, Wang XL, Liu CX and Zhang
C: Shenmai injection in heart failure patients: A systematic review
and Meta-analysis. Zhong Guo Xun Zheng Yi Xue Za Zhhi She.
10:939–945. 2010.
|
|
11
|
Zhang L, Wang BH, Hu J and Shang HC:
Shenmai injection for children with viral myocarditis: A systematic
review. Zhong Guo Xun Zheng Yi Xue Za Zhi She. 10:700–706.
2010.
|
|
12
|
Li KJ: Shenmai injection for acute
ischemic stroke: A systematic review of randomized controlled
trial. Zhong Yi Yao Xue Bao. 34:4–7. 2006.
|
|
13
|
Liu JG: Systematic review on Shenmai
Injection treatment of dilated cardiomyopathy. Zhong Cheng Yao.
34:1456–1461. 2012.
|
|
14
|
Hu J, Zhang W, Xie YM, Wang LX, Nie XL and
Zhang YL: Meta-analysis of Shenmai injection treatment for acute
myocardial infarction. Zhong Guo Zhong Yao Za Zhi She.
37:2760–2767. 2012.
|
|
15
|
Gong W and Hu Z: Clinical application of
Shenmai injection. Yi Yao Dao Bao. 19:181–183. 2000.(In
Chinese).
|
|
16
|
Zhang L, Hu J, Xiao L, Zhang Y, Zhao W,
Zheng W and Shang H: Adverse drug reactions of Shenmai injection: A
systematic review. J Evid-Based Med. 3:177–182. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bai CX, Takahashi K, Masumiya H,
Sawanobori T and Furukawa T: Nitric oxide-dependent modulation of
the delayed rectifier K+ current and the L-type
Ca2+ current by ginsenoside Re, an ingredient of
Panax ginseng, in guinea-pig cardiomyocytes. Br J Pharmacol.
142:567–575. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Scott GI, Colligan PB, Ren BH and Ren J:
Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat
ventricular myocytes: Role of nitric oxide. Br J Pharmacol.
134:1159–1165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bai CX, Sunami A, Namiki T, Sawanobori T
and Furukawa T: Electrophysiological effects of ginseng and
ginsenoside Re in guinea pig ventricular myocytes. Eur J Pharmacol.
476:35–44. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Z, Li Z and Liu X: Effect of
ginsenoside Re on cardiomyocyte apoptosis and expression of
Bcl-2/Bax gene after ischemia and reperfusion in rats. J Huazhong
Univ Sci Technolog Med Sci. 22:305–309. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He L, Sun S and Fan J: Effect of Shenmai
injection on neurocyte apoptosis and change of cytoplasmic calcium.
Zhong Guo Zhong Xi Yi Jie He Za Zhi. 21:605–607. 2001.(In
Chinese).
|
|
22
|
Hou M and Ao D: Study on the mechanism of
ginsenosides against ischemia-reperfusion injury of myocardium.
Zhong Guo Xiong Xin Xue Guan Wai Ke Lin Chuang Za Zhi. 7:256–9.
2000.(In Chinese).
|
|
23
|
Li Y, Cui X, Pan L, Li Z, Ji C and Du K:
Electron microscopic observation and Ca2+ analysis on
protective effect of ginsenoside from fruit on myocardium of
hemorrhagic shock in dogs. Bai Qiu En Yi Ke Da Xue Xue Bao.
24:452–454. 1998.(In Chinese).
|
|
24
|
Zhao X, Li Z, Cao Y, Geng Q, An L and Tang
H: (2001) The influence of ginsenosides on intracellular free
calcium concentration during hypoxia in guinea pigs. J Chin Med
Univ. 30:431–434. 2001.
|
|
25
|
He Q, Sun J, Wang Q, Wang W and He B:
Neuroprotective effects of ginsenoside Rg1 against oxygen-glucose
deprivation in cultured hippocampal neurons. J Chin Med Assoc.
77:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu D, Wu L, Li CR, Wang XW, Ma YJ, Zhong
ZY, Zhao HB, Cui J, Xun SF, Huang XL, et al: Ginsenoside Rg1
protects rat cardiomyocyte from hypoxia/reoxygenation oxidative
injury via antioxidant and intracellular calcium homeostasis. J
Cell Biochem. 108:117–124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Simpson P and Savion S: Differentiation of
rat myocytes in single cell cultures with and without proliferating
nonmyocardial cells. Cross-striations, ultrastructure and
chronotropic response to isoproterenol. Circ Res. 50:101–116. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stamm C, Friehs I, Cowan DB, CaoDanh H,
Choi YH, Duebener LF, McGowan FX and del Nido PJ: Dopamine
treatment of postischemic contractile dysfunction rapidly induces
calcium-dependent pro-apoptotic signaling. Circulation.
106:I290–I298. 2002.PubMed/NCBI
|
|
29
|
Cao S, Nie L, Wang G and Lin R: Study on
ginsenosides contents of Shenmai injection and its intermediates by
UPLC. Yao Wu Fen Xi Za Zhi. 7:1264–68. 2014.(In Chinese).
|
|
30
|
Chen Y, Hao R and Huang Q: Shenmai
injection protects rat cardiomyocytes from angiotensin II-induced
apoptosis in vitro. Zhong Guo Bing Li Sheng Li Za Zhi. 9:76–80.
2004.(In Chinese).
|
|
31
|
Hao R, Lou J, Zhang Y, Zheng H and Huang
Q: The effect of Shenmai injection on cardiac myocyte apoptosis
after hypoxia. Zhong Guo Bing Li Sheng Li Za Zhi. 4:660–663.
2007.(In Chinese).
|
|
32
|
Koss KL, Ponniah S, Jones WK, Grupp IL and
Kranias EG: Differential phospholamban gene expression in murine
cardiac compartments. Molecular and physiological analyses. Circ
Res. 77:342–353. 1995.PubMed/NCBI
|
|
33
|
Ito K, Yan X, Feng X, Manning WJ, Dillmann
WH and Lorell BH: Transgenic expression of sarcoplasmic reticulum
Ca(2+) ATPase modifies the transition from hypertrophy to early
heart failure. Circ Res. 89:422–429. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schmidt U, del Monte F, Miyamoto MI,
Matsui T, Gwathmey JK, Rosenzweig A and Hajjar RJ: Restoration of
diastolic function in senescent rat hearts through adenoviral gene
transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation.
101:790–796. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Plank DM, Yatani A, Ritsu H, Witt S,
Glascock B, Lalli MJ, Periasamy M, Fiset C, Benkusky N, Valdivia HH
and Sussman MA: Calcium dynamics in the failing heart: Restoration
by beta-adrenergic receptor blockade. Am J Physiol Heart Circ
Physiol. 285:H305–H315. 2003. View Article : Google Scholar : PubMed/NCBI
|