Introduction
Encephalitis with prominent neuropsychiatric
symptoms and post-mortem evidence of inflammatory lesions was
described in the 1960s as limbic encephalitis (LE) (1). Subsequent studies identified an
association between LE and antibodies directed against tumor and
brain tissue, establishing LE as a paraneoplastic disease. Typical
clinical features include disturbance of consciousness, short-term
memory, psychosis and seizures. The antibodies are directed against
intracellular antigens and many of these onconeuronal antibodies
are associated with certain malignancies, such as small cell lung
cancer. Cancer is typically diagnosed in ≥95% of patients with
these antibodies (2) (Table I). However, it is unlikely that these
antibodies are directly pathogenic due to their intracellular
targets. Antibody transfer (anti-Yo) failed to provoke respective
histopathological or typical clinical features (3), and neuronal loss appears to be T-cell
driven (4). These antibodies can be
divided into three subgroups: Ia, containing the classical
onconeuronal antibodies, such as anti-Hu, anti-Yo and anti-Ri; Ib,
cancer-associated antibodies (SOX and ZIC) lacking an association
with an immune response causing a paraneoplastic syndrome (PNS);
and Ic, non-PNS antibodies, including glutamate decarboxylase,
associated with cerebellar ataxia. Antibodies in the Ia group are
attributed to the majority of paraneoplastic syndromes (PNS) with
anti-Hu and anti-Yo as the most common, accounting for up to ~50%
of all PNS antibodies (5).
 | Table I.Onconeuronal and neuronal surface
antigen antibodies. Clinical syndrome, common associated tumors and
rate of tumor diagnosis for onconeuronal antibodies in comparison
with surface antibodies. |
Table I.
Onconeuronal and neuronal surface
antigen antibodies. Clinical syndrome, common associated tumors and
rate of tumor diagnosis for onconeuronal antibodies in comparison
with surface antibodies.
| A, Onconeuronal
antibodies |
|
|---|
|
|---|
| Antibody | Neurological
syndromes | Tumors | Probability of
cancer (%) |
|---|
| Hu | Encephalomyelitis,
cerebellar degeneration, limbic encephalitis, brainstem
encephalitis | SCLC | 98 |
| CV2 | Encephalomyelitis,
chorea, cerebellar degeneration, limbic encephalitis | SCLC | 96 |
| Amphiphysin | Stiff-person
syndrome, myelopathy and myoclonus, encephalonus | Breast SCLC | 95 |
| Ri | Brainstem
encephalitis, opsoclonus myoclonus | Breast, SCLC | 97 |
| Yo | Cerebellar
degeneration | Ovarian,
breast | 98 |
| Ma2 | Limbic
encephalitis, brainstem encephalitis | Testicular | 96 |
|
|---|
| B, Surface
antibodies |
|
|
|---|
| Antibody | Neurological
syndromes | CNS pleocytosis
(%) | Tumors | Probability of
cancer (%) |
|
|---|
| VGKC | Limbic
encephalitis, Morvan's syndrome, Creutzfeld-Jakob disease-like
syndrome | 41 | SCLC, thymoma | 31 |
| NMDA | Encephalitis with
neuropsychiatric features, catatonia, aphasia, hypoventilation | 91 | Ovarian,
teratoma | 9–56 |
| AMPA | Limbic
encephalitis, atypical psychosis | 90 |
| 70 |
|
GABAB | Limbic
encephalitis | 80 |
| 47 |
| Glycine | Encephalomyelitis,
stiff person syndrome | Unknown |
| 3 |
After the year 2000, a second set of autoantibodies
was described (6–9), which are directed against surface
antigen epitopes, primarily ion channels. In addition to possessing
different target antigen locations, these antibodies exhibit a
lower coincidence with malignancies, varying from 3% (glycine AB)
up to 70% (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
antibodies (AMPA) (2). In anti-NMDA
receptor encephalitis, younger patients are at a reduced risk of
presenting with a tumor (10).
Surface target structures are associated with the voltage-gated
potassium channel (VGKC; antibodies LGI1 and Caspr2), ligand
dependent ion channels, such as ionotropic glutamate receptors
antibodies (NMDA, AMPA), GABAA, GABAB, and
glycine receptor antibodies. Present in ~4% of all
autoimmune-mediated encephalitis, the anti-NMDA receptor is the
most common. Large case series studies involving >200 patients
have been published on the disease course, therapeutic intervention
and role of the NMDA receptor antibody (11–13). A
direct pathogenic role of these antibodies can be assumed, as
immunotherapy mitigates the clinical symptoms and improves
neurological outcome (11,14–16).
Furthermore, cell-culture experiments have shown a reversible
downregulation of NMDA receptors on antibody-exposed cells,
mediated by titer-dependent capping and internalization. Thereby
the surface expression of the NMDA receptor is diminished
prominently, affecting the temporal lobes and hippocampus (12) and disturbing cell communication (for
example, GABAergic and dopamine) pathways. Neuropsychiatric
symptoms are the common clinical feature of anti-NMDA receptor
encephalitis. The distinct involvement of the peripheral nervous
system may be explained by the varying expression patterns of the
surface antigen (17); for example,
neuromyotonia is more common in Caspr2- compared with LGI1-mediated
disease. Anti-NMDA receptor antibodies are directed against an
extracellular epitope of the Glu-N1 unit of the NMDA receptor. To
date, 7 different subunits in 3 subfamilies (Glu-N1, Glu-N2a-d and
Glu-N3a+b) have been identified. The NMDA receptor is a
heterotetrameric assembly of an NR1 subunit, usually in combination
with modulatory NR2 and/or NR3 units. The NR2 subunit composition
primarily defines the gating and functional differences of the
receptor (18,19). Its composition varies between cell
type (neuron, oligodendrocyte, astrocyte) and site of expression
(20–23).
Materials and methods
Patients
The present study included 7 patients with anti-NMDA
receptor encephalitis admitted to the hospital of Hannover Medical
School (Hannover, Germany) between 2008 and 2014. This study was
approved by the local Ethics Committee of Hannover Medical School
and patients or their carers provided written informed consent. The
details of patient 1 have been previously reported in part
(24) and more recently in
association with brain metabolic changes in autoimmune encephalitis
(25).
Testing and diagnosis
Routine blood tests included blood count and
measurement of serum electrolytes, liver enzymes, creatine kinase
(CK), glucose, partial thromboplastin time (PTT) and international
normalized ratio (INR). Cerebrospinal fluid (CSF) was available for
extraction in 6 patients, and subjected to a variety of analyses,
including cell count, glucose, lactate, total protein, albumin,
IgG, IgA, IgM, oligoclonal bands, and microbiological and
virological analysis. NMDA receptor IgG antibodies were detected by
immobilizing human embryonic kidney cells (HEK 293) transfected
with the NR1 subunit of the NMDA receptor on BIOCHIPS (Euroimmun
AG, Lübeck, Germany), and incubating them with patient sera and/or
cerebrospinal fluid. Primary dilutions were 1:10 in serum and 1:1
in CSF. Quantification was obtained via the specific fluorescence
intensities using an indirect immunofluorescence test, and
expressed as end-point titers. Patients were diagnosed with
anti-NMDA receptor encephalitis when presenting typical clinical
features in combination with CSF and/or serum NMDA receptor IgG
antibodies. Magnetic resonance imaging (MRI) fields, including
contrast application, were obtained using a 1.5 or 3.0-Tesla MRI
instrument. Clinical outcome was rated using the modified Rankin
scale (mRS).
Results
Patient admission
A total of 7 female patients admitted to the
hospital between 2008 and 2014 were diagnosed with anti-NMDA
receptor encephalitis. On admission, patients were aged between 23
and 57 years old. Hospital admission was via the accident and
emergency department in 4/7 cases, due to the subacute onset of
neuropsychiatric symptoms, including personality changes.
Case repor
Case 1 (patient 2)
A 34-year-old woman with no pre-existing illness was
admitted to the hospital with the subacute onset of personality
change, anxiety and psychotic-hallucinatory perception. The results
of cranial MRI and thoracic/abdominal computed tomography (CT)
examination appeared normal. In the electroencephalography (EEG), a
continuous slow activity with an intermittent right temporal focus
was observed. Within 2 days after admission, the patient developed
orofacial dyskinesia, and severe focal and generalized seizures,
which were only interrupted using high doses of phenobarbital.
Eventually, the patient developed central hypoventilation with
respiratory insufficiency and required ventilation (mRS 5). The
results of a fludeoxyglucose positron emission tomography (FDG-PET)
examination were unremarkable regarding tumor screening; however,
an abdominal ultrasound revealed a suspicious lesion in the right
ovary. In response to this observation, an ovariectomy was
conducted 9 days after patient admission, which revealed a
teratoma. CSF analysis revealed oligoclonal bands without
pleocytosis, and anti-NMDA receptor antibodies were detectable in
the CSF (1:100) and serum (1:800). In the intensive care unit
(ICU), 2 weeks after admission, therapy was initiated with
immunoabsorption (6 applications) followed by methylprednisolone (1
g/day for 5 days). However, the patient developed a severe
autonomic dysfunction, with cardiac arrhythmia, blood pressure
dysregulation, disturbed thermoregulation and hypersalivation. EEG
and particularly the cardiorespiratory situation worsened to a
critical condition within the next month. An additional left
ovariectomy was performed, revealing no teratoma, and a second
cycle of immunoabsorption (6 applications) followed by 4 cycles of
cyclophosphamide (600 mg/m2) was administered at monthly
intervals. Towards the fourth cycle of treatment, the patient
started to improve steadily. The immunosuppressive therapy was
de-escalated to immunoglobulins (0.4 g/kg/day for 5 days).
Repetitive analysis of CSF and serum NMDA receptor antibodies
showed decreasing antibody titers (Table II). Thoracic and abdominal CT was
repeated after 6 months, in addition to MRI of the pelvis. After 7
months in the ICU, the patient was discharged for rehabilitation.
At this time point the patient was oriented only to herself but had
lost orientation in time and space, and showed fluctuating
vigilance and cooperation. The patient was followed up for 3 years
and her clinical state gradually improved. On the final visit the
patient was fully orientated, cooperative and able to look after
herself (mRS 1).
 | Table II.Analysis of NMDA receptor antibody
titers in CSF and serum at admission and during follow-up with
respective CSF/serum protein levels and antibody index value. |
Table II.
Analysis of NMDA receptor antibody
titers in CSF and serum at admission and during follow-up with
respective CSF/serum protein levels and antibody index value.
| Patient | Follow-up
(months) | Oligoclonal
bands | CSF titer | Serum titer | IgG serum
(g/l) | IgG CSF (g/l) | CSF titer/protein
(mg/l) | Serum titer/protein
(g/l) | Antibody index |
|---|
| 1 | 0 | Positive | ND |
1:1,600 | 7.67 | 0.152 | NA | 80.7 | NA |
|
| 10 |
|
1:1,600 |
1:51,200 | 6.11 | 0.049 | 32.7 | 8,379.7 | 3.9 |
|
| 11 |
|
1:800 |
1:25,600 | 2.37 | 0.014 | 57.1 | 10,801.7 | 5.29 |
|
| 22 |
|
1:400 |
1:25,600 | 4.11 | 0.012 | 32.3 | 6,228.7 | 4.13 |
|
| 24 |
| 1:50 |
1:1,600 | 2.83 | 0.022 | 2.3 | 565.4 | 4.02 |
| 2 | 0 | Positive |
1:100 | 1:800 | 9.68 | 0.051 | 2.0 | 103.3 | 23.68 |
|
| 4 |
| 1:10 | 1:400 | 5.40 | 0.015 | 0.7 | 74.1 | 9 |
|
| 7 |
| 1:10 | 1:200 | 10.10 | 0.018 | 0.6 | 19.8 | 28.6 |
| 3 | 0 | Negative | 1:50 | Negative | 12.10 | 0.048 | 1.0 | NA | NA |
|
| 1 |
| 1:10 | Negative | 8.59 | 0.023 | 0.4 | NA | NA |
| 4 | 0 | Positive | Negative |
1:100 | 8.86 | 0.156 | NA | 11.2 | NA |
|
| 2 |
| ND |
1:800 | ND | ND | NA | NA | NA |
|
| 7 |
| ND |
1:400 | 5.35 | 0.041 | NA | 74.8 | NA |
| 5 | 0 | Positive | 1:100 |
1:800 | ND | ND | NA | NA | NA |
|
| 1 |
| ND |
1:800 | ND | ND | NA | NA | NA |
|
| 2 |
| ND |
1:800 | ND | ND | NA | NA | NA |
| 6 | −21 |
| ND | 1:10 | ND | ND | NA | NA | NA |
|
| 0 | Positive | ND |
1:100 | ND | ND | NA | NA | NA |
|
| 6 |
| ND |
1:100 | ND | ND | NA | NA | NA |
|
| 7 |
| ND |
1:800 | ND | ND | NA | NA | NA |
|
| 10 |
| ND | 1:50 | ND | ND | NA | NA | NA |
| 7 | 3 | Positive | 1:200 |
1:200 | 12.80 | 0.097 | 2.1 | 15.6 | 131.55 |
Case 2 (patient 5)
A 44-year-old woman was referred to the hospital for
confirmation of a diagnosis of anti-NMDA receptor encephalitis.
Three months previously, the patient had initially been admitted to
a different hospital with a right temporal anopsy, preceded by a
mild generalized headache and mood changes with latent aggressive
behavior. Alpha activity with signs of varying vigilance were
described in the EEG. Within a week, the patient developed aphasia
with semantic paraphrasia, severe apraxia, anxiety, hallucinations
and reduced orientation to person, time, place and situation. The
results of a cranial MRI examination were unremarkable. CSF
analysis revealed 156 cells/µl, positive oligoclonal bands, a
positive Epstein-Barr virus polymerase chain reaction (PCR)
analysis and elevated 14-3-3 protein concentrations. Infectious
meningoencephalitis was suspected and antiviral and antibiotic
therapy was administered. After 1 week, the patient began to
develop orofacial dyskinesia. Final results from CSF revealed NMDA
receptor IgG antibodies in the CSF and serum (Table II). The patient was administered
haloperidol (2×2.5 mg) and prednisolone (100 mg, orally), but
subsequently developed a neuroleptic malignant syndrome. The
haloperidol was immediately discontinued and the rigor symptoms
eased; however, the patient developed a respiratory insufficiency
and required ventilation. The patient's clinical condition worsened
and after 2 weeks she rapidly developed a trismus-like spasm of the
jaw, fracturing several maxillary teeth, and complex focal
seizures. The patient was at this point transferred to the hospital
at the Hannover Medical School. PET/CT scan results were normal
regarding neoplasia; however, due to age and the aggressive
clinical symptoms, prophylactic ovariectomy was performed, but
revealed no teratoma. The patient was treated with 5 cycles of
plasmapheresis, followed by cyclophosphamide (6 cycles of 750
mg/m2 in monthly intervals), in combination with
rituximab (4 cycles of 375 mg/m2 every week for 1
month). The patient was discharged for rehabilitation after 2
months in the hospital. At the time of transfer, the patient
exhibited variable vigilance, fluctuating aggression and fecal and
urinary incontinence and was fully dependent on assisted care for
everyday functions (mRS 4). During the rehabilitative treatment,
vigilance and clinical symptoms gradually improved, as the patient
relearned everyday skills and her mood stabilized. On the final
visit, 21 months after onset, the patient was fully independent and
was undergoing work rehabilitation for her job as a translator (mRS
1). As immunosuppressive treatment with cyclophosphamide continued
up to 9 months after onset, and an ovariectomy had been performed,
no further immunosuppressive treatment was administered, while
close clinical monitoring was maintained.
Case 3 (patient 6)
A 23 year old woman was admitted via an emergency
department due to phasic loss of orientation to time, place and
situation, abnormal fearful behavior and stereotypical repetitive
movements (mRS 3). Two years previously, the patient had been
referred to a psychiatric department with intermittent behavioral
disorder. Despite a pleocytosis of 51 cells/µl and oligoclonal
bands in the CSF, further encephalitis work-up investigations
including cranial MRI and CSF analysis for viral pathogens did not
elucidate the cause of the patient's symptoms, which resolved
within 6 weeks.
At admission repeated cranial MRI results were
normal, as were MRI of the pelvis and PET/CT scans regarding tumor
screening. A microinvasive laparoscopy was performed, and ovary
biopsy revealed no signs of teratoma. The EEG revealed a slow alpha
rhythm with a paroxysmal frontal delta wave focus, suggesting an
ictal focus for the stereotype movements, and therefore
levetiracetam treatment was initiated (final dose, 2×750 mg).
Neuropsychological deficits included memory and executive
functions. CSF analysis showed a mild pleocytosis, with 18 cells/µl
and oligoclonal bands. NMDA receptor antibodies were tested for
only in serum and were found to be positive (1:100). Notably, a
serum sample stored from the first episode 2 years previously was
analyzed and NMDA receptor antibodies (1:10) were detected. Due to
the pleocytosis, acyclovir treatment was administered for 10 days,
and after the receipt of the NMDA receptor antibody result 14 days
after admission methylprednisolone (1 g/day for 5 days) was
administered. The patient's clinical symptoms and EEG results
improved rapidly and the patient was discharged after 28 days.
However, the patient was readmitted for a planned second course of
methylprednisolone (1 g/day for 5 days) 1 month later. At that
time, neuropsychological memory deficits remained detectable. A
final course of methylprednisolone was administered at 6 months
after admission. Towards the final hospital admission, the
anti-NMDA receptor antibody concentration decreased and the memory
deficits exhibited full remission (mRS 0), enabling the patient to
continue an apprenticeship as an industrial management assistant.
Due to the mild treatment course, no long term immunosuppressive
treatment was initiated.
Assessment of CSF
As a defining condition, anti-NMDA receptor IgG
antibodies were found in the CSF and/or serum in all patients.
Matching serum/CSF samples could be analyzed in 6 patients. Serum
and CSF were screened for NMDA receptor antibodies, followed by
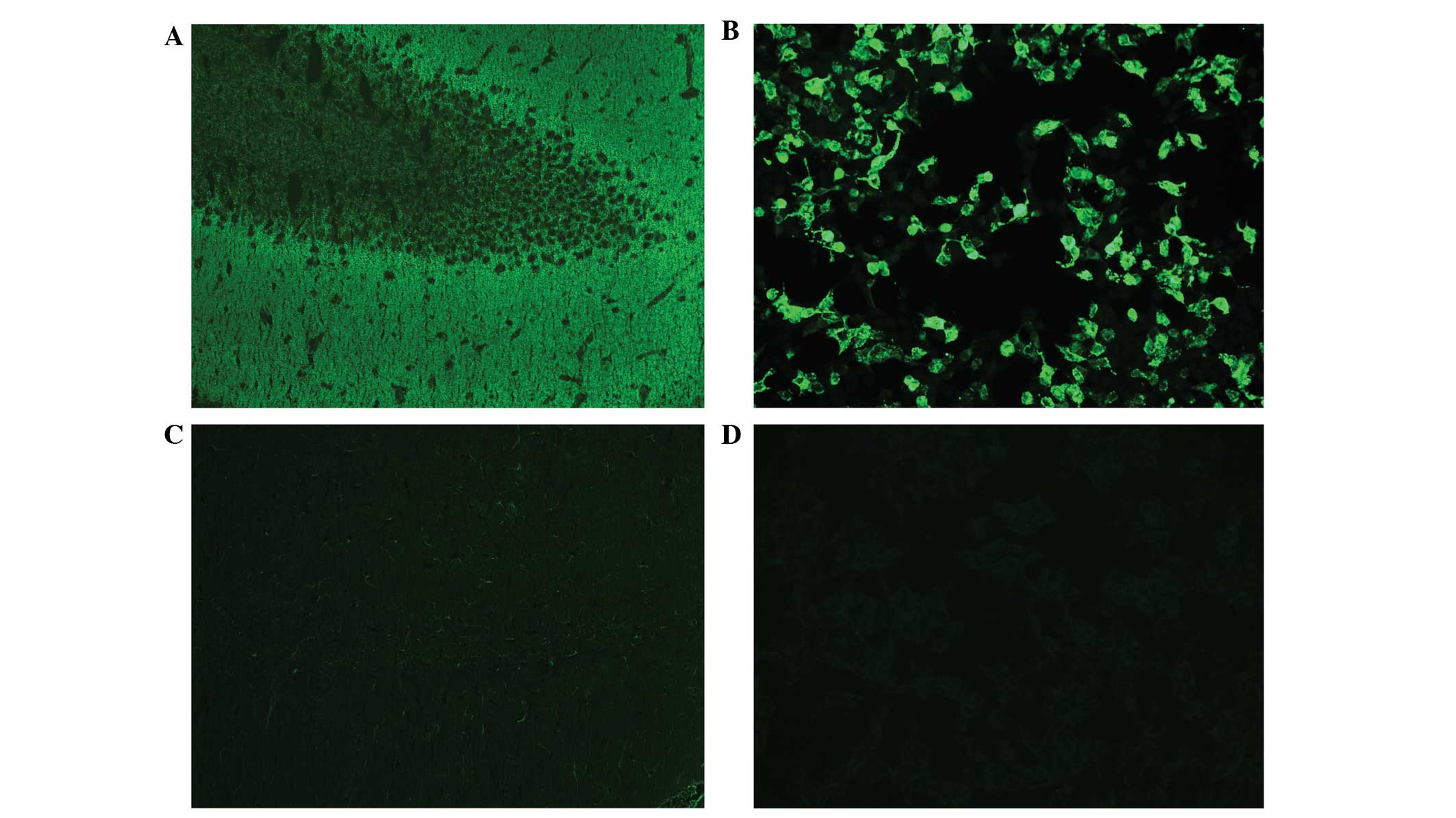
end-point titration on transfected HEK 293 cells (Fig. 1). In patient 6, only serum antibody
titers were available. In patient 3, NMDA receptor antibodies were
only detected in the CSF, which is uncommon but not unusual as
serum antibodies are negative in ~15% of patients using detection
techniques such as rat brain slice and transfected HEK cells
(26). In patients 1 and 2,
calculation of the antibody specific index revealed an intrathecal
synthesis of antibodies. In patient 4, a matching CSF/serum sample
was obtained at the onset of the disease; however, no antibodies
were detected in the CSF following repeated analysis. This is a
highly unusual observation, as antibodies in the central nervous
system (CNS) appear to mediate the clinical symptoms, and patients
with anti-NMDA receptor encephalitis without CSF NMDA receptor
antibodies have not been described. However, NMDA receptor
seropositivity alone has been reported in patients with herpes
simplex virus (HSV) encephalitis (13). The highest CSF and serum titers were
detected in patient 1, who was one of the two patients that
exhibited the least clinical improvement (24,25).
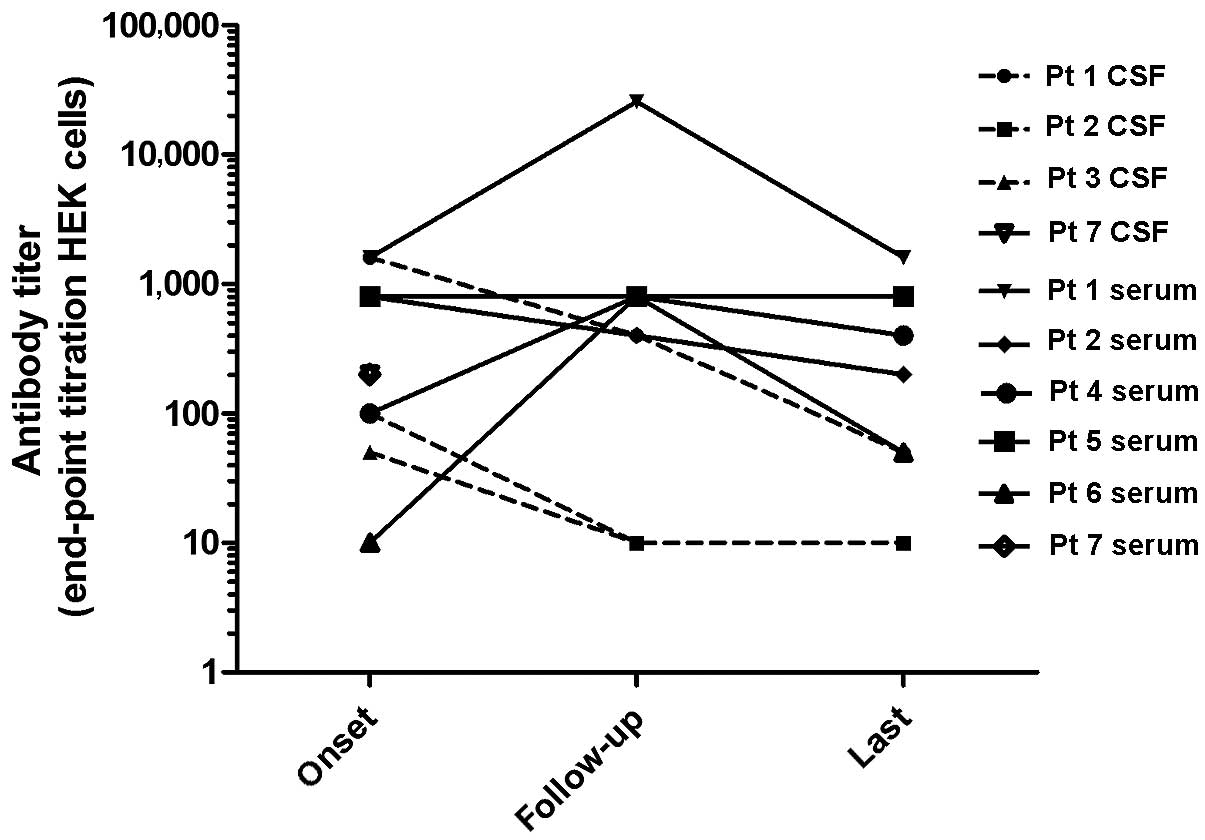
Notably, in contrast to the clinical symptoms, the CSF and serum
NMDA receptor antibody titer declined between month 10 and 11
during immunosuppressive treatment (Table II). However, simultaneously CSF and
serum protein concentrations notably decreased. Therefore, the
antibody titer was adjusted by the protein levels to determine the
CSF titer/CSF protein and serum titer/serum ratios. As presented in
Table II, these ratios increase
until the clinical symptoms are alleviated, then decrease according
to clinical improvement. In patient 4, the antibody titer in the
serum and the ratio serum titer/serum protein increased between
admission and the end of follow-up. This patient failed to improve
clinically, despite antibody titers in the serum decreasing between
months 2 and 7. In patients 2 and 3, the antibody titers and the
titer/protein ratio decreased in correlation with clinical
improvement. In patients 1, 4, 6 and 7 an increase in antibody
titers was detected during the course of the disease (Fig. 2).
Additional diagnostic investigations
MRI scans revealed age-appropriate normal results in
4 patients. In patient 3, a single small unspecific periventricular
T2 hyperintense lesion was observed despite severe clinical
impairment at that time (mRS 5). In patient 1, hippocampal
T2-hyper-intensities and a bilateral diffusion weighted signal
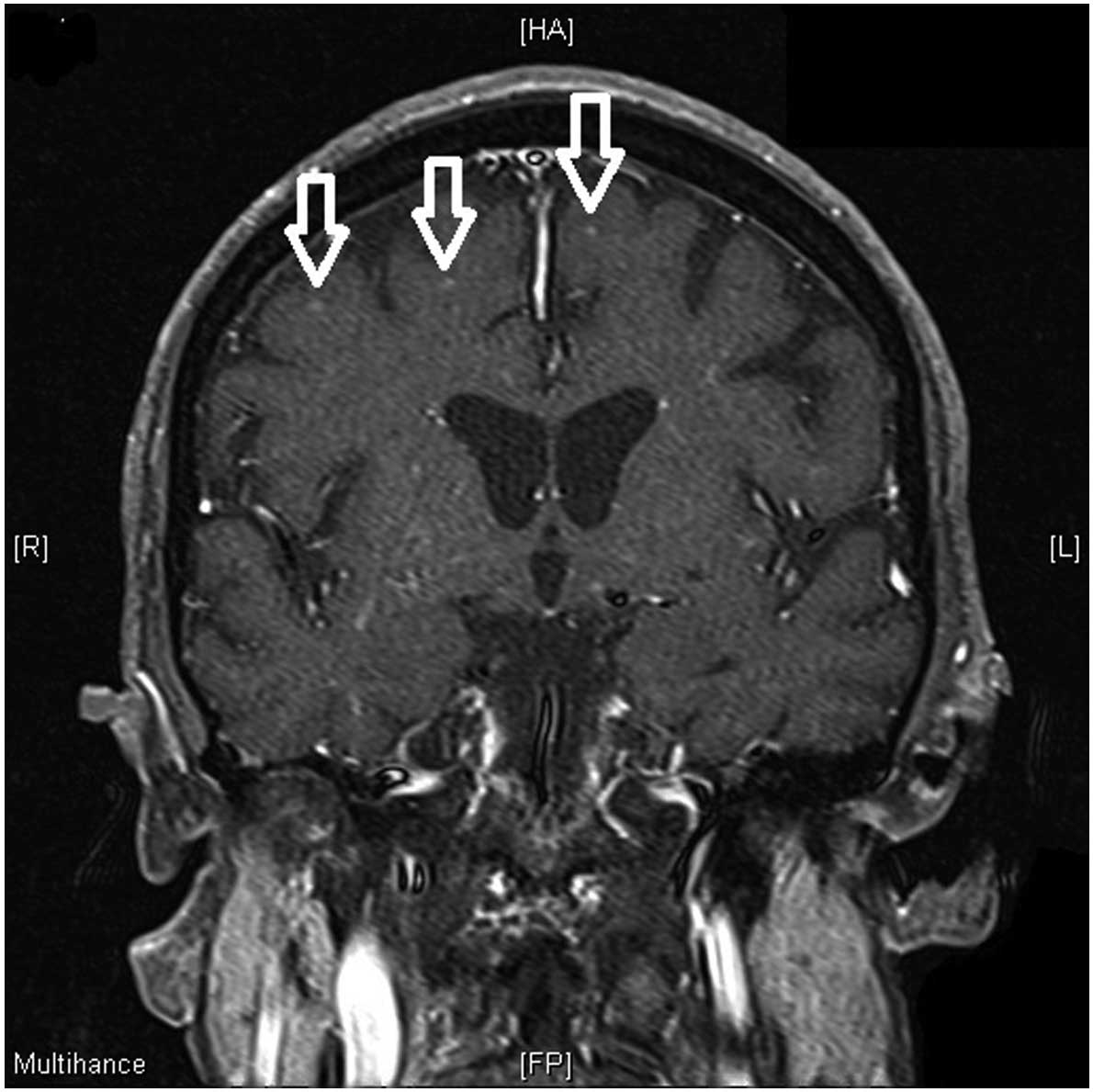
elevation were observed. In patient 4 subcortical
T2-hyper-intensities with contrast enhancement were noted (Fig. 3). In addition to tumor screening with
routine diagnostics, such as chest X-ray and abdominal ultrasound,
all patients received FDG-PET. Oophorectomy was performed in
patients 1–5. Ovarian teratoma were detected in patients 1 and 2;
90% of tumors associated with this disease are teratomas, with an
incidence of 25–56% (11,12,27).
Discussion
The present case series illustrates the
characteristics of the multi-stage clinical presentation and the
range of severity of anti-NMDA receptor encephalitis, in context
with their anti-NMDA receptor antibodies. The majority of patients
were premenopausal women, which is a typical feature of this
disease entity; however, anti-NMDA receptor antibodies have been
reported in men and women of all ages (28).
In patients with the typical symptoms of sub-acute
onset of psychic disturbances, differences in personality, memory
loss and seizures, the range of differential diagnoses is limited.
Clinical presentation and verification of NMDA receptor antibodies
define the diagnosis of anti-NMDA receptor encephalitis. MRI alters
only in ~33% of patients, and although EEG and CSF abnormalities
are common in 90 and 79% of cases, respectively, the results are
unspecific (11). Infectious causes
of encephalitis, particularly HSV and less commonly
Varicella-Zoster virus or cytomegalovirus, must be excluded via PCR
and/or antibody index using CSF examination. Metabolic causes, such
as uremic and hepatic encephalopathy, may be assessed by routine
laboratory tests. More often, diagnostic uncertainty occurs if
symptoms are mild or psychic changes are dominated by negative
symptoms, such as depression or avolition, such as during the first
episode of patient 6. In young patients, toxic causes due to drug
or alcohol abuse with Wernicke's encephalopathy may considered
(Table III).
 | Table III.Differential diagnoses, common
clinical features and useful diagnostic methods to separate these
diagnoses from anti-NMDA receptor encephalitis. |
Table III.
Differential diagnoses, common
clinical features and useful diagnostic methods to separate these
diagnoses from anti-NMDA receptor encephalitis.
| Differential
diagnosis | Clinical
presentation | Diagnostic key |
|---|
| Anti-NMDA
encephalitis | Phase 1: Prodromal
stage (headache, fever). Phase 2: Behavioral changes, seizures,
fluctuating vigilance, hypoventilation. Phase 3: movement
abnormalities, vegetative dysfunction | NMDA antibodies in
CSF or serum (15% false negative) |
| Other autoimmune
encephalitis | Depending on
antibody: Behavioral changes, seizures, amnesia, cerebellar
degeneration, Morvan's syndrome, stiff person syndrome | Anti-neuronal
antibodies, SCLC, thymoma, breast, ovarian or testicular
cancer |
| Infectious
encephalitis (HSV, VZV, syphilis, HIV) | Fever, seizures,
focal deficits, | Identification of
pathogen (PCR), triphasic waves in CJD |
| Metabolic
encephalopathy | Cognitive
dysfunction, reduced vigilance, flapping tremor | Hyperammonemia,
uremia, electrolyte imbalance, MRI T2 white matter lesions |
| Wernicke's
encephalopathy | Cognitive
dysfunction, reduced vigilance, ocular motor disturbances,
ataxia | MRI T2 lesions of
the corpora mamillaria, response to thiamin |
| SREAT (Hashimoto's
encephalopathy) | Psychosis,
seizures, cognitive dysfunction, reduced vigilance, focal deficits,
ataxia | TPO and MAK
antibodies, response to corticosteroids |
| Vasculitis (lupus
erythematosus, Sjögren's syndrome) | Fever, myalgia,
focal/multifocal deficits, | Ischemic MRI
lesions, autoantibodies: dsDNA, SS-A/Ro, SS-A/LA, PR3, MPO |
| Intoxication | Drug-dependent
psychosis, reduced vigilance | Drug screening |
Other autoimmune diseases, such as steroid
responsive encephalopathy associated with autoimmune thyroiditis
(SREAT, formerly known as Hashimoto encephalopathy) and CNS
vasculitis, may be considered as possible differential diagnoses.
For example, in patient 4, who presented with subcortical
T2-hyperintensities and contrast enhancement in MRI, a vasculitis
was suspected and excluded as a possibility by brain biopsy.
Considering SREAT, thyroid peroxidase antibodies are identified in
5% of healthy individuals and clinical symptoms respond rapidly to
steroid treatment. In older patients the rapid mental deterioration
of Creutzfeldt-Jacob disease may resemble the neuropsychiatric
symptoms of anti-NMDA receptor encephalitis; however, elevated
levels of 14-3-3 protein may be present in the CSF in both
diseases.
Commonly in anti-NMDA receptor encephalitis the
clinical presentation passes through different stages. During the
first stage, the prodromal stage which precedes the next stage by
~2 weeks, 70% of patients suffer from unspecific flu-like symptoms,
including fever, headache, nausea and unrest (12,28). In
the second phase, neuropsychiatric symptoms are overt and seizures
occur. All of the present patients exhibited psychological
abnormalities, ranging from unrest and disorientation to fear,
affective disturbances and manifested psychosis, hallucinations and
loss of short-term memory. Furthermore, all the present patients
suffered from generalized or focal seizures. This is in accordance
with the prior literature, as psychiatric disturbances are a
defining symptom of the disease and the incidence of seizures is
70–80% (12,27). In particular, the symptom combination
of neuropsychiatric abnormalities and generalized seizures is
distinct from that of other types of autoimmune encephalitis, such
as anti-LGI1 encephalitis, in which neuropsychiatric symptoms are
less common and mild and predominantly focal seizures occur.
Central hypoventilation is another common feature of the early
stages of anti-NMDA receptor encephalitis.
The third phase, after 10–20 days (27), is defined by dyskinesias and
vegetative dysregulation. Patients 1–3 suffered from autonomic
dysfunction with cardiac arrhythmia, blood pressure deregulation,
disturbed thermoregulation and hypersalivation.
The initial symptoms of anti-NMDA receptor
encephalitis are unspecific flu-like symptoms that usually precede
the neuropsychiatric manifestations by 1–2 weeks. This indicates a
rapid onset of antibody production, which resembles infectious or
acute onset fast-progressing autoimmune diseases such as acute
demyelinating encephalomyelitis. Potential associations have been
indicated between NMDA receptor antibody production and
Mycoplasma pneumoniae (29)
and HSV, possibly by autoantigen presentation following neuronal
cell death (13). A study
demonstrated that in a small subgroup of patients, the presence of
NMDA receptor antibodies is associated with aquaporin 4 and myelin
oligodendrocyte glycoprotein antibodies, which are also observed in
patients with neuromyelitis optica or other demyelinating
syndromes, suggesting connected immune processes (30). Other than the association with
ovarian teratoma, the reason for the predominance of women among
patients with anti-NMDA receptor encephalitis remains unclear;
however, it is possible that estrogen via stimulated antibody
production may be involved (31).
In animal experiments it has been demonstrated that
the injection of NMDA receptor antibodies in rats causes
corticomotor hyperexcitability (32), which may be the underlying cause of
the agitation and seizures observed in the early stage of the
disease. Internalization of NMDA surface receptors decreases the
neuronal excitability of GABAergic neurons, which highly express
NMDA receptors; therefore, it has been speculated that positive
symptoms such as dyskinesias are generated by disinhibiting
extrapyramidal networks (10,33). It
has been hypothesized that the disruption of the blood-brain
barrier is a relevant factor for anti-NMDA receptor associated
neuropsychiatric diseases (34).
Furthermore, it has been suggested that the central hypoventilation
observed in patients with anti-NMDA receptor encephalitis may be
caused by the disruption of ponto-medullary respiratory reflexes,
as occurs in an NMDA receptor blockade (35). Therefore, the time-course of symptoms
supports a primarily cortical/hippocampal mechanistic pathway, with
secondary subcortical alteration and disturbance of
corticostriatal/brainstem pathways. The fact that the NMDA receptor
is expressed in hippocampal, cortical and cerebellar neurons, in
addition to glial cells such as oligodendrocytes and astrocytes, in
varying concentrations and subunit composition may be of further
pathophysiological relevance to the clinical presentation and time
course of the disease (18,21,23,36,37. The
expression varies under different pathophysiological conditions,
such as ischemia (38). Memory
deficits, as a typical feature of anti-NMDA receptor encephalitis,
may be explained by the increased expression of the NMDA receptor
in hippocampal neurons (12,39).
It has been demonstrated that the titer of NMDA
receptor antibodies correlates with clinical outcome, and that high
antibody titers are more common in patients with poor outcome or
tumors (12,26). In the present case series, the
highest antibody titer was detected in a patient with occult
teratoma who failed to be detected by ultrasound, CT, MRI and PET.
High antibody titers may be an indicator of an underlying tumor,
particularly if there is no decline following immunosuppressive
treatment. Despite the correlation between antibody titers,
severity of symptoms and the antibody-driven pathogenesis, a number
of patients, such as patients 1 and 4 in the patient case series
and others in the literature (12,26),
failed to improve despite decreasing antibody titers. As shown in
the present cases, NMDA receptor antibodies are part of the overall
protein concentration measured in the CSF or serum. Reduced
antibody titers may therefore correlate with an overall reduction
in protein concentration. This is relevant for patients with
anti-NMDA receptor encephalitis, since plasma exchange or
immunoabsorption in particular, but also ICU treatment and a
long-lasting catabolic state significantly decrease the protein
concentration in serum and CSF (Table
II). This may be avoided by calculating the antibody/protein
ratio as opposed to the antibody titers alone. This also elucidates
the importance of follow-up CSF analysis as a marker for disease
activity and prognosis during the course of this disease and other
types of autoimmune and infectious encephalitis (40,41).
The relevance of IgA and IgM anti-NMDA receptor
antibodies remains unclear, as they are present in ~9% of healthy
individuals. IgG anti-NMDA receptor antibodies are present in ~1%
of healthy individuals only (42).
Notably, IgA and IgM are more frequently observed in patients with
neuropsychiatric disease and a history of blood-brain barrier
disruption (43). Furthermore, an
association between anti-NMDA receptor IgA and cognitive
dysfunction has been detected (44).
In all patients, with the exception of patient 4,
immunotherapy was initiated within 14 days after the onset of
psychiatric symptoms. The latter patient was referred to the
hospital with unknown encephalopathy for >1 year, receiving no
immunotherapy during that time. All patients received intravenous
methylprednisolone 1 g/day for 5 days. Further primary treatment
was 0.4 g/kg intravenous immunoglobulin (IVIG) per day for 5 days
and/or plasmapheresis, and the number of repetitions was
symptom-dependent. In 4 patients a second-line therapy consisted of
4 cycles rituximab with 375 mg/m2 body surface area
(BSM) at weekly intervals in combination with 6 cycles
cyclophosphamide 750 mg/BSM at monthly intervals. One patient
received only first-line therapy (patient 6), the 2 remaining
patients received only cyclophosphamide (patient 2) and only
azathioprine (patient 4) as second-line therapies. ICU admission
was necessary for 4 patients due to central hypoventilation during
the course of the disease.
Treatment outcome can be positively influenced by
early treatment initiation, and the use of intensified
(second-line) treatment if initial therapy fails (observed in 45%
of patients) (11). Treatment
failure can be assumed if no sustained improvement is observed 4
weeks after first-line therapy. However, all patients were at least
moderately disabled during their disease course, 4 patients had a
good clinical outcome (mRS ≤1) at last follow-up. Patient 7
improved from mRS 5 to mRS 2 in 3 months of observation time.
However, the 2 remaining patients were relevantly disabled (mRs 4).
Possible explanations of poorer response to treatment may include
deferred diagnosis with delayed treatment initiation in case 4, and
delayed diagnosis of teratoma in case 1, as imaging including
FDG-PET and explorative laparotomy with ovarian biopsy yielded
negative results. However, the late ovariectomy that confirmed
teratoma histologically exhibited limited improvement. Controlled
trials are lacking. However, the commonly used and recommended
treatment regime, as administered to the majority of the present
patients, comprises first line methylprednisolone and IVIG and/or
plasmapheresis followed by second line rituximab and/or
cyclophosphamide, as this treatment protocol reduces clinical
symptoms and the levels of anti-NMDA receptor antibodies.
Approximately 12% of patients with anti-NMDA receptor encephalitis
suffer from relapses; however, immunosuppressive treatment during
the first episode and second line immunosuppressive treatment in
patients without a tumor is associated with a lower frequency of
relapses (11).
In patients with a new subacute onset of
neuropsychiatric symptoms, the differential diagnosis of anti-NMDA
receptor encephalitis should be considered and CSF analysis may aid
the detection of anti-NMDA receptor antibodies. Although multistage
clinical presentation is a common feature of the disease, the
severity of symptoms is highly variable. Cranial MRI is typically
necessary for differential diagnosis; however, in anti-NMDA
receptor encephalitis results are unspecific. Depending on the
symptoms, other diagnoses may be excluded (Table III). If anti-NMDA receptor
encephalitis is confirmed, an intensive search for tumors,
particularly teratomas, is required. Immunosuppressive treatment is
required immediately, as a good clinical outcome is associated with
early therapy for decreasing anti-NMDA receptor antibody
concentrations.
Acknowledgements
The authors thank Karin Fricke for technical
support.
References
|
1
|
Brierley JB, Corsellis JAN, Hierons R and
Nevin S: Subacute encephalitis of later adult life. Mainly
affecting the limbic areas. Brain. 83:357–368. 1960. View Article : Google Scholar
|
|
2
|
Graus F, Saiz A and Dalmau J: Antibodies
and neuronal autoimmune disorders of the CNS. J Neurol.
257:509–517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Graus F, Illa I, Agusti M, Ribalta T,
Cruz-Sanchez F and Juarez C: Effect of intraventricular injection
of an anti-Purkinje cell antibody (anti-Yo) in a guinea pig model.
J Neurol Sci. 106:82–87. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bien CG, Vincent A, Barnett MH, Becker AJ,
Blümcke I, Graus F, Jellinger KA, Reuss DE, Ribalta T, Schlegel J,
et al: Immunopathology of autoantibody-associated encephalitides:
Clues for pathogenesis. Brain. 135:1622–1638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giometto B, Grisold W, Vitaliani R, Graus
F, Honnorat J and Bertolini G: PNS Euronetwork: Paraneoplastic
neurologic syndrome in the PNS Euronetwork database: A European
study from 20 centers. Arch Neurol. 67:330–335. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ances BM, Vitaliani R, Taylor RA,
Liebeskind DS, Voloschin A, Houghton DJ, Galetta SL, Dichter M,
Alavi A, Rosenfeld MR and Dalmau J: Treatment-responsive limbic
encephalitis identified by neuropil antibodies: MRI and PET
correlates. Brain. 128:1764–1777. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bataller L, Kleopa KA, Wu GF, Rossi JE,
Rosenfeld MR and Dalmau J: Autoimmune limbic encephalitis in 39
patients: Immunophenotypes and outcomes. J Neurol Neurosurg
Psychiatry. 78:381–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dalmau J, Tuzün E, Wu HY, Masjuan J, Rossi
JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, et al:
Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis
associated with ovarian teratoma. Ann Neurol. 61:25–36. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gold M, Pul R, Bach JP, Stangel M and
Dodel R: Pathogenic and physiological autoantibodies in the central
nervous system. Immunol Rev. 248:68–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dalmau J, Lancaster E, Martinez-Hernandez
E, Rosenfeld MR and Balice-Gordon R: Clinical experience and
laboratory investigations in patients with anti-NMDAR encephalitis.
Lancet Neurol. 10:63–74. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Titulaer MJ, McCracken L, Gabilondo I,
Armangué T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I,
Martinez-Hernandez E, et al: Treatment and prognostic factors for
long-term outcome in patients with anti-NMDA receptor encephalitis:
An observational cohort study. Lancet Neurol. 12:157–165. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dalmau J, Gleichman AJ, Hughes EG, Rossi
JE, Peng X, Lai M, Dessain SK, Rosenfeld MR, Balice-Gordon R, Lynch
DR, et al: Anti-NMDA-receptor encephalitis: Case series and
analysis of the effects of antibodies. Lancet Neurol. 7:1091–1098.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pruss H, Finke C, Höltje M, Hofmann J,
Klingbeil C, Probst C, Borowski K, Ahnert-Hilger G, Harms L, Schwab
JM, et al: N-methyl-D-aspartate receptor antibodies in herpes
simplex encephalitis. Ann Neurol. 72:902–911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lancaster E, Lai M, Peng X, Hughes E,
Constantinescu R, Raizer J, Friedman D, Skeen MB, Grisold W, Kimura
A, et al: Antibodies to the GABA (B) receptor in limbic
encephalitis with seizures: Case series and characterisation of the
antigen. Lancet Neurol. 9:67–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai M, Hughes EG, Peng X, Zhou L,
Gleichman AJ, Shu H, Matà S, Kremens D, Vitaliani R, Geschwind MD,
et al: AMPA receptor antibodies in limbic encephalitis alter
synaptic receptor location. Ann Neurol. 65:424–434. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wong SH, Saunders MD, Larner AJ, Das K and
Hart IK: An effective immunotherapy regimen for VGKC
antibody-positive limbic encephalitis. J Neurol Neurosurg
Psychiatry. 81:1167–1169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kleopa KA, Elman LB, Lang B, Vincent A and
Scherer SS: Neuromyotonia and limbic encephalitis sera target
mature Shaker-type K+ channels: Subunit specificity
correlates with clinical manifestations. Brain. 129:1570–1584.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paoletti P, Bellone C and Zhou Q: NMDA
receptor subunit diversity: Impact on receptor properties, synaptic
plasticity and disease. Nat Rev Neurosci. 14:383–400. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Traynelis SF, Wollmuth LP, McBain CJ,
Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ and
Dingledine R: Glutamate receptor ion channels: Structure,
regulation and function. Pharmacol Rev. 62:405–496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dzamba D, Honsa P and Anderova M: NMDA
Receptors in glial cells: Pending questions. Curr Neuropharmacol.
11:250–262. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee MC, Ting KK, Adams S, Brew BJ, Chung R
and Guillemin GJ: Characterisation of the expression of NMDA
receptors in human astrocytes. PLoS One. 5:e141232010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verkhratsky A and Kirchhoff F:
Glutamate-mediated neuronal-glial transmission. J Anat.
210:651–660. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Verkhratsky A and Kirchhoff F: NMDA
Receptors in glia. Neuroscientist. 13:28–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Boeck AL, Logemann F, Krauß T, Hussein K,
Bültmann E, Trebst C and Stangel M: Ovarectomy despite negative
imaging in anti-NMDA receptor encephalitis: Effective even late.
Case Rep Neurol Med. 2013:8431922013.PubMed/NCBI
|
|
25
|
Wegner F, Wilke F, Raab P, Tayeb SB, Boeck
AL, Haense C, Trebst C, Voss E, Schrader C, Logemann F, et al:
Anti-leucine rich glioma inactivated 1 protein and
anti-N-methyl-D-aspartate receptor encephalitis show distinct
patterns of brain glucose metabolism in
18F-fluoro-2-deoxy-d-glucose positron emission
tomography. BMC Neurol. 14:1362014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gresa Arribas, Titulaer MJ, Torrents A,
Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R,
Rosenfeld MR, Lynch D, et al: Antibody titres at diagnosis and
during follow-up of anti-NMDA receptor encephalitis: A
retrospective study. Lancet Neurol. 13:167–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Irani SR, Bera K, Waters P, Zuliani L,
Maxwell S, Zandi MS, Friese MA, Galea I, Kullmann DM, Beeson D, et
al: N-methyl-D-aspartate antibody encephalitis: Temporal
progression of clinical and paraclinical observations in a
predominantly non-paraneoplastic disorder of both sexes. Brain.
133:1655–1667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Day GS, High SM, Cot B and Tang-Wai DF:
Anti-NMDA-receptor encephalitis: Case report and literature review
of an under-recognized condition. J Gen Intern Med. 26:811–816.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gable MS, Gavali S, Radner A, Tilley DH,
Lee B, Dyner L, Collins A, Dengel A, Dalmau J and Glaser CA:
Anti-NMDA receptor encephalitis: Report of ten cases and comparison
with viral encephalitis. Eur J Clin Microbiol Infect Dis.
28:1421–1429. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Titulaer MJ, Hoftberger R, Iizuka T,
Leypoldt F, McCracken L, Cellucci T, Benson LA, Shu H, Irioka T,
Hirano M, et al: Overlapping demyelinating syndromes and
anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol:.
75:411–428. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Whitacre CC: Sex differences in autoimmune
disease. Nat Immunol. 2:777–780. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Manto M, Dalmau J, Didelot A, Rogemond V
and Honnorat J: Afferent facilitation of corticomotor responses is
increased by IgGs of patients with NMDA-receptor antibodies. J
Neurol. 258:27–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kleinig TJ, Thompson PD, Matar W, Duggins
A, Kimber TE, Morris JG, Kneebone CS and Blumbergs PC: The
distinctive movement disorder of ovarian teratoma-associated
encephalitis. Mov Disord. 23:1256–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hammer C, Zerche M, Schneider A, Begemann
M, Nave KA and Ehrenreich H: Apolipoprotein E4 carrier status plus
circulating anti-NMDAR1 autoantibodies: Association with
schizoaffective disorder. Mol Psychiatry. 19:1054–1056. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dutschmann M, Morschel M, Rybak IA and
Dick TE: Learning to breathe: Control of the inspiratory-expiratory
phase transition shifts from sensory- to central-dominated during
postnatal development in rats. J Physiol. 587:4931–4948. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henson MA, Roberts AC, Pérez-Otaño I and
Philpot BD: Influence of the NR3A subunit on NMDA receptor
functions. Prog Neurobiol. 91:23–37. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Burzomato V, Frugier G, Pérez-Otano I,
Kittler JT and Attwell D: The receptor subunits generating NMDA
receptor mediated currents in oligodendrocytes. J Physiol.
588:3403–3414. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krebs C, Fernandes HB, Sheldon C, Raymond
LA and Baimbridge KG: Functional NMDA receptor subtype 2B is
expressed in astrocytes after ischemia in vivo and anoxia
in vitro. J Neurosci. 23:3364–3372. 2003.PubMed/NCBI
|
|
39
|
Irani SR, Alexander S, Waters P, Kleopa
KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B and Vincent
A: Antibodies to Kv1 potassium channel-complex proteins
leucine-rich, glioma inactivated 1 protein and contactin-associated
protein-2 in limbic encephalitis, Morvan's syndrome and acquired
neuromyotonia. Brain. 133:2734–2748. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Skripuletz T, Schwenkenbecher P, Pars K,
Stoll M, Conzen J, Bolat S, Pul R, Vonberg RP, Sedlacek L, Wurster
U, et al: Importance of follow-up cerebrospinal fluid analysis in
cryptococcal meningoencephalitis. Dis Markers. 2014:1625762014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen B, Wang Y, Geng Y, Huang Y, Guo S and
Mao X: Marked improvement of anti-N-methyl-D-aspartate receptor
encephalitis by large-dose methylprednisolone and plasmapheresis
therapy combined with F-fluorodeoxyglucose positron emission
tomography imaging: A case report. Exp Ther Med. 8:1167–1169.
2014.PubMed/NCBI
|
|
42
|
Dahm L, Ott C, Steiner J, Stepniak B,
Teegen B, Saschenbrecker S, Hammer C, Borowski K, Begemann M, Lemke
S, et al: Seroprevalence of autoantibodies against brain antigens
in health and disease. Ann Neurol. 76:82–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hammer C, Stepniak B, Schneider A, Papiol
S, Tantra M, Begemann M, Sirén AL, Pardo LA, Sperling S, Mohd
Jofrry S, et al: Neuropsychiatric disease relevance of circulating
anti-NMDA receptor autoantibodies depends on blood-brain barrier
integrity. Mol Psychiatry. 19:1143–1149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pruss H, Hültje M, Maier N, Gomez A,
Buchert R, Harms L, Ahnert-Hilger G, Schmitz D, Terborg C, Kopp U,
et al: IgA NMDA receptor antibodies are markers of synaptic
immunity in slow cognitive impairment. Neurology. 78:1743–1753.
2012. View Article : Google Scholar : PubMed/NCBI
|