Introduction
Preeclampsia (PE) is the most serious
pregnancy-specific disease in the world (1). It is characterized by hypertension,
edema and proteinuria. The exact etiology and pathogenic mechanism
of this syndrome are far from being understood; however, it is
widely accepted that PE is an autoimmune disease induced in
pregnancy due to an immune imbalance at the maternal-fetal
interface (2,3). Several studies have indicated that the
T-helper 1 (Th1)-type cytokine tumor necrosis factor-α (TNF-α) is
associated with PE (4–6). It has been reported that TNF-α can
activate the autoantibody-mediated angiotensin receptor. The
production of two critical etiological factors in PE, soluble
fms-like tyrosine-1 (sFlt-1) and soluble endoglin, can be affected
by both TNF-α and the autoantibody-mediated angiotensin receptor
(7,8). It has therefore been proposed that
TNF-α is involved in the pathogenesis of PE.
A number of approaches have been developed to target
specific factors associated with the pathogenesis of PE. The
application of vascular endothelial growth factor 121 to Sprague
Dawley (SD) rats exhibiting elevated sFlt-1 levels has been shown
to successfully ameliorate the main characteristics of PE,
including hypertension, proteinuria and glomerular endotheliosis
(9). Regarding the treatment of PE,
no therapeutic intervention has been proven to be successful to
date; however, we hypothesize that focusing on the immune disorder
of PE may be a reasonable approach.
Mesenchymal stem cells (MSCs) were first identified
by Friedenstein et al in 1966 in the bone marrow (BM)
(10). MSCs, which are adult stem
cells with self-renewal and multilineage differentiation
potentials, have received increasing attention over the last
decade. Human MSCs have been demonstrated to exert
anti-inflammatory, immunoregulatory and repair effects in a variety
of animal models, including models of cardiac disease, lung injury
and pulmonary hypertension (11–13). BM
is a major source of MSCs in most investigations; however, the
collection of BM is invasive and the number of MSCs reduces with
age, which significantly limits the clinical application of MSCs
(14,15). In 2004, Wang et al (16) demonstrated that the human umbilical
cord blood is rich in MSCs that have surface markers and are
capable of induced differentiation, similar to MSCs from various
tissues including the BM. Thus, umbilical cord blood-derived MSCs
have been widely applied in various experimental models, including
models of Parkinson's disease, spinal cord injury, lung injury and
pulmonary hypertension (13,17–19).
Furthermore, there is a functional similarity between the pulmonary
circulation and the placenta system; however, whether MSCs can be
applied to treat endotoxin-induced hypertension during pregnancy
remains unknown. PE can be established in rats via the intravenous
administration of endotoxin; this disease model exhibits a similar
pathogenesis to that of human PE (20). In the present study, an
endotoxin-induced rat model infused with umbilical cord
blood-derived MSCs was used to investigate the therapeutic effect
of umbilical cord blood-derived MSCs in PE, in order to provide
experimental evidence for the future clinical application of stem
cells.
Materials and methods
Isolation and culture of human
MSCs
Human umbilical cords were collected from local
maternity hospitals, following the provision of written informed
consent by all donors. The human tissue collection for research was
approved by the institutional review board of the Chinese Academy
of Medical Science and Peking Union Medical College (Beijing,
China). Each umbilical cord was cut into 1- to 3-mm pieces, which
were transferred to Dulbecco's modified Eagle's medium (DMEM)/F12
(Gibco-BRL, Grand Island, NY, USA) containing 10% fetal bovine
serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin
(Invitrogen Life Technologies, Carlsbad, CA, USA). The tissue was
placed in a mixture of 5 U/ml hyaluronidase, 125 U/ml collagenase
and 50 U/ml dispase (Sigma-Aldrich, St. Louis, MO, USA) at 37°C for
30 min with gentle agitation. The cells were subsequently pelleted
through low-speed centrifugation (2,000 × g for 5 min), suspended
in fresh medium and transferred to T-75 cm2 cell plates
containing DMEM/F12 along with 20% FBS in a humidified environment
with 5% CO2 at saturated humidity. When the cell
confluence reached ~80%, the cells were digested with 0.25% trypsin
and passaging was performed at a ratio of 1:3. Cells of the fifth
to eighth generation were subjected to flow cytometry for
identification and used for differentiation induction and
transplantation.
Flow cytometry
In the fluorescence-activated cell sorting (FACS)
analysis, the following human monoclonal antibodies were used:
Human anti-cluster of differentiation (CD) 90 (cat. no. 555596),
anti-CD105 (cat. no. 560839), anti-CD73 (cat. no. 550257),
anti-CD19 (cat. no. 555412), anti-CD45 (cat. no. 555483),
anti-human leukocyte antigen (HLA)-DR (cat. no. 555812), anti-CD11b
(cat. no. 555388) and anti-CD34 (cat. no. 555821) (all BD
Biosciences, San Jose, CA, USA). The antibodies were either
purified or directly conjugated with phycoerythrin or fluorescein
isothiocyanate. Data from 10,000 single cell events were collected
using a standard FACSCalibur™ flow cytometer (Immunocytometry
Systems; Becton Dickinson, Franklin Lakes, NJ, USA) for the
fluorescence measurements. Analysis of the obtained data was
performed using CellQuest™ software (Becton Dickinson).
Osteogenic and adipogenic
differentiation
Osteogenic differentiation was induced using an
induction medium of DMEM-F12 supplemented with 10% FBS, 3.06 mg/ml
β-glycerophosphate, 100 nmol/l dexamethasone, 10 nmol/l
1,25-dihydroxyvitamin D3 and 0.15 mmol/l ascorbic acid-2-phosphate
(all from Sigma-Aldrich). To induce adipogenic differentiation,
DMEM supplemented with 10% FBS, 1 µmol/l dexamethasone, 5 µg/ml
insulin, 0.5 mmol/l isobutylmethylxanthine and 60 µmol/l
indomethacin (all from Sigma-Aldrich) was used. A control was
established by maintaining cells in regular growth medium. After
3–4 weeks of osteogenic or adipogenic induction, the presence of
calcium deposition in osteocytes or neutral lipid vacuoles in
adipocytes was assessed by staining the cells with alizarin red or
oil red solution, respectively.
Preparation of the rat PE model and
cell transplantation
The rat model of PE was established according to the
protocol described by Faas et al (21). All research involving animals was
carried out strictly in accordance with the recommendations in the
Guide for the Care and Use of Laboratory Animals approved by China
Medical University (Shenyang, China). Specific pathogen-free
7–8-week-old SD rats (Institute of Laboratory Animal Science, CAMS
& PUMC, Beijing, China) were kept under specific pathogen-free
conditions in a room with a 12-h light/dark cycle and a temperature
maintained at 18–22°C. Standard laboratory pelleted formula and tap
water were provided. Separate rack-mounted wire cages, all on the
same shelf, were used to house each group of rats The experiments
were conducted in accordance with the institutional animal ethics
guidelines (China Medical University), and all efforts were made to
minimize the suffering of the animals.
Pregnancy was achieved by housing two female SD rats
on the night of proestrus with a fertile male for 1 night The next
day, when spermatozoa were detected in the smear, was considered as
day 0 of pregnancy, and the plugged females were removed from the
breeding cages. The pregnant rats were randomly divided into three
groups (control, endotoxin-treated and endotoxin + MSCs), with 7
rats in each group. The pregnant rats subsequently received an
infusion of either 2 ml endotoxin solution (Sigma-Aldrich; 1.0 µg
endotoxin/kg body weight; endotoxin-treated and endotoxin + MSCs
groups) or 2 ml saline (control group) for 1 h via the vena
caudalis. In the endotoxin + MSCs group, the 7 pregnant rats were
injected with MSCs suspended in 100 µl phosphate-buffered saline
(2×106 cells/100 µl) on day 14.
Systolic blood pressure was measured in calm, warmed
and restrained rats using the tail-cuff method on gestation days
(DGs) 8, 15 and 19. Urine samples were analyzed for proteinuria
using a pyrogallol phenol red kit (Sigma-Aldrich) in an automatic
biochemical analyzer (Hitachi 7600-020; Hitachi, Tokyo, Japan) on
DGs 9, 16 and 20. In addition, 20 µl blood was collected and used
to determine the total white blood cell count, which was measured
with a microcell counter (Sysmex F800; Toa Medical Electronics Co.,
Ltd., Kobe, Japan).
ELISA
ELISA kits (R&D Systems, Minneapolis, MN, USA)
were used to detect the levels of interleukin (IL)-1β, IL-10 and
TNF-α in the serum according to manufacturer's instructions.
Statistical analysis
The statistical analysis was performed using SPSS
version 14.0 statistical software (SPSS, Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation. Comparisons
were conducted with one-way analysis of variance, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Culture and identification of MSCs
derived from human umbilical cords
The MSCs formed a monolayer of long, spindle-shaped
cells within 1 week and exhibited potent proliferation activity
(Fig. 1A). The flow cytometry showed
that the umbilical cord-derived MSCs were symmetric with phenotypic
surface antigens. The cells were positive for CD105, CD73 and CD90
and negative for CD19, CD45, CD11b, HLA-DR and CD34 (Fig. 1B). In addition, the differentiation
induction experiment showed that the MSCs had the capacity for
adipogenesis (Fig. 1C) and
osteogenesis (Fig. 1D), which was
detected using oil red O staining and by the calcification of the
alizarin red staining, respectively. These findings suggested that
the cells were MSCs.
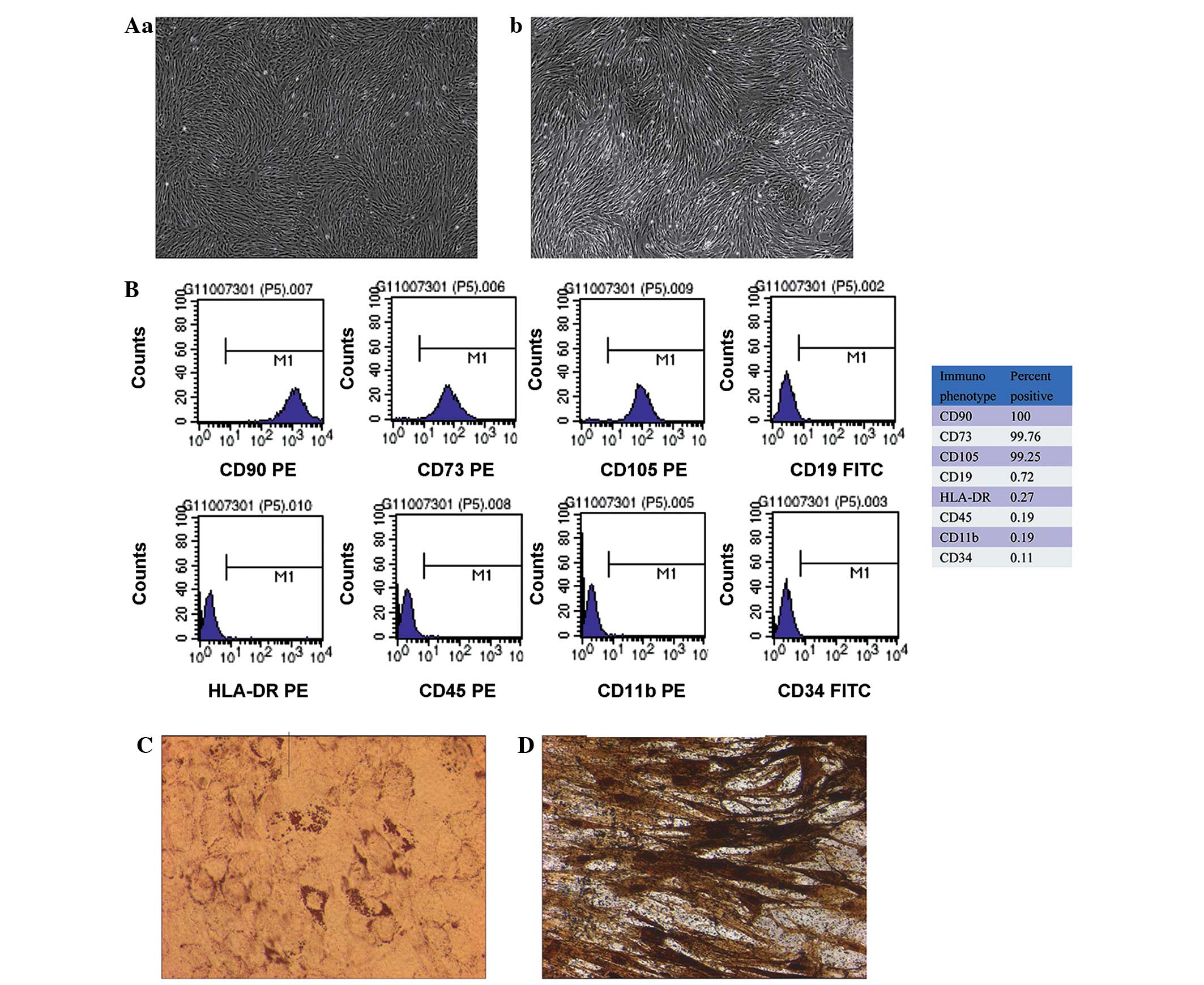 | Figure 1.Isolation and characterization of MSCs
derived from human umbilical cord tissue. (A) Morphology of passage
(a) 3 and (b) 8 umbilical cord-derived MSCs: The cells exhibited
uniform spindle-shape morphology and were ranked compactly in a
parallel or vortex form (magnification, ×40). (B) Flow cytometric
characterization of isolated umbilical cord-derived MSCs during
passage. Expression of the surface antigens CD90, CD73, CD105,
CD19, HLA-DR, CD45, CD11b and CD34 was detected using flow
cytometry. The positive percentage of each marker is shown on the
right. Data are representative of three independent experiments.
(C) Detection of adipogenic differentiation using oil red O
staining. Adipogenic differentiation was evidenced by the formation
of lipid vacuoles by oil-red O staining in umbilical cord-derived
MSCs following adipogenic induction (magnification, ×200). (D)
Osteogenic differentiation was assayed using alizarin red staining
(magnification, ×200). Osteogenic differentiation was evidenced by
the formation of mineralized matrix in the umbilical cord-derived
MSCs following osteogenic induction. MSC, mesenchymal stem cell;
CD, cluster of differentiation; HLA, human leukocyte antigen; PE,
phycoerythrin; FITC, fluorescein isothiocyanate. |
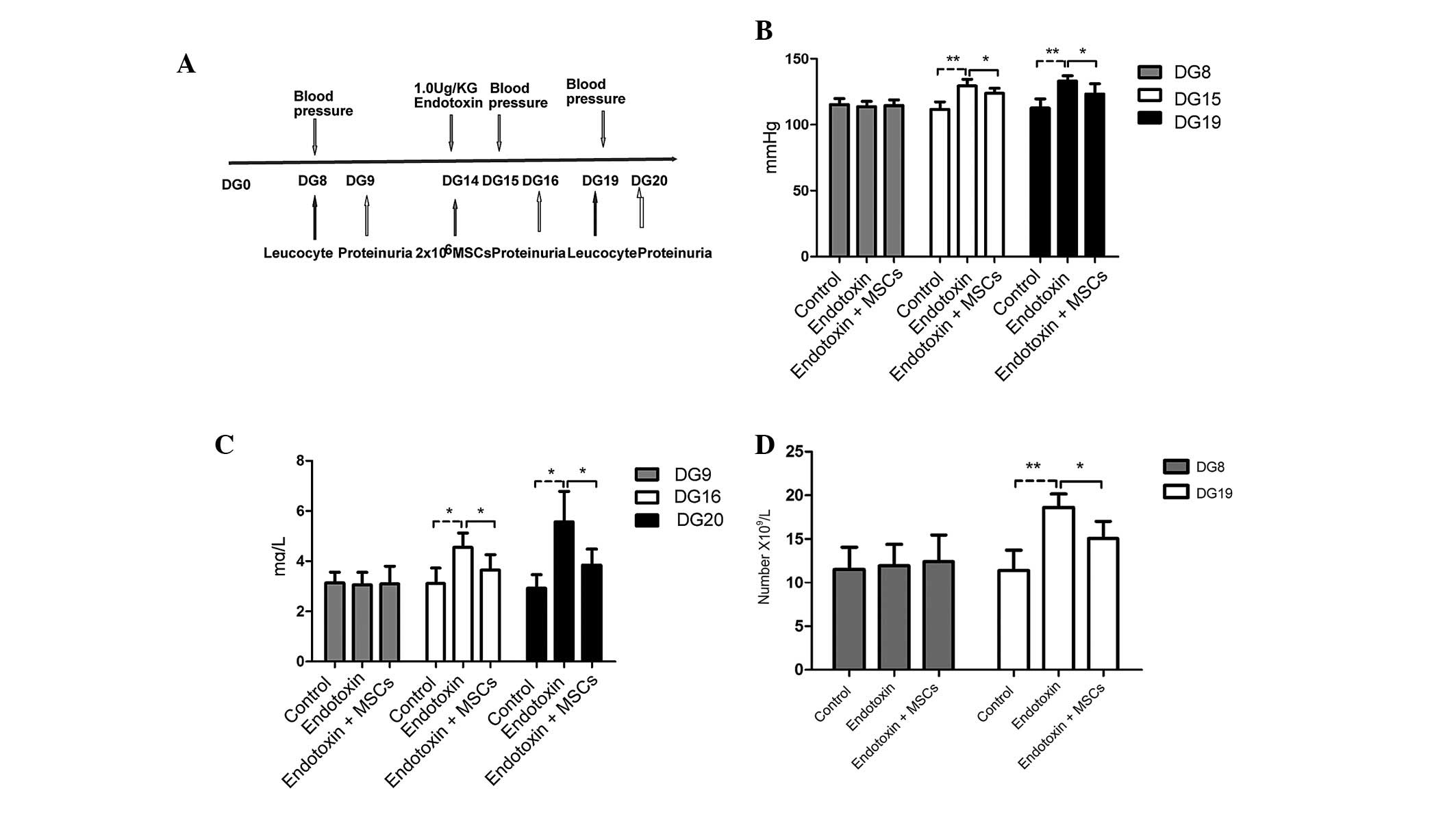
MSC-based therapy ameliorates the
symptoms of PE
Following the positive identification of the MSCs,
PE model rats were established and treated with a suspension of
MSCs in order to investigate the potential therapeutic effects of
the MSCs in PE (Fig. 2A). The
characteristic features of increased blood pressure (Fig. 2B), proteinuria (Fig. 2C) and an increased white blood cell
count (Fig. 2D) indicated that the
rat model of PE had been successfully established. Notably, MSC
infusion was found to ameliorate the symptoms exhibited by the PE
model rats, eliciting a decrease in the blood pressure and
proteinuria (Fig. 2B–D).
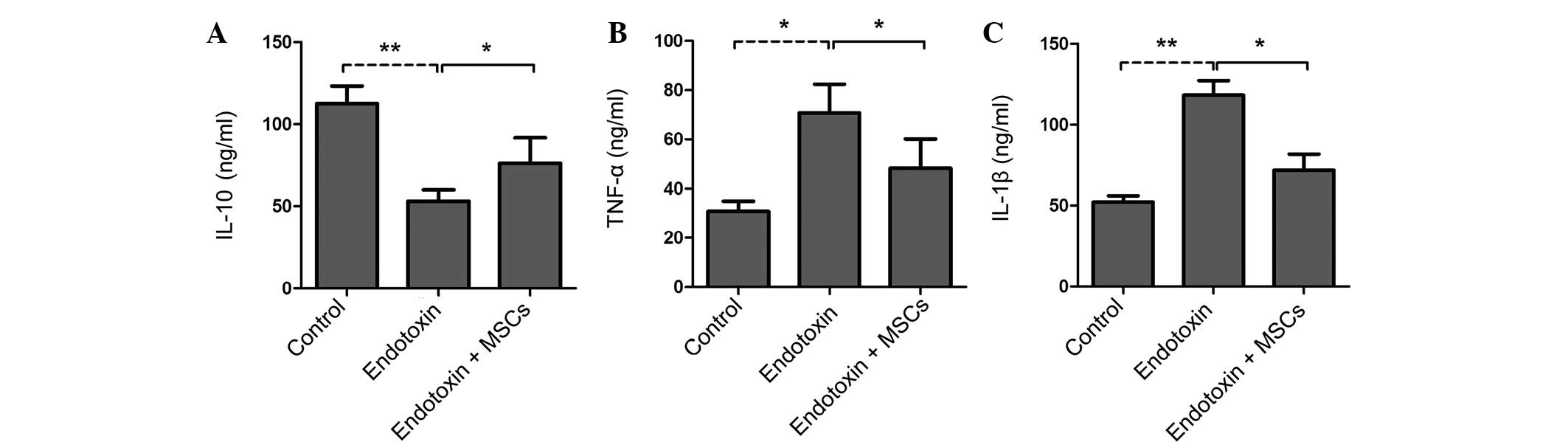
Therapeutic effect of MSCs is mainly
dependent on the serum levels of inflammatory cytokines
Compared with the control rats, the
endotoxin-induced PE rat group showed increased expression levels
of IL-1β and TNF-α but decreased levels of IL-10 in the serum
(Fig. 3A–C). Of note, the infusion
of MSCs significantly attenuated the increases in IL-1β and TNF-α
expression and increased the IL-10 levels in the serum (Fig. 3A–C). These findings suggest that MSCs
may ameliorate endotoxin-induced PE mainly via the suppression of
the levels of inflammatory factors.
Discussion
PE is specific to pregnancy, and is the most common
serious complication of pregnancy in the world (1). Although the exact etiology and
pathogenesis of PE remain unclear, there is now consensus that an
activated inflammatory response is involved (22). It has been confirmed that MSCs exert
anti-inflammatory and immunoregulatory effects in animal models,
including models of arthritis, colitis and autoimmune
encephalomyelitis (23–25); however, the therapeutic efficacy of
human umbilical cord-derived MSCs in a rat model of PE and a
detailed understanding of any associated cellular and molecular
mechanisms have yet to be established. In the present study, the
question of whether human umbilical cord-derived MSCs could be
applied for the treatment of PE was addressed.
To date, MSCs have been successfully cultivated from
a range of tissues, including trabecular bone, adipose tissue,
placenta, pancreas and umbilical cord blood (26–32). In
the present study, fibroblast-like cells were isolated from the
umbilical cord tissue of newborns by a modified enzymatic digestion
procedure and cultured (21,33). The human umbilical cord-derived MSCs
adhered to the cell culture plates and showed a high expression of
CD105, CD73 and CD90, the characteristic MSC surface markers; by
contrast, neither CD34 nor CD45 expression was observed. The
umbilical cord-derived MSCs were induced to differentiate into
either bone or adipose tissue, demonstrating that the MSCs isolated
from newborn umbilical cord tissue met the criteria used to
identify MSCs (34).
It has been suggested that PE is a pregnancy-induced
autoimmune disease. Compared with non-pregnant women, the
circulating levels of TNF-α and IL-6 are further raised in patients
with PE (35). It was indicated that
Th1 cytokines, which are believed to be the main cause of PE, could
directly damage organs (36).
Consistent with previous findings that TNF-α is a key player in the
etiology of PE (37), it was found
in the present study that the levels of IL-1β and TNF-α were
increased in the blood of the pregnant endotoxin-treated rats,
suggesting that increased levels of Th1 pro-inflammatory cytokines,
including IL-1β and TNF-α, play a crucial role in control of
PE.
An important characteristic of MSCs is their
immunosuppressive properties (38).
Several studies have indicated that MSCs can suppress the
expression of pro-inflammatory cytokines, such as IL-1β and TNF-α,
in vitro and in vivo (39–41). A
reduction in the level of TNF-α has been observed following the
co-culture of MSCs with anti-CD3/CD28-activated peripheral blood
mononuclear cells (39). Since MSCs
primarily mediate a downregulation of Th1 pro-inflammatory
cytokines, we hypothesized that an MSC infusion could be a suitable
strategy to promote immune balance and effectively control PE.
Notably, the results of the present study showed that the infusion
of MSCs significantly reduced the IL-1β and TNF-α expression and
increased the IL-10 levels in the serum. These findings suggest
that MSCs may ameliorate the symptoms of endotoxin-induced PE rats
mainly via the suppression of pro-inflammatory factors.
In conclusion, the present study demonstrated that
human umbilical cord-derived MSC-based therapy significantly
ameliorated the symptoms of an endotoxin-induced PE rat model, as
evidenced by decreased blood pressure and proteinuria. Furthermore,
the therapy reversed abnormal IL-1β and TNF-α expression in the
serum. These data suggest that MSCs may play an important role in
the immune balance at the maternal-fetal interface and that human
umbilical cord-derived MSC-based therapy may provide a potential
therapeutic strategy for PE.
Acknowledgements
The authors are grateful for the support of the
Natural Science Foundation of China (grant no. 30872618).
References
|
1
|
Chesley LC: Hypertensive disorders in
pregnancy. J Nurse Midwifery. 30:99–104. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kanasaki K and Kalluri R: The biology of
preeclampsia. Kidney Int. 76:831–837. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Goldman-Wohl DS and Yagel S: Examination
of distinct fetal and maternal molecular pathways suggests a
mechanism for the development of preeclampsia. J Reprod Immunol.
76:54–60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Conrad KP and Benyo DF: Placental
cytokines and the pathogenesis of preeclampsia. Am J Reprod
Immunol. 37:240–249. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gulati R: Raised serum TNF-alpha, blood
sugar and uric acid in preeclampsia in third trimester of
pregnancy. JNMA J Nepal Med Assoc. 44:36–38. 2005.PubMed/NCBI
|
|
6
|
Peraçoli MT, Bannwart CF, Cristofalo R, et
al: Increased reactive oxygen species and tumor necrosis
factor-alpha production by monocytes are associated with elevated
levels of uric acid in pre-eclamptic women. Am J Reprod Immunol.
66:460–467. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Irani RA, Zhang Y, Zhou CC, et al:
Autoantibody-mediated angiotensin receptor activation contributes
to preeclampsia through tumor necrosis factor-alpha signaling.
Hypertension. 55:1246–1253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Parrish MR, Murphy SR, Rutland S, et al:
The effect of immune factors, tumor necrosis factor-alpha, and
agonistic autoantibodies to the angiotensin II type I receptor on
soluble fms-like tyrosine-1 and soluble endoglin production in
response to hypertension during pregnancy. Am J Hypertens.
23:911–916. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Z, Zhang Y, Ying Ma J, et al:
Recombinant vascular endothelial growth factor 121 attenuates
hypertension and improves kidney damage in a rat model of
preeclampsia. Hypertension. 50:686–692. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Friedenstein AJ, Piatetzky-Shapiro II and
Petrakova KV: Osteogenesis in transplants of bone marrow cells. J
Embryol Exp Morphol. 16:381–390. 1966.PubMed/NCBI
|
|
11
|
Williams AR and Hare JM: Mesenchymal stem
cells: Biology, pathophysiology, translational findings, and
therapeutic implications for cardiac disease. Circ Res.
109:923–940. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kode JA, Mukherjee S, Joglekar MV and
Hardikar AA: Mesenchymal stem cells: Immunobiology and role in
immunomodulation and tissue regeneration. Cytotherapy. 11:377–391.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pullamsetti SS, Schermuly R, Ghofrani A,
et al: Novel and emerging therapies for pulmonary hypertension. Am
J Respir Crit Care Med. 189:394–400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishida S, Endo N, Yamagiwa H, et al:
Number of osteoprogenitor cells in human bone marrow markedly
decreases after skeletal maturation. J Bone Miner Metab.
17:171–177. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mueller SM and Glowacki J: Age-related
decline in the osteogenic potential of human bone marrow cells
cultured in three-dimensional collagen sponges. J Cell Biochem.
82:583–590. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang HS, Hung SC, Peng ST, et al:
Mesenchymal stem cells in the Wharton's jelly of the human
umbilical cord. Stem Cells. 22:1330–1337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fu YS, Cheng YC, Lin MY, et al: Conversion
of human umbilical cord mesenchymal stem cells in Wharton's jelly
to dopaminergic neurons in vitro: Potential therapeutic application
for Parkinsonism. Stem Cells. 24:115–124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang CC, Shih YH, Ko MH, et al:
Transplantation of human umbilical mesenchymal stem cells from
Wharton's jelly after complete transection of the rat spinal cord.
PLoS One. 3:e33362008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moodley Y, Atienza D, Manuelpillai U, et
al: Human umbilical cord mesenchymal stem cells reduce fibrosis of
bleomycin-induced lung injury. Am J Pathol. 175:303–313. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Faas MM, Schuiling GA, Baller JF, et al: A
new animal model for human preeclampsia: Ultra-low-dose endotoxin
infusion in pregnant rats. Am J Obstet Gynecol. 171:158–164. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Faas MM, Broekema M, Moes H, et al:
Altered monocyte function in experimental preeclampsia in the rat.
Am J Obstet Gynecol. 191:1192–1198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sacks GP, Studena K, Sargent K and Redman
CW: Normal pregnancy and preeclampsia both produce inflammatory
changes in peripheral blood leukocytes akin to those of sepsis. Am
J Obstet Gynecol. 179:80–86. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
González MA, Gonzalez-Rey E, Rico L, et
al: Treatment of experimental arthritis by inducing immune
tolerance with human adipose-derived mesenchymal stem cells.
Arthritis Rheum. 60:1006–1019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gonzalez-Rey E, Anderson P, González MA,
et al: Human adult stem cells derived from adipose tissue protect
against experimental colitis and sepsis. Gut. 58:929–939. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J, Li Y, Chen J, et al: Human bone
marrow stromal cell treatment improves neurological functional
recovery in EAE mice. Exp Neurol. 195:16–26. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuli R, Tuli S, Nandi S, et al:
Characterization of multipotential mesenchymal progenitor cells
derived from human trabecular bone. Stem Cells. 21:681–693. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zuk PA, Zhu M, Mizuno H, et al:
Multilineage cells from human adipose tissue: Implications for
cell-based therapies. Tissue Eng. 7:211–228. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukuchi Y, Nakajima H, Sugiyama D, et al:
Human placenta-derived cells have mesenchymal stem/progenitor cell
potential. Stem Cells. 22:649–658. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
In't Anker PS, Scherjon SA, der Keur
Kleijburg-van C, et al: Isolation of mesenchymal stem cells of
fetal or maternal origin from human placenta. Stem Cells.
22:1338–1345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu Y, Liao L, Wang Q, et al: Isolation and
identification of mesenchymal stem cells from human fetal pancreas.
J Lab Clin Med. 141:342–349. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bieback K, Kern S, Klüter H and Eichler H:
Critical parameters for the isolation of mesenchymal stem cells
from umbilical cord blood. Stem Cells. 22:625–634. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han YF, Tao R, Sun TJ, et al: Optimization
of human umbilical cord mesenchymal stem cell isolation and culture
methods. Cytotechnology. 65:819–827. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dominici M, Le Blanc K, Mueller I, et al:
Minimal criteria for defining multipotent mesenchymal stromal
cells. The International Society for Cellular Therapy position
statement. Cytotherapy. 8:315–317. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Conrad KP, Miles TM and Benyo DF:
Circulating levels of immunoreactive cytokines in women with
preeclampsia. Am J Reprod Immunol. 40:102–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zenclussen AC, Fest S, Joachim R, et al:
Introducing a mouse model for pre-eclampsia: Adoptive transfer of
activated Th1 cells leads to pre-eclampsia-like symptoms
exclusively in pregnant mice. Eur J Immunol. 34:377–387. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Redman CWG, Sacks GP and Sargent IL:
Preeclampsia: An excessive maternal inflammatory response to
pregnancy. Am J Obstet Gynecol. 180:499–506. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shi Y, Hu G, Su J, et al: Mesenchymal stem
cells: A new strategy for immunosuppression and tissue repair. Cell
Res. 20:510–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Carter D, Tyrell A, Bubnic S, et al:
Characterization of MSC potential to treat GVHD using molecular
markers linked to MSC-mediated immunosuppression in vitro. Blood.
106:160b2005.
|
|
40
|
Gupta N, Su X, Popov B, et al:
Intrapulmonary delivery of bone marrow-derived mesenchymal stem
cells improves survival and attenuates endotoxin-induced acute lung
injury in mice. J Immunol. 179:1855–1863. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rafei M, Campeau PM, Aguilar-Mahecha A, et
al: Mesenchymal stromal cells ameliorate experimental autoimmune
encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine
ligand 2-dependent manner. J Immunol. 182:5994–6002. 2009.
View Article : Google Scholar : PubMed/NCBI
|