Introduction
Chronic hand eczema (CHE) is a type of eczema that
persists for >3 months or occurs at least twice a year despite
adequate treatment and treatment adherence (1). CHE is a common dermatological condition
with frequent relapses, occurs possibly as a result of long-term
exposure to various irritants and allergens (2,3). Up to
70% of cases of CHE are severe (4),
and its treatment has proven to be a considerably difficult task
for dermatologists. Topical use of corticosteroids, alone or in
combination with keratolytic agents (such as salicylic acid and
benzoic acid), retinoic acid and emollients, is the first-line
treatment for CHE. Other treatment strategies, including
calcineurin inhibitors and phototherapy, are used in patients with
contraindications for steroids or those dissatisfied with the
effects of conventional treatments. Few studies have evaluated the
effectiveness of calcineurin inhibitors in hand eczema (5,6).
Psoralen plus ultraviolet therapy may be considered as a
second-line option when topical corticosteroids are ineffective.
Although its efficacy is limited, it is considered to be relatively
safe (3,7). Systemic treatments are indicated for
refractory CHE, the majority of which have not been investigated in
randomized clinical trials and are therefore prescribed off-label
(2,3,8). At
present, the only treatments approved are alitretinoin, which may
be considered a second-line option as it has shown good response
rates in clinical trials and observational studies (9–12). In
addition, cyclosporin, azathioprine, methotrexate and mycophenolate
mofetil may all be considered third-line treatments (13,14).
However, a poor response to various treatments is still observed in
certain CHE cases with severe keratinization (14).
The Daivobet ointment (LEO Pharma A/S, Ballerup,
Denmark), a complex product that consists of calcipotriol and
betamethasone, has been proven successful in the treatment of
plaque-type psoriasis during the last decade. However, the effect
of Daivobet on other skin disorders of abnormal keratinization,
such as eczema, remains unclear, since the literature is
limited.
Subsequent to obtaining informed consent from all
the patients, 3 cases of refractory hyperkeratotic eczema of the
hand between February 2012 and July 2013 were successfully treated
at the Department of Dermatology of Beijing Hospital (Beijing,
China). In all cases, CHE was diagnosed based on the following
definition: Eczema lasting for >3 months, or which occurs at
least twice a year despite adequate treatment (1). All patients were initially topically
treated with a number of corticosteroids (such as
chloroflumethasone cream) alone or in combination with keratolytic
agents (such as salicylic acid or benzoic acid) or retinoic acid.
The therapeutic effect in each case was evaluated using
photographic comparison, and each patient was evaluated by the same
physician at every visit.
Case report
Case 1
A 45-year-old male patient with no history of trauma
or exposure to irritants was admitted to the hospital in February
2012 presenting with itchy hyperkeratotic plaques on both hands,
which had developed 2 years prior to admission. Since then, the
lesion had gradually spread, thickened and hardened, and painful
skin fissures had appeared during the winter. The patient had been
topically treated with various corticosteroids and other agents,
but little improvement was observed. Upon physical examination, the
patient was found to have symmetrical hyperkeratotic erythematous
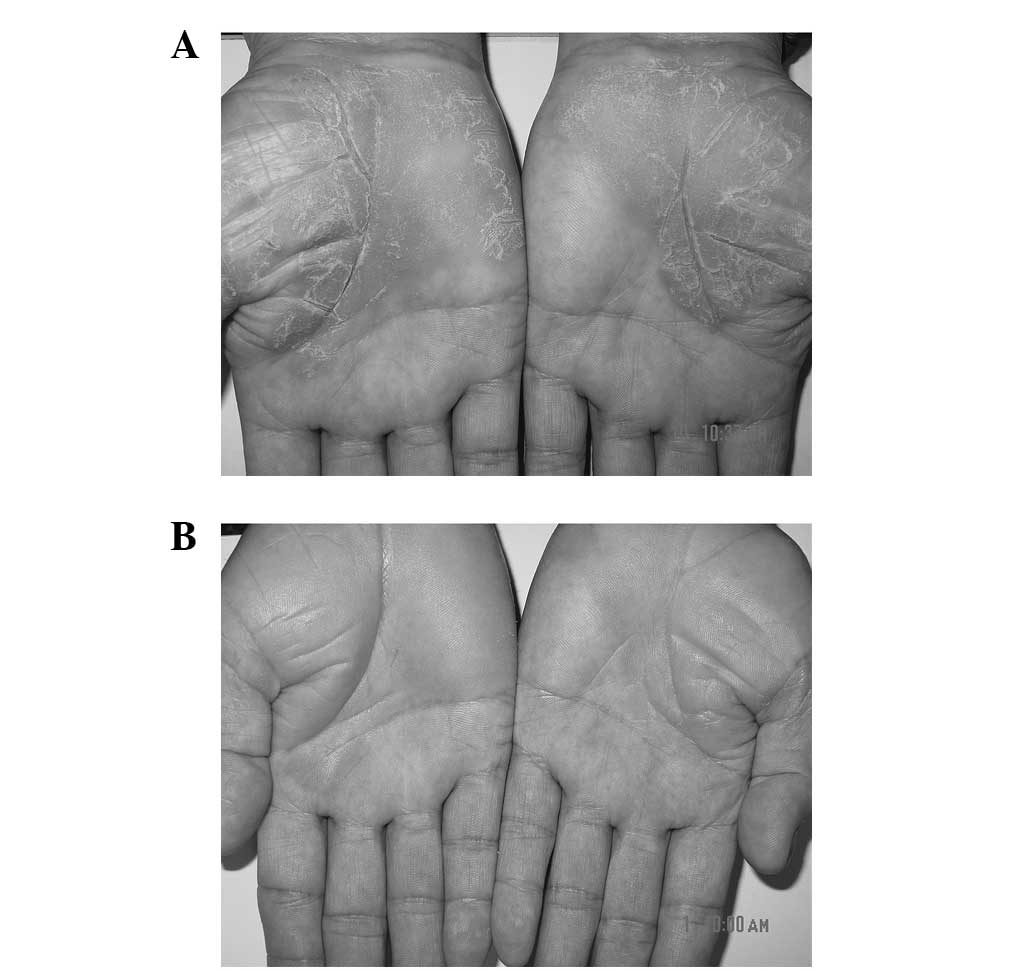
plaques with a well-demarcated margin on the palms. A little
excoriation was noted on the plaques (Fig. 1A). Treatment with 0.25 g Daivobet was
administered twice daily. The itching subsided 2 weeks later and an
almost complete regression (90%) had been achieved by week 12.
Daivobet was then applied once daily for 4 weeks. By week 16, the
skin lesions had completely disappeared (Fig. 1B). No recurrence was detected at a
subsequent 6-month follow-up examination following the end of
treatment.
Case 2
A 44-year-old male patient was admitted in June 2012
presenting with itching hyperkeratotic plaques on the back of his
right hand, which had developed 2 years prior to admission. The
patient had previously undergone various topical therapies with no
signs of improvement. Upon physical examination, hyperkeratotic
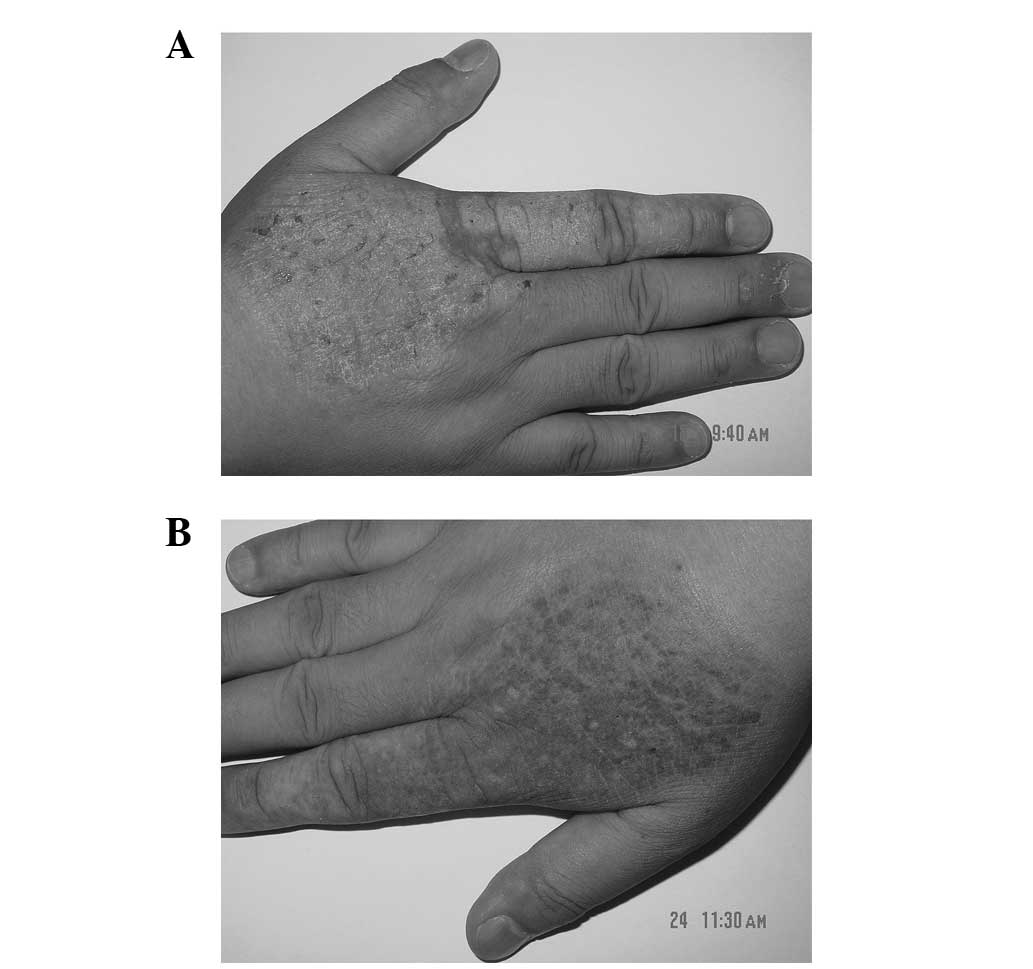
erythematous plaques with a clear margin were observed on the
extensor surface of the right hand of the patient, with some
visible excoriation on the surface of the lesion (Fig. 2A). Daivobet was applied twice daily.
The itching subsided and the lesions became darker and thinner
after 1 week. The application of Daivobet was then reduced to once
daily. After a further 2 weeks, the lesion had completely
disappeared and only post-inflammatory pigmentation was observed
(Fig. 2B). No recurrence was
detected at a subsequent 6-month follow-up examination following
the end of treatment.
Case 3
A 33-year-old male patient was admitted to the
hospital in December 2012 presenting with itchy hyperkeratotic
erythematous plaques on both palms, which had been developing for
2.5 years. Itchy, small erythematous plaques initially presented on
the dorsum of the right hand. Several months later, the lesion had
increased in size and extended to the left hand, and the itching
had exacerbated. Various topical agents were administered, with
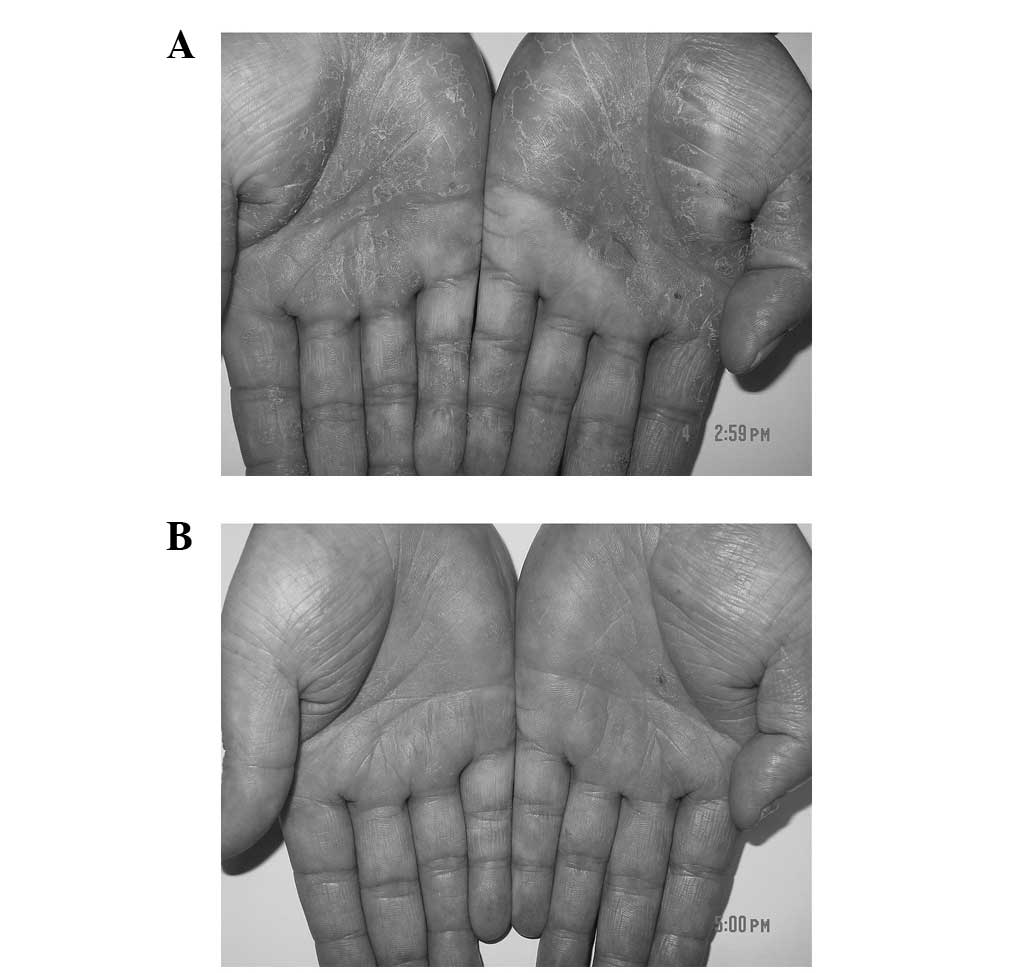
little or no improvement. Upon physical examination, symmetrical
hyperkeratotic erythematous plaques with some scaling and fissuring
were observed on both palms and the flexor surface of the fingers
(Fig. 3A). Daivobet was initially
applied twice daily. The itching subsided after 2 weeks. Fearing
possible adverse reactions, the patient decided to discontinue the
use of Daivobet. There is a possibility that
hypothalamo-pituitary-axis (HPA) suppression may occur if a potent
corticosteroid component is absorbed to a sufficient extent. As
betamethasone dipropionate is potent class 2 corticosteroid, there
is a possibility that it may cause HPA axis suppression if
significant systemic absorption occurs. After 10 weeks, the lesion
and subjective symptoms had deteriorated. Subsequently, the patient
began to reuse Daivobet twice daily and 80% of the lesion was
resolved within 8 weeks. The application was then reduced to once
daily, and ~90% of the lesion was resolved within a further 4 weeks
(Fig. 3B). The 2-month follow-up did
not show a recurrence of the lesion.
None of the 3 patients had a history of systemic
diseases, allergies to corticosteroids and vitamin D derivatives,
psoriasis or other inflammatory disorders, and patch and fungal
test results were negative. No other lesions, with the exception of
those on the hands, were observed during the patients' visits to
the hospital. All topical therapies were discontinued 2 weeks prior
to the administration of Daivobet. No adverse reactions, such as
contact dermatitis or HPA axis suppression, occurred during or
following treatment.
Discussion
CHE is one of the most commonly diagnosed skin
disorders and its treatment poses a challenge to the majority of
dermatologists, due to its uncertain etiology and pathogenesis
(14). Egawa (15) reported the successful treatment of 5
patients with hyperkeratotic palmoplantar eczema with a topical
vitamin D3 derivative (calcipotriol or maxacalcitol). Of
these cases, 4 patients were almost cured after 2–8 weeks of
treatment, and in the fifth patient, the lesion improved markedly
following a 7-week treatment. No adverse reactions occurred during
or following the treatment, and when relapses occurred, the
patients responded well to treatment (15). The successful treatment of these
previous cases prompted us to attempt the application of a
calcipotriol/betamethasone ointment for refractory CHE. All 3
patients in the present study were male, aged 33–45 years, with a
disease course of 2–2.5 years. In 2 of the cases, the lesions were
localized on the palms and in 1 case on the dorsum of the hand. The
treatment duration was 5–24 weeks. In total, 2 patients were
completely cured and 1 patient experienced a remarkable improvement
(90% resolution of the lesion) following treatment. The subjective
symptoms (itching) in all 3 patients disappeared within 1–2
weeks.
The Daivobet ointment used for the treatment of
these cases is a complex product containing the vitamin
D3 derivative calcipotriol (50 µg/g) and the ultrapotent
corticosteroid betamethasone (0.5 mg/g) (16). Vitamin D3 derivatives can
bond with vitamin D receptors on the surface of immune cells, which
contributes to the following biological effects: i) Suppression of
keratinocyte proliferation and regulation of keratinocyte
differentiation; ii) suppression of angiogenesis; iii) regulation
of the release of cytokines, including suppression of the
generation of inflammatory cytokines; and iv) regulation of
inflammatory and immune responses in allergic contact dermatitis by
decreasing the hapten-induced activation of the dendritic cells
(15,16).
The anti-inflammatory and anti-immunity effect of
betamethasone is wide and non-specific; it suppresses inflammatory
reactions at all stages of a disease. Furthermore, it can
effectively suppress various immune responses, including the
reduction of antibody generation, antigen uptake and antigen
presentation through the interaction with macrophages, and
inhibition of lymphocyte proliferation (17).
Thus far, Daivobet has been primarily used
clinically for patients with psoriasis (18,19). Two
large scale randomized controlled trials confirmed that the
Daivobet has numerous advantages compared with calcipotriol,
tacalcitol, betamethasone and placebo, including safety, tolerance,
rapid onset and effectiveness (20,21). All
patients with CHE who were enrolled in the present study exhibited
a severe hyperkeratotic lesion and showed poor responses to
corticosteroids or other agents. Psoriasis and CHE with
hyperkeratinization are considered to share common
histopathological features, such as marked abnormal keratinization,
psoriasis-like epidermal proliferation, angiectasis of the upper
dermis and chronic inflammatory cell infiltration surrounding the
vessel. These common features provide histological evidence that
Daivobet can be regarded as an alternative therapy for chronic
refractory eczema with abnormal keratinization, when other
treatments are inefficient.
In conclusion, in the present study, Daivobet was
used to successfully treat 3 patients with CHE, and thus is a safe
and effective choice for the treatment of refractory CHE. However,
the present study had a limited sample of cases. Therefore,
large-sample randomized controlled trials supporting these results
are required.
References
|
1
|
Menné T, Johansen JD, Sommerlund M and
Veien NK: Danish Contact Dermatitis Group: Hand eczema guidelines
based on the Danish guidelines for the diagnosis and treatment of
hand eczema. Contact Dermatitis. 65:3–12. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Diepgen TL, Elsner P, Schliemann S,
Fartasch M, Köllner A, Skudlik C, John SM and Worm M: Deutsche
Dermatologische Gesellschaft: Guideline on the management of hand
eczema ICD-10 Code: L20 L23. L24. L25. L30. J Dtsch Dermatol Ges.
7(Suppl 3): S1–16. 2009.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
English J, Aldridge R, Gawkrodger DJ,
Kownacki S, Statham B, White JM and Williams J: Consensus statement
on the management of chronic hand eczema. Clin Exp Dermatol.
34:761–769. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Apfelbacher C, Molin S, Weisshaar E, Bauer
A, Elsner P, Mahler V, Weiss M, Ruzicka T and Diepgen TL:
Characteristics and provision of care in patients with chronic hand
eczema: Updated data from the CARPE registry. Acta Derm Venereol.
94:163–167. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Krejci-Manwaring J, McCarty MA, Camacho F,
Manuel J, Hartle J, Fleischer A Jr and Feldman SR: Topical
tacrolimus 0.1% improves symptoms of hand dermatitis in patients
treated with a prednisone taper. J Drugs Dermatol. 7:643–646.
2008.PubMed/NCBI
|
|
6
|
Bauer A, Lange N, Matterne U, Meurer M,
Braeutigam M and Diepgen TL: Efficacy of pimecrolimus 1% cream in
the long term management of atopic hand dermatitis. A double-blind
RCT. J Dtsch Dermatol Ges. 10:426–433. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gritiyarangsan P, Sukhum A, Tresukosol P
and Kullavanijaya P: Topical PUVA therapy for chronic hand eczema.
J Dermatol. 25:299–301. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lynde C, Guenther L, Diepgen TL,
Sasseville D, Poulin Y, Gulliver W, Agner T, Barber K, Bissonnette
R, Ho V, et al: Canadian hand dermatitis management guidelines. J
Cutan Med Surg. 14:267–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schindler M, Drozdenko G, Kühl AA and Worm
M: Immunomodulation in patients with chronic hand eczema treated
with oral alitretinoin. Int Arch Allergy Immunol. 165:18–26. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ham K, Maini P and Gooderham MJ:
Real-world experience with alitretinoin in a community dermatology
practice setting in patients with chronic hand dermatitis. J Cutan
Med Surg. 18:332–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gola M, Milanesi N and Derme AM: Clinical
evaluation and assessment of the therapeutic efficacy of
alitretinoin in a group of patients with chronic hand eczema
refractory to topical steroid therapy. G Ital Dermatol Venereol.
149:435–439. 2014.PubMed/NCBI
|
|
12
|
Schmith GD, Singh R, Gomeni R, Graff O,
Hamedani AG, Troughton JS and Learned SM: Use of longitudinal
dose-response modeling to support the efficacy and tolerability of
alitretinoin in severe refractory chronic hand eczema (CHE). CPT
Pharmacometrics Syst Pharmacol. 4:255–262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mrowietz U, Klein CE, Reich K, Rosenbach
T, Ruzicka T, Sebastian M and Werfel T: Cyclosporine therapy in
dermatology. J Dtsch Dermatol Ges. 7:474–479. 2009.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
de León FJ, Berbegal L and Silvestre JF:
Management of chronic hand eczema. Actas Dermosifiliogr. May
21–2015.(Epub ahead of print) (In Spanish).
|
|
15
|
Egawa K: Topical vitamin D3 derivatives in
treating hyperkeratotic palmoplantar eczema: A report of five
patients. J Dermatol. 32:381–386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Simonsen LI, Høy G, Didriksen E, Persson
J, Melchior N and Hansen J: Development of a new formulation
combining calcipotriol and betamethasone dipropionate in an
ointment vehicle. Drug Dev Ind Pharm. 30:1095–1102. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gola M, D'Erme AM and Milanesi N: Clinical
efficacy of two topical corticosteroids in the management of
chronic hand eczema. G Ital Dermatol Venereol. 150:293–296.
2015.PubMed/NCBI
|
|
18
|
Vakirlis E, Kastanis A and Ioannides D:
Calcipotriol/betamethasone dipropionate in the treatment of
psoriasis vulgaris. Ther Clin Risk Manag. 4:141–148.
2008.PubMed/NCBI
|
|
19
|
McCormack PL: Calcipotriol/betamethasone
dipropionate: A review of its use in the treatment of psoriasis
vulgaris of the trunk, limbs and scalp. Drugs. 71:709–730. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McCormack PL: Spotlight on
calcipotriene/betamethasone dipropionate in psoriasis vulgaris of
the trunk, limbs and scalp. Am J Clin Dermatol. 12:421–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kragballe K, Austad J, Barnes L, Bibby A,
de la Brassinne M, Cambazard F, Fleming C, Heikkilä H, Williams Z,
Rey J Peyri, et al: Efficacy results of a 52-week, randomised,
double-blind, safety study of a calcipotriol/betamethasone
dipropionate two-compound product (Daivobet/Dovobet/Taclonex) in
the treatment of psoriasis vulgaris. Dermatology. 213:319–326.
2006. View Article : Google Scholar : PubMed/NCBI
|