Introduction
Non-alcoholic fatty liver disease (NAFLD) is a
clinical syndrome characterized by the accumulation of excess fat
in the liver of individuals that drink little or no alcohol. The
prevalence of NAFLD is 15–30% in Western populations (1–4) and
6–25% in Asian populations (5).
NAFLD is associated with metabolic syndrome, obesity, diabetes and
cardiovascular disease (6).
Currently, no standard for the treatment of NAFLD exists, and
treating risk factors remains the focus of managing NAFLD (7).
Liraglutide is a potent, long-acting synthetic
analogue of the human glucagon-like peptide 1 (GLP-1) molecule. It
shares 97% sequence identity with human GLP-1. Liraglutide has
previously been used for the treatment of diabetes (8); however, a recent study reported that
liraglutide could improve oxidative stress and lipid peroxidation
and decrease transforming growth factor-β1 and tumor necrosis
factor-α expression in rats with NAFLD (9). In a study by Olaywi et al
(10), it was revealed that
liraglutide induced improvements in transaminases, as well as
histology, in patients with non-alcoholic steatohepatitis. These
studies have suggested the potential function of liraglutide in
improving NAFLD.
The AMP-activated protein kinase (AMPK) signaling
pathway plays a key role in regulating hepatic lipid metabolism. It
has been revealed that AMPK coordinates the long-term adaptation of
lipid metabolism by downregulating the transcriptional factor
sterol regulatory element binding protein 1 (SREBP1) (11,12).
To the best of our knowledge, studies on the
molecular mechanisms underlying the liraglutide-induced improvement
in NAFLD are limited (13). Whether
liraglutide reduces fatty degeneration in hepatic cells via the
AMPK/SREBP1 pathway remains unclear; therefore, a well-described
model of in vitro NAFLD was established in the present study
using a normal human hepatocyte-derived cell line, with the aim of
investigating the role of the AMPK/SREBP1 pathway in mediating the
liraglutide-induced reduction in fatty degeneration.
Materials and methods
Cell culture
The L-02 human normal liver cell line was purchased
from the American Type Culture Collection (Manassas, VA, USA). The
cells were cultured in Dulbecco's modified Eagle's medium
(Gibco-BRL, Grand Island, NY, USA) supplemented with 10% fetal
bovine serum (Gibco-BRL) at 37°C with 5% CO2. Oleic acid
(OA) and palmitic acid (PA) were purchased from Sigma-Aldrich (St.
Louis, MO, USA) and added to the culture medium at the
concentration of 0.5 mM in a 2:1 molar ratio. Liraglutide was
provided by Novo Nordisk (Copenhagen, Denmark) and diluted to
concentrations of 0.1, 0.5, 1.0 and 2.0 nM. Compound C was obtained
from Calbiochem (San Diego, CA, USA) and used at a concentration of
20 µM. Cells were incubated with free fatty acids (FFAs),
liraglutide or compound C alone or in combination.
Oil red O staining
The cells were fixed with 10% formaldehyde for 10
min and then washed with phosphate-buffered saline (PBS). Oil red O
solution (60% oil red O dye and 40% water) (Sigma-Aldrich) was
filtered and added to stain the cells at room temperature for 15
min. PBS was then used to remove the unbound dye, and the cells
were observed under the microscope (TE200; Nikon Corp., Tokyo,
Japan).
Measurement of triglyceride (TG) and
total cholesterol (TC)
The TG quantification and cholesterol assay kits
were purchased from Abcam (Cambridge, MA, USA). The levels of TG
and TC were determined according to the manufacturer's
instructions. Briefly, a standard curve was prepared, and the cells
were lysed. For the measurement of TG, 2 µl lipase was added to
each well and incubated at room temperature for 20 min. A total of
50 µl reaction mix, including 46 µl TG assay buffer, 2 µl TG probe
and 2 µl TG enzyme mix, was then added and incubated at room
temperature for 30–60 min. For the measurement of TC, 50 µl
reaction mix, containing 44 µl cholesterol assay buffer, 2 µl
cholesterol probe, 2 µl enzyme mix and 2 µl cholesterol esterase,
was added to each well and incubated at 37°C for 60 min. The
optical density at 570 nm was measured using a microtiter plate
reader (ELx800NB; BioTek Instruments, Inc., Winooski, VT, USA).
Western blot analysis
The cells were lysed in radioimmunoprecipitation
assay buffer (Sangon Biotech, Shanghai, China), and protein
concentrations were quantified via the bicinchoninic acid (BCA)
assay method using a BCA Protein Assay kit (Beyotime Institute of
Biotechnology, Shanghai, China). Proteins were separated using 12%
sodium dodecyl sulfate-polyacrylamide gel and transferred onto
polyvinylidene difluoride membranes (Millipore, Billerica, MA,
USA). The membranes were incubated in Tris-buffered saline
containing 5% non-fat milk at 37°C for 2 h. The membranes were then
incubated at 37°C for 1 h with the following primary antibodies:
Rabbit monoclonal to AMPKα1 (1:1000; ab32047), rabbit polyclonal to
AMPKα1 (phospho S487) (1:1,000; ab131357), rabbit monoclonal to
AMPKα1 (phospho S496) (1:1,000; ab92701), rabbit polyclonal to
SREBP1 (1:500; ab193318), rabbit polyclonal to SREBP1 (phospho
S372) (1:500; ab140483), rabbit polyclonal to SREBP1 (phospho S439)
(1:500; ab138663), rabbit monoclonal to liver-type fatty
acid-binding protein (L-FABP) (1:1,000; ab129203) (all Abcam) and
mouse monoclonal to β-actin (1:1,000; sc-47778; Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA). After washing with PBS,
the membranes were incubated with the secondary antibodies [goat
anti-rabbit IgG/horseradish peroxidase (HRP) and goat anti-mouse
IgG/HRP; Santa Cruz Biotechnology, Inc.] at 37°C for 1 h. The
signals were detected using enhanced chemiluminescence (ECL) (ECL
western blotting kit; Pierce, Rockford, IL, USA).
Statistical analysis
Data were analyzed using SPSS software, version 19.0
(IBM SPSS, Armonk, NY, USA). Data are expressed as the mean ±
standard deviation. The differences between two groups were
analyzed using the Student's t-test. P<0.05 was considered to
indicate a statistically significant difference.
Results
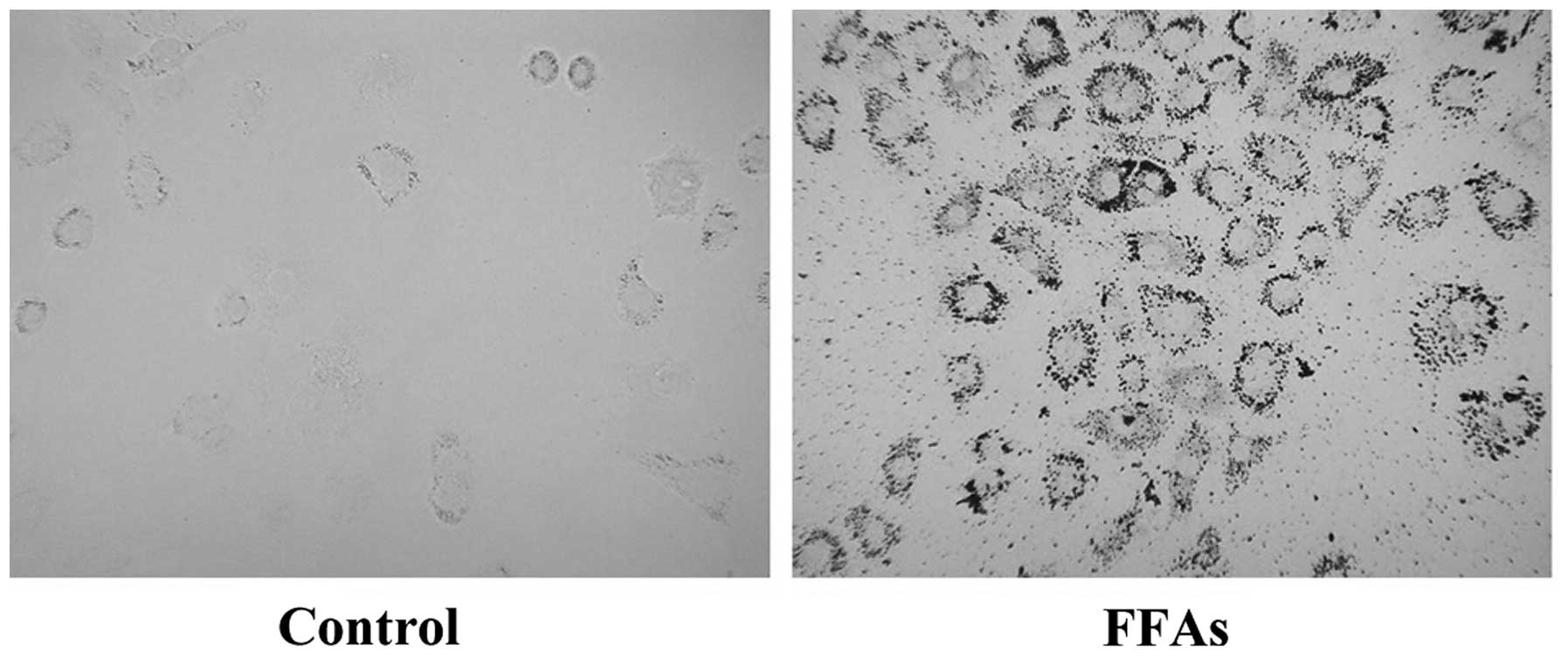
FFA treatment induces lipid
accumulation in L-02 cells
L-02 cells were treated with 0.5 mM FFAs for 24 h,
and oil red O staining was then performed. As shown in Fig. 1, no lipid droplets were detected in
the L-02 cells in the control group; however, a large quantity of
red lipid droplets was observed in the L-02 cells in the FFA group.
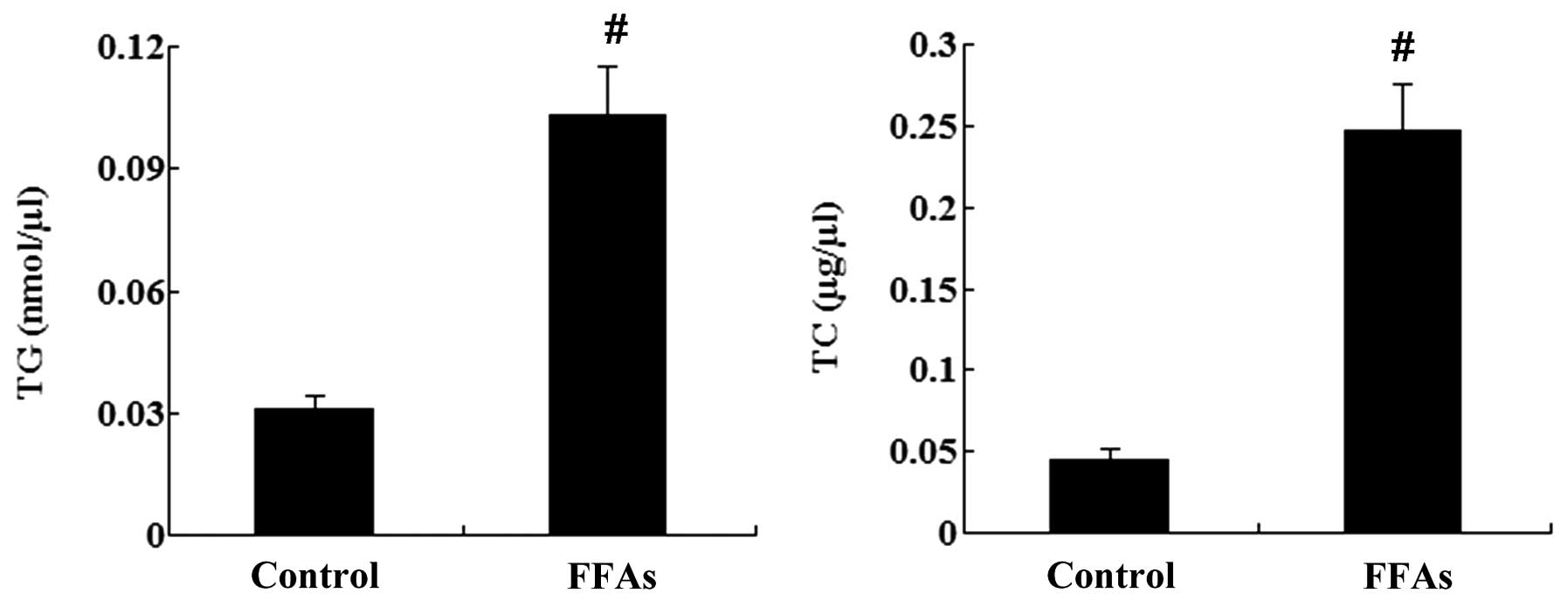
In addition, the intracellular levels of TG and TC were
significantly increased by treatment with FFAs (P<0.01; Fig. 2).
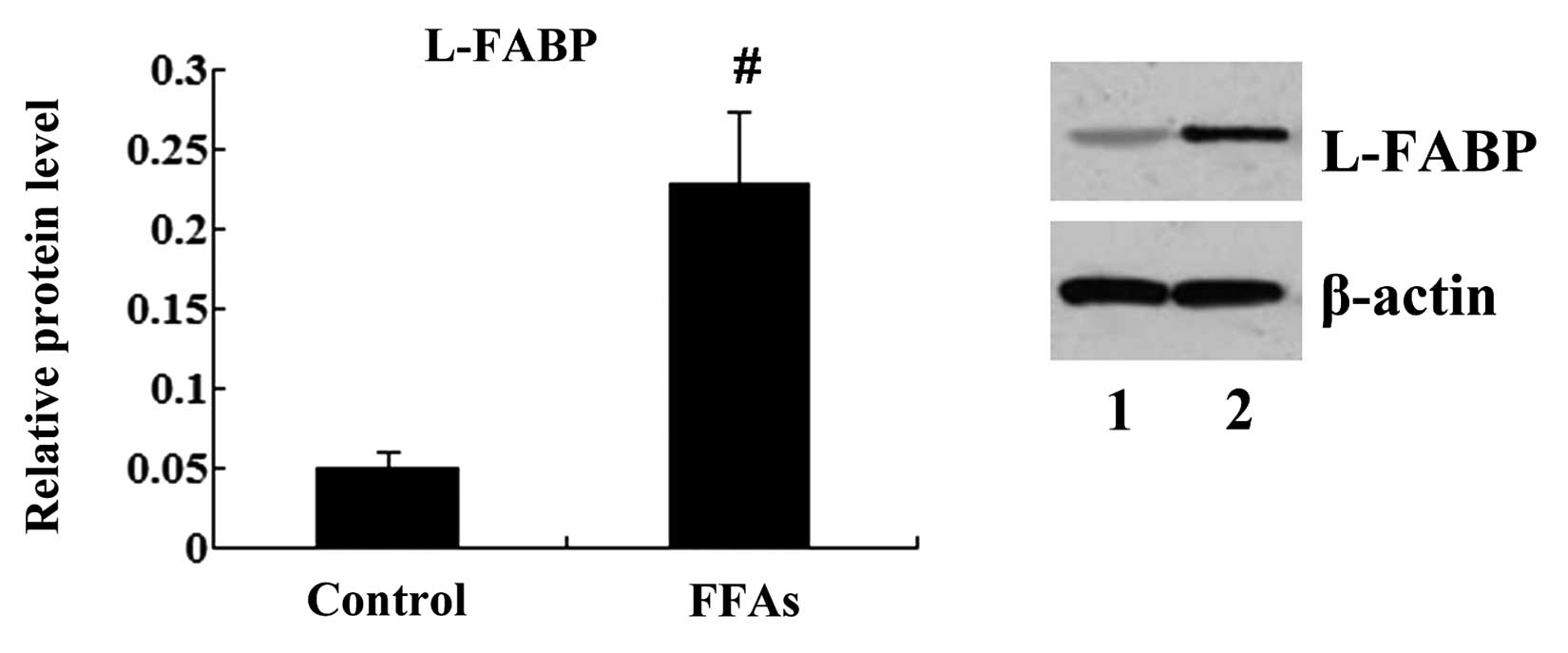
FFA treatment induces L-FABP
expression in L-02 cells
L-02 cells were treated with 0.5 mM FFAs for 24 h,
and the expression of L-FABP in the L-02 cells was then analyzed
using western blot analysis. It was found that the L-FABP
expression was significantly different between the control and the
FFA group. The protein expression of L-FABP was significantly
upregulated following FFA treatment (P<0.01; Fig. 3).
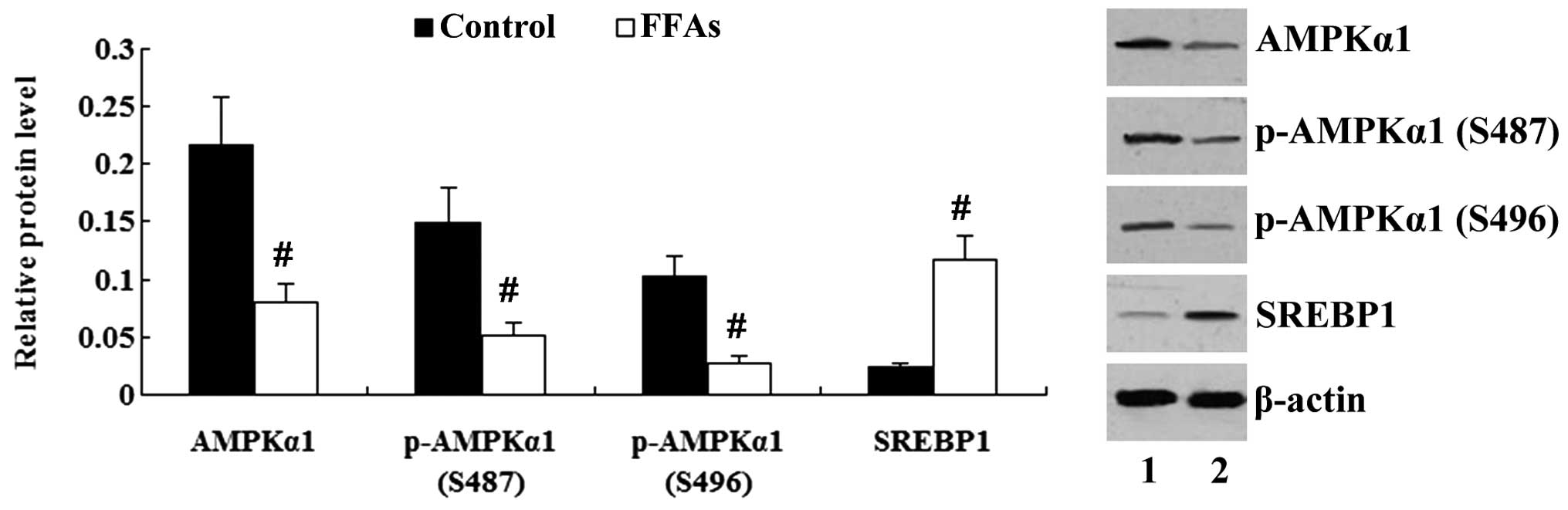
FFA treatment affects the AMPK/SREBP1
pathway in L-02 cells
As shown in Fig. 4,
incubation of the L-02 cells with FFAs for 24 h resulted in the
decreased expression of AMPKα1 and a reduction in the
phosphorylation of AMPKα1 (P<0.01). The expression of SREBP1 was
increased significantly by treatment with FFAs (P<0.01).
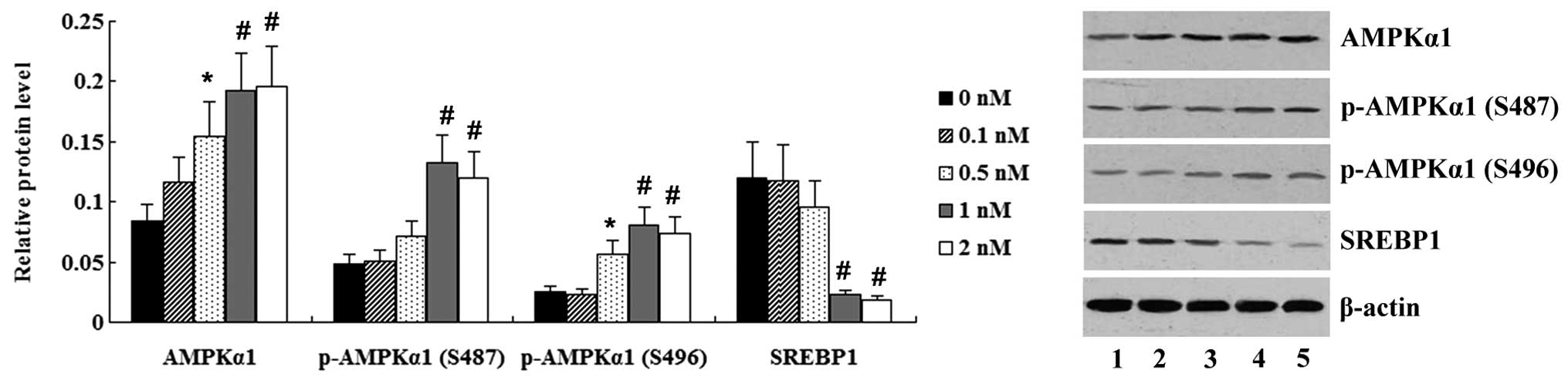
Liraglutide activates the AMPK/SREBP1
pathway in FFA-exposed L-02 cells
To determine the effect of liraglutide on the
AMPK/SREBP1 pathway, FFA-exposed L-02 cells were incubated with
liraglutide at different concentrations (0.1, 0.5, 1.0 and 2.0 nM).
The expression of AMPKα1 and SREBP1 was determined using western
blot analysis. As shown in Fig. 5,
the expression of AMPKα1 and phosphorylated (p-)AMPKα1 (S496) was
significantly increased in the FFA-exposed L-02 cells following
treatment with liraglutide (0.5–2.0 nM) (P<0.05). Liraglutide at
the concentrations of 1.0 and 2.0 nM significantly upregulated
p-AMPKα1 (S487) expression (P<0.01); however, the expression of
SREBP1 in FFA-exposed L-02 cells was significantly decreased by
treatment with 1.0 and 2.0 nM liraglutide (P<0.01) (Fig. 5).
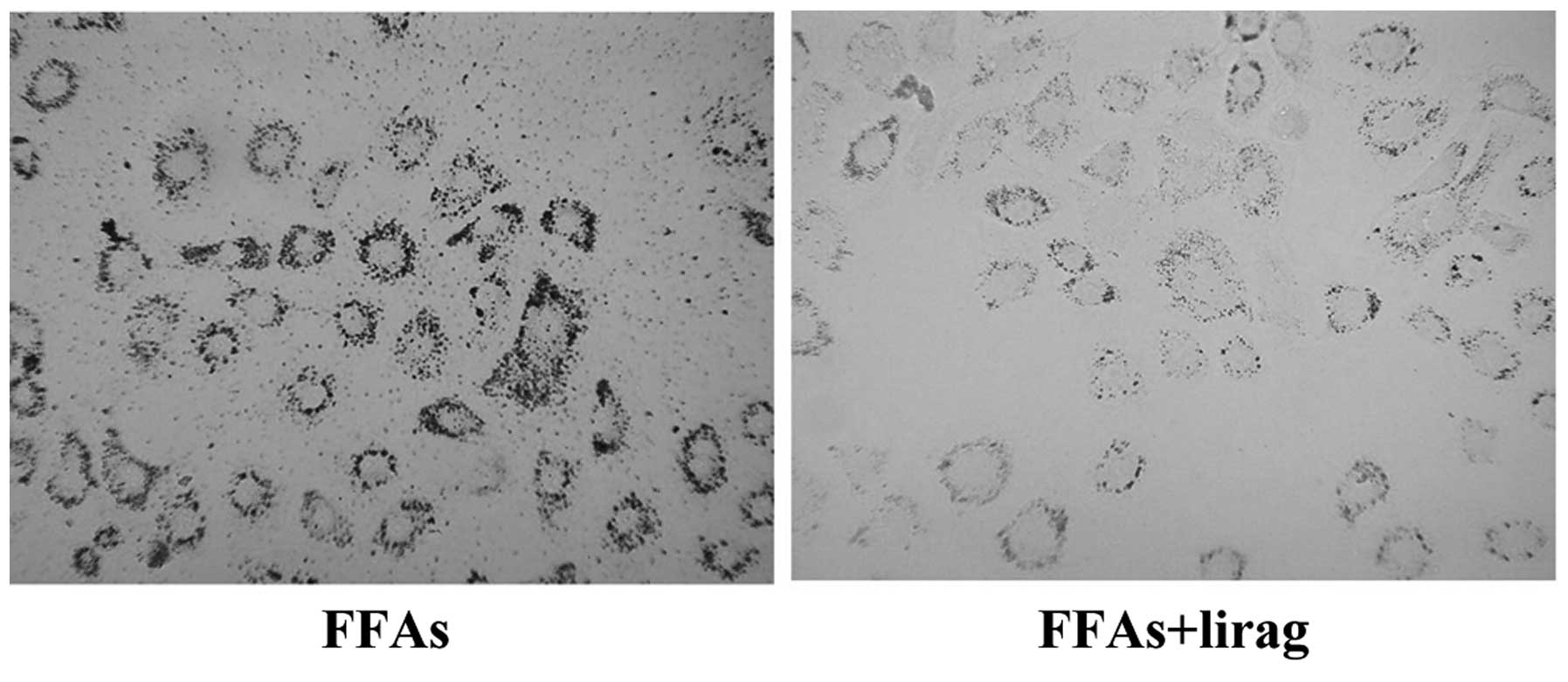
Liraglutide reduces lipid accumulation
and L-FABP expression in FFA-exposed L-02 cells
Next, the effect of liraglutide on lipid
accumulation and L-FABP expression in FFA-exposed L-02 cells was
investigated. FFA-exposed L-02 cells were incubated with
liraglutide (1.0 nM), and oil red O staining was then performed. As
shown in Fig. 6, liraglutide
decreased the number of lipid droplets in the FFA-exposed L-02
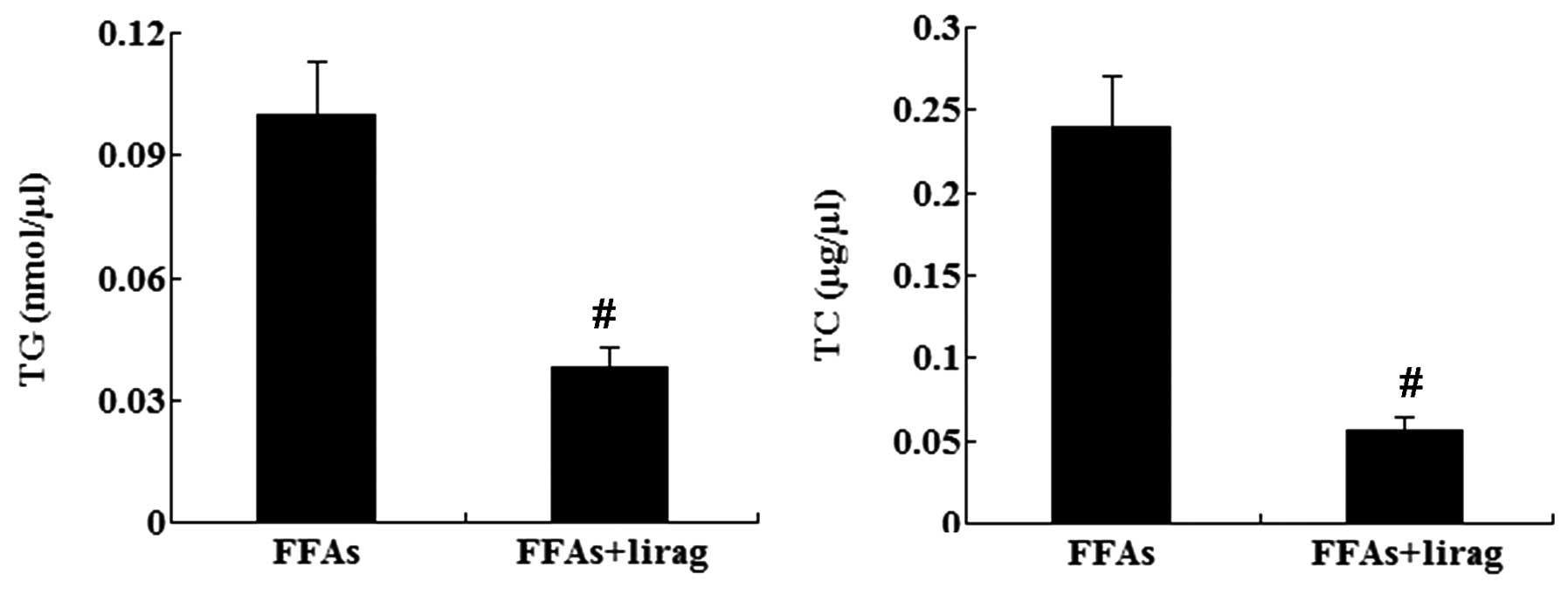
cells. The intracellular levels of TG and TC were also decreased
significantly in the FFA-exposed L-02 cells following treatment
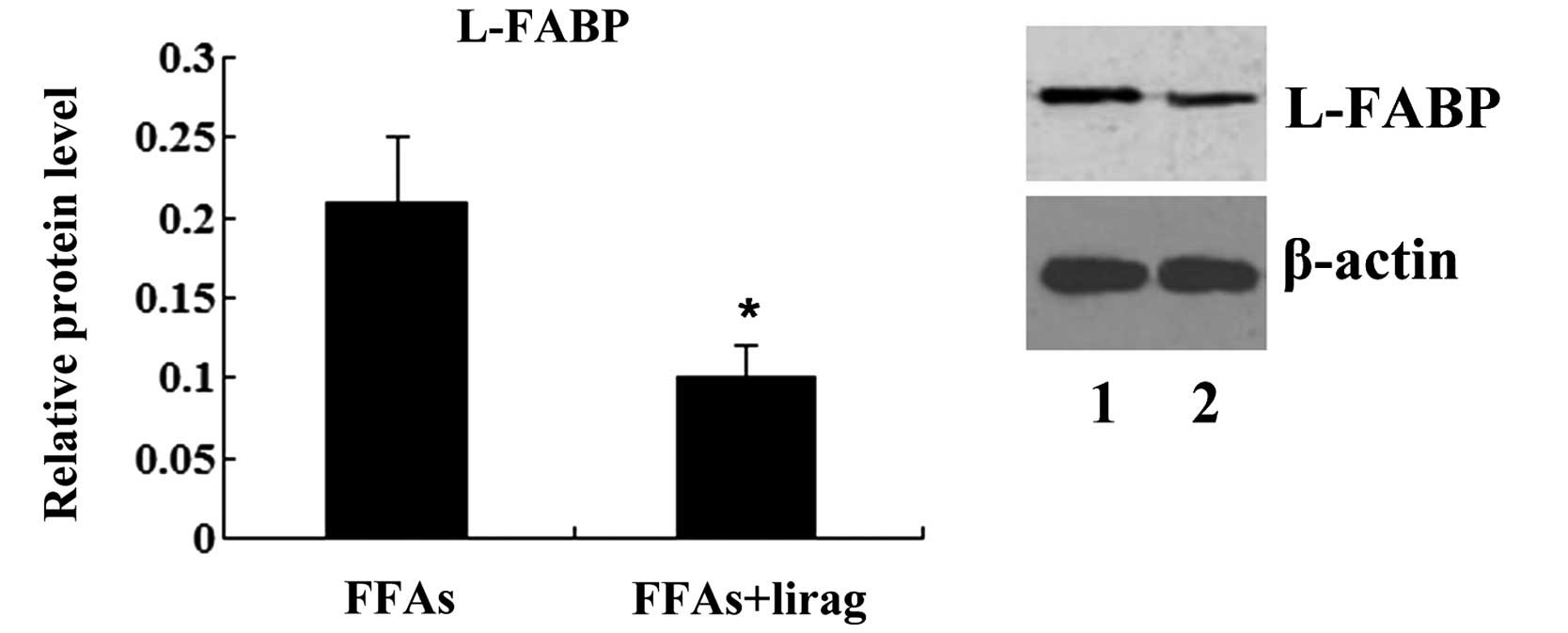
with liraglutide (1.0 nM) (P<0.01; Fig. 7). Western blotting results
demonstrated that the incubation of FFA-exposed L-02 cells with
liraglutide resulted in a decreased expression of L-FABP protein
(P<0.05; Fig. 8).
AMPK/SREBP1 pathway is involved in the
effect of liraglutide on lipid accumulation and L-FABP expression
in FFA-exposed L-02 cells
To investigate whether the AMPK/SREBP1 pathway was
involved in the effect of liraglutide on lipid accumulation and
L-FABP expression in the FFA-exposed L-02 cells, compound C, an
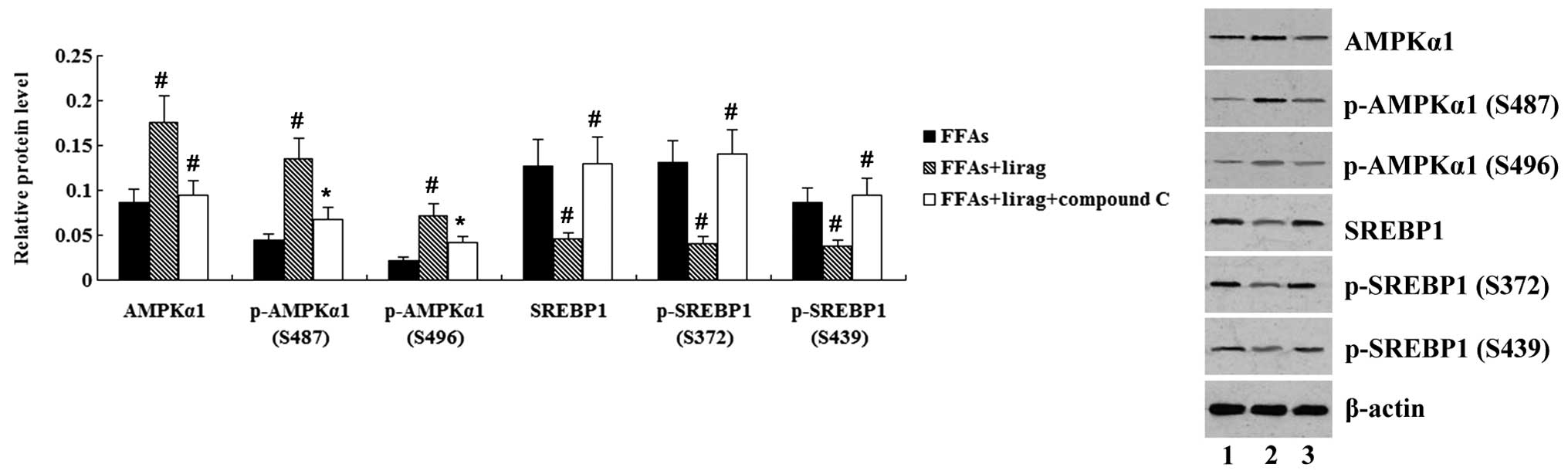
AMPK inhibitor was added to treat the cells. As indicated in
Fig. 9, AMPKα1 and p-AMPKα1 protein
levels were significantly decreased (P<0.05), while SREBP1 and
p-SREBP1 protein levels were significantly increased (P<0.01),
in the FFA-exposed L-02 cells in the liraglutide + compound C group
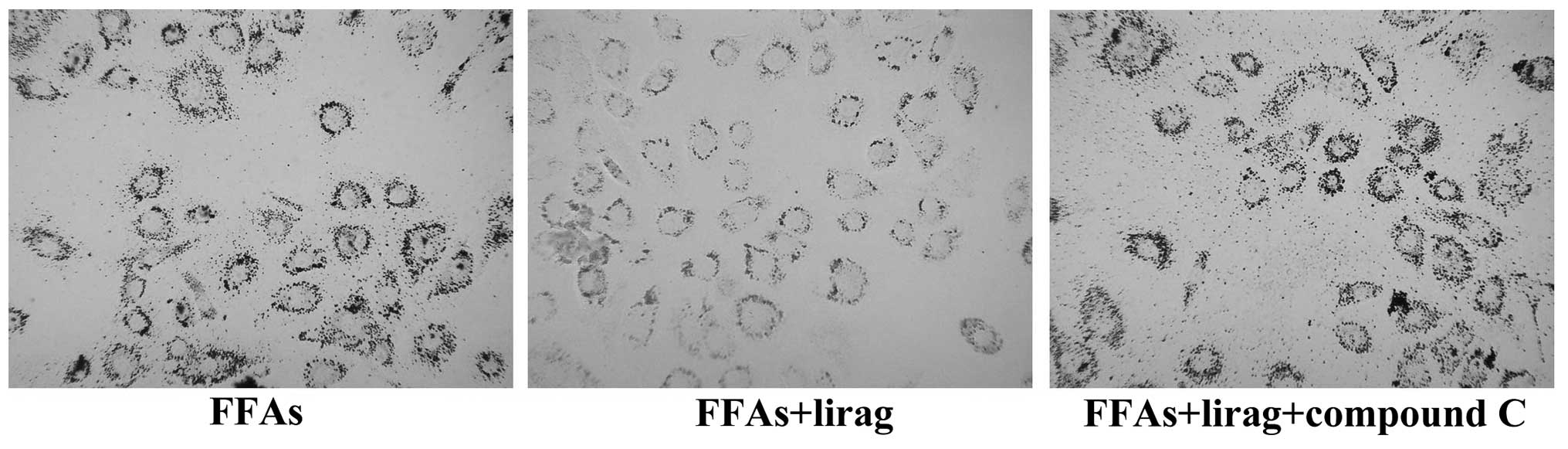
compared with those in the liraglutide group. Oil red O staining
showed that liraglutide reduced the number of lipid droplets in the
FFA-exposed L-02 cells; however, this effect was attenuated by
treatment with 20 µM compound C (Fig.
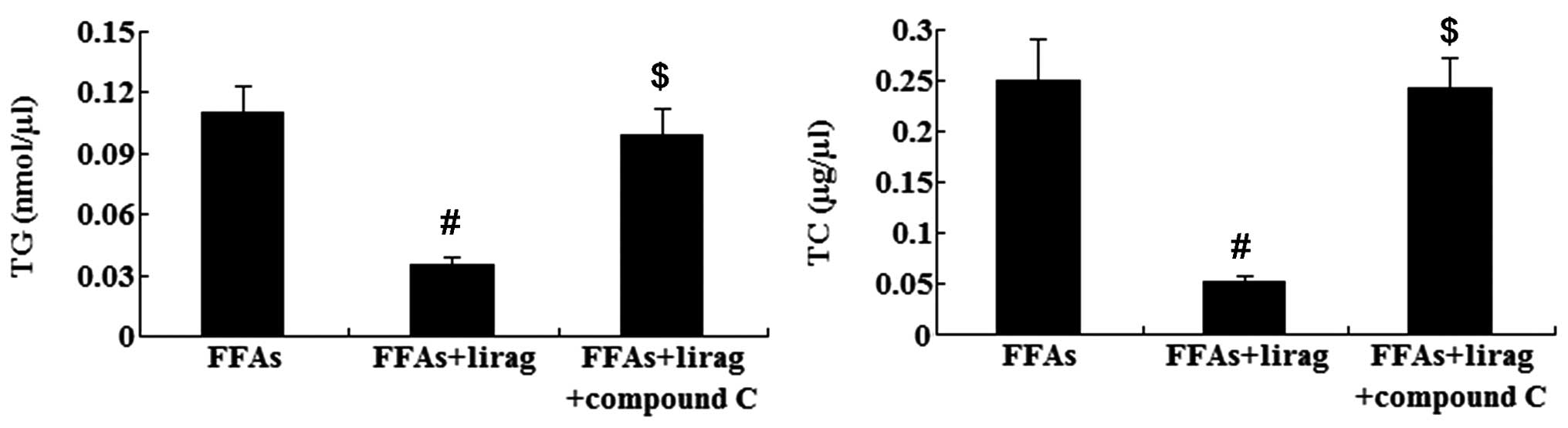
10). Furthermore, it was found that, compared with the
liraglutide group, treatment with 20 µM compound C induced elevated
levels of TG and TC in the FFA-exposed L-02 cells (P<0.01)
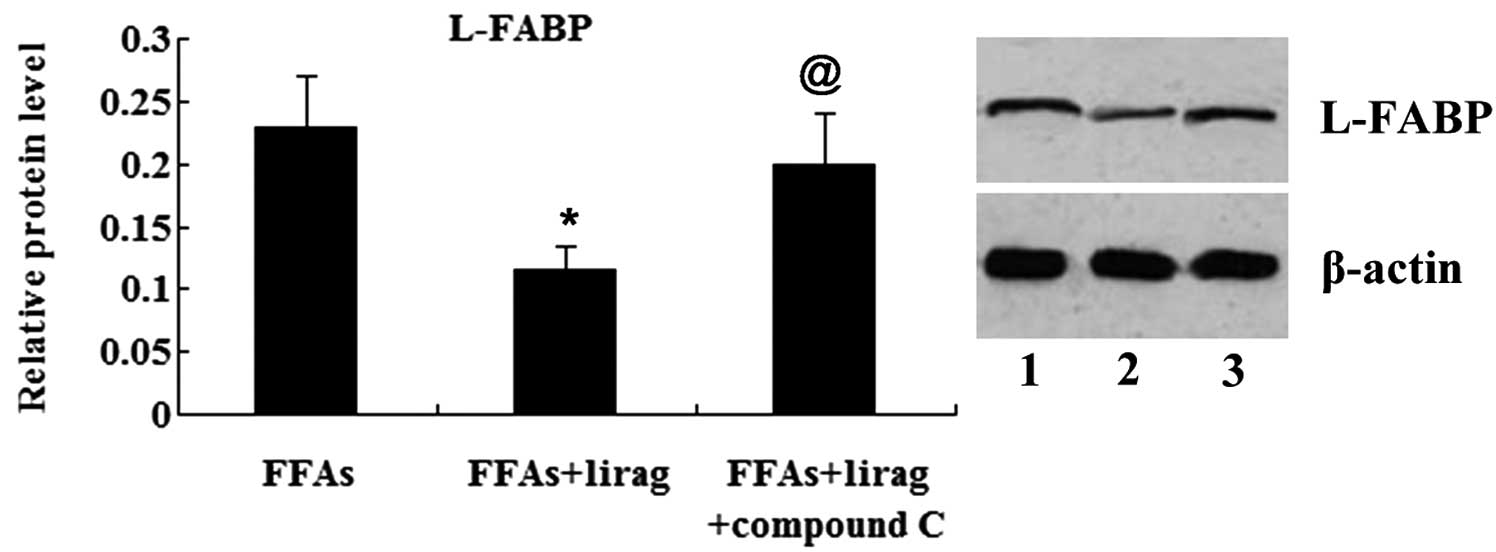
(Fig. 11). The results from the
western blot analysis revealed that the expression of L-FABP was
suppressed by liraglutide; however, this effect was abolished by
the treatment of the FFA-exposed L-02 cells with compound C
(Fig. 12).
Discussion
To simulate the NAFLD condition in vitro,
L-02 normal human hepatocyte-derived cells were treated with 0.5 mM
FFAs (PA and OA in a 1:2 molar ratio) for 24 h. The effect of
liraglutide on the development of NAFLD was then examined in
vitro.
The results from liraglutide clinical trials have
demonstrated the effect of liraglutide on reductions in TG,
low-density lipoprotein cholesterol and FFAs (14–19).
Consistent with the previous in vivo studies, oil red O
staining in the present study demonstrated that liraglutide
attenuated the significant increase in intracellular lipid
accumulation. In addition, a significant decrease in the content of
TG and TC was observed when the FFA-exposed L-02 cells were
incubated with liraglutide, as indicated.
L-FABP plays a key role in the fatty acid metabolism
of the liver. It has recently been reported that L-FABP constitutes
a novel diagnostic marker for detecting NAFLD, and the serum and
hepatic expression levels of L-FABP were significantly upregulated
in NAFLD patients compared with those in control subjects (20,21). In
the present study, it was found that the increased expression of
L-FABP in FFA-exposed L-02 cells could be suppressed by
liraglutide. These findings together demonstrate that liraglutide
has a potential function in improving NAFLD in vitro.
The AMPK/SREBP1 pathway plays an important role in
the development of orotic acid-induced fatty liver (22). AMPK is a heterotrimeric enzyme
complex that is involved in a variety of biological activities that
normalize glucose, lipid and energy imbalances. SREBP1 is a
transcription factor responsible for fatty acid synthesis (23). It has been demonstrated that AMPK
phosphorylation can inhibit the expression of SREBP1 (12,24,25),
whereas the inhibition of AMPK augments the expression of SREBP1,
thus leading to the activation of lipogenesis (26). In the present study, FFA-exposed L-02
cells were treated with various concentrations of liraglutide, and
the results from the western blot analysis revealed that
liraglutide affected the AMPK/SREBP1 pathway in a dose-dependent
manner. The aim of the next part of the study was to examine
whether the AMPK/SREBP1 pathway mediated the liraglutide-induced
reduction in fatty degeneration.
The AMPK-mediated mechanism could be suppressed by
compound C, an inhibitor of AMPK (27,28). In
the present study, it was found that compound C blocked the
inhibitory effect of liraglutide on the increases in intracellular
lipid accumulation, TG and TC content, as well as the elevated
expression of L-FABP induced by FFAs. These results indicate that
the effect of liraglutide on reducing fatty degeneration was
mediated by the AMPK/SREBP1 pathway.
In conclusion, the present study has demonstrated
that liraglutide can reduce the fatty degeneration induced by FFAs
in hepatocytes. This effect may be partially mediated by the
AMPK/SREBP1 pathway.
References
|
1
|
Clark JM and Diehl AM: Hepatic steatosis
and type 2 diabetes mellitus. Curr Diab Rep. 2:210–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bedogni G, Miglioli L, Masutti F,
Tiribelli C, Marchesini G and Bellentani S: Prevalence of and risk
factors for nonalcoholic fatty liver disease: The Dionysos
nutrition and liver study. Hepatology. 42:44–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Browning JD, Szczepaniak LS, Dobbins R,
Nuremberg P, Horton JD, Cohen JC, Grundy SM and Hobbs HH:
Prevalence of hepatic steatosis in an urban population in the
United States: Impact of ethnicity. Hepatology. 40:1387–1395. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: The epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan JG: An introduction of strategies for
the management of nonalcoholic fatty liver disease (NAFLD)
recommended by Asia Pacific Working Party on NAFLD. Zhonghua Gan
Zang Bing Za Zhi. 15:552–553. 2007.(In Chinese). PubMed/NCBI
|
|
6
|
Petta S, Muratore C and Craxì A:
Non-alcoholic fatty liver disease pathogenesis: The present and the
future. Dig Liver Dis. 41:615–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schwenger KJ and Allard JP: Clinical
approaches to non-alcoholic fatty liver disease. World J
Gastroenterol. 20:1712–1723. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Knudsen LB, Nielsen PF, Huusfeldt PO,
Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M and
Agersø H: Potent derivatives of glucagon-like peptide-1 with
pharmacokinetic properties suitable for once daily administration.
J Med Chem. 43:1664–1669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao H, Xu L, Li D, Guang L and Deng W:
Effects of glucagon-like peptide-1 on liver oxidative stress, TNF-α
and TGF-β1 in rats with non-alcoholic fatty liver disease. Nan Fang
Yi Ke Da Xue Xue Bao. 33:1661–1664. 2013.(In Chinese). PubMed/NCBI
|
|
10
|
Olaywi M, Bhatia T, Anand S and Singhal S:
Novel anti-diabetic agents in non-alcoholic fatty liver disease: A
mini-review. Hepatobiliary Pancreat Dis Int. 12:584–588. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Morrison A, Yan X, Tong C and Li J: Acute
rosiglitazone treatment is cardioprotective against
ischemia-reperfusion injury by modulating AMPK, Akt, and JNK
signaling in nondiabetic mice. Am J Physiol Heart Circ Physiol.
301:H895–H902. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhou G, Myers R, Li Y, Chen Y, Shen X,
Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al: Role of
AMP-activated protein kinase in mechanism of metformin action. J
Clin Invest. 108:1167–1174. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Yang M, Ren H, Hu H, Boden G, Li
L and Yang G: GLP-1 analogue prevents NAFLD in ApoE KO mice with
diet and Acrp30 knockdown by inhibiting c-JNK. Liver Int.
33:794–804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buse JB, Rosenstock J, Sesti G, Schmidt
WE, Montanya E, Brett JH, Zychma M and Blonde L: LEAD-6 Study
Group: Liraglutide once a day versus exenatide twice a day for type
2 diabetes: A 26-week randomised, parallel-group, multinational,
open-label trial (LEAD-6). Lancet. 374:39–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marre M, Shaw J, Brändle M, Bebakar WM,
Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD and Colagiuri S:
LEAD-1 SU Study Group: Liraglutide, a once-daily human GLP-1
analogue, added to a sulphonylurea over 26 weeks produces greater
improvements in glycaemic and weight control compared with adding
rosiglitazone or placebo in subjects with Type 2 diabetes (LEAD-1
SU). Diabet Med. 26:268–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nauck M, Frid A, Hermansen K, Shah NS,
Tankova T, Mitha IH, Zdravkovic M, Düring M and Matthews DR: LEAD-2
Study Group: Efficacy and safety comparison of liraglutide,
glimepiride, and placebo, all in combination with metformin, in
type 2 diabetes: The LEAD (liraglutide effect and action in
diabetes)-2 study. Diabetes Care. 32:84–90. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Garber A, Henry R, Ratner R,
Garcia-Hernandez PA, Rodriguez-Pattzi H, Olvera-Alvarez I, Hale PM,
Zdravkovic M and Bode B: LEAD-3 (Mono) Study Group: Liraglutide
versus glimepiride monotherapy for type 2 diabetes (LEAD-3 Mono): A
randomised, 52-week, phase III, double-blind, parallel-treatment
trial. Lancet. 373:473–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zinman B, Gerich J, Buse JB, Lewin A,
Schwartz S, Raskin P, Hale PM, Zdravkovic M and Blonde L: LEAD-4
Study Investigators: Efficacy and safety of the human glucagon-like
peptide-1 analog liraglutide in combination with metformin and
thiazolidinedione in patients with type 2 diabetes (LEAD-4
Met+TZD). Diabetes Care. 32:1224–1230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Russell-Jones D, Vaag A, Schmitz O, Sethi
BK, Lalic N, Antic S, Zdravkovic M, Ravn GM and Simó R: Liraglutide
Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group:
Liraglutide vs insulin glargine and placebo in combination with
metformin and sulfonylurea therapy in type 2 diabetes mellitus
(LEAD-5 met+SU): A randomised controlled trial. Diabetologia.
52:2046–2055. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Özenirler S, Degertekin CK, Erkan G, Elbeğ
Ş, Tuncer C, Kandilc U and Akyol G: Serum liver fatty acid binding
protein shows good correlation with liver histology in NASH.
Hepatogastroenterology. 60:1095–1100. 2013.PubMed/NCBI
|
|
21
|
Higuchi N, Kato M, Tanaka M, Miyazaki M,
Takao S, Kohjima M, Kotoh K, Enjoji M, Nakamuta M and Takayanagi R:
Effects of insulin resistance and hepatic lipid accumulation on
hepatic mRNA expression levels of apoB, MTP and L-FABP in
non-alcoholic fatty liver disease. Exp Ther Med. 2:1077–1081.
2011.PubMed/NCBI
|
|
22
|
Jung EJ, Kwon SW, Jung BH, Oh SH and Lee
BH: Role of the AMPK/SREBP-1 pathway in the development of orotic
acid-induced fatty liver. J Lipid Res. 52:1617–1625. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Srivastava RA, Pinkosky SL, Filippov S,
Hanselman JC, Cramer CT and Newton RS: AMP-activated protein
kinase: An emerging drug target to regulate imbalances in lipid and
carbohydrate metabolism to treat cardio-metabolic diseases. J Lipid
Res. 53:2490–2514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Porstmann T, Santos CR, Griffiths B, Cully
M, Wu M, Leevers S, Griffiths JR, Chung YL and Schulze A: SREBP
activity is regulated by mTORC1 and contributes to Akt-dependent
cell growth. Cell Metab. 8:224–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shklyaev S, Aslanidi G, Tennant M, Prima
V, Kohlbrenner E, Kroutov V, Campbell-Thompson M, Crawford J, Shek
EW, Scarpace PJ and Zolotukhin S: Sustained peripheral expression
of transgene adiponectin offsets the development of diet-induced
obesity in rats. Proc Natl Acad Sci USA. 100:14217–14222. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
You M, Matsumoto M, Pacold CM, Cho WK and
Crabb DW: The role of AMP-activated protein kinase in the action of
ethanol in the liver. Gastroenterology. 127:1798–1808. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gundewar S, Calvert JW, Jha S,
Toedt-Pingel I, Ji SY, Nunez D, Ramachandran A, Anaya-Cisneros M,
Tian R and Lefer DJ: Activation of AMP-activated protein kinase by
metformin improves left ventricular function and survival in heart
failure. Circ Res. 104:403–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sasaki H, Asanuma H, Fujita M, Takahama H,
Wakeno M, Ito S, Ogai A, Asakura M, Kim J, Minamino T, et al:
Metformin prevents progression of heart failure in dogs: Role of
AMP-activated protein kinase. Circulation. 119:2568–2577. 2009.
View Article : Google Scholar : PubMed/NCBI
|