Introduction
C-X-C chemokine receptor 4 (CXCR4) is an exclusive
receptor for stromal cell-derived factor-1 (SDF-1, also known as
CXCL12) that has been widely recognized to function in tumor
progression. The orphan receptor CXCR7 has been identified as
another high-affinity CXCL12 receptor that can also bind CXCL11
(1–3). The biological effects of CXCR4 may,
therefore, be attributed to CXCR7 (4).
CXCR7 is an atypical, chemokine-specific
seven-transmembrane guanosine-binding protein-coupled receptor
that, unlike other CXCRs, does not induce intracellular
Ca2+ release following ligand binding (1,5).
Previous characterization of CXCR7-deficient mice has suggested
that the receptor plays an innate role in fetal cardiac development
and B-cell localization (1,6–8). In
addition, studies have indicated that, like CXCR4, CXCR7 is weakly
expressed or absent in the majority of normal tissues but is highly
expressed in several types of cancer, including prostate, lung,
liver, melanoma, rhabdosarcoma, brain, breast and pancreas
(1,7,9).
Furthermore, CXCR7 expression is necessary for cancer cell
survival, tumor development and metastases (1,7,9).
Little is currently known about the role of CXCR7 in
colon cancer growth and metastasis. The aim of the present study
was to investigate the localization of CXCR7 in human colon cancer
cell lines and to explore its function in cell survival and
migration by reducing its expression using a CXCR7-small
interfering RNA (siRNA) recombinant lentivirus.
Materials and methods
Screening of cell lines
Cell culture
The HT-29 and SW-480 human colon cancer cell lines
(Cell Center of Xiangya Medical College of Central South
University, Changsha, China) were cultured in RPMI-1640 medium
(Gibco™; Life Technologies, Carlsbad, CA, USA) supplemented with
10% fetal bovine serum (Gibco), 100 U/ml penicillin and 100 µg/ml
streptomycin. Cells were maintained at 37°C in an incubator with 5%
CO2 and saturated humidity.
Quantitative polymerase chain reaction (qPCR) of
CXCR7 mRNA
Total RNA was extracted from HT-29 and SW-480 cells
in the logarithmic growth phase using TRIzol® according to the
manufacturer's instructions (Invitrogen™; Life Technologies,
Paisley, UK). cDNA was synthesized using a RevertAid™ kit (MBI
Fermentas, Vilnius, Lithuania). Fluorescence qPCR was performed in
a 25-µl reaction mixture containing 12.5 µl SYBR® Premix Ex Taq™
(Takara Bio, Otsu, Japan) (2X), 1 µl 10 µM primer pair mixture, 2
µl cDNA template and double-distilled H2O. Amplification
was initiated by predegeneration at 95°C for 5 min, followed by 40
cycles of denaturation at 94°C for 20 sec, annealing at 53°C for 20
sec and extension at 72°C for 20 sec, with a final extension at
72°C for 5 min. The CXCR7 primer sequences were as follows:
Forward, 5′-ctg cgt cca aca atg ag-3′ and reverse, 5′-gga agt aga
aga cag cga ta-3′ (Telebio Biomedical Co., Ltd., Shanghai, China).
18S rRNA was used as an internal normalized reference (forward,
5′-acg gac agg att gac aga tt-3′; reverse, 5′-gcc act tgt ccc tct
aag aa-3′). CXCR7 gene expression was analyzed using the ΔΔCt
method, as follows: 2−ΔΔCT [ΔΔCT = (Ct(CXCR7) - Ct(18S
rRNA))target - (Ct(CXCR7) - Ct(18S rRNA))internal
standard]. The cell line with the highest CXCR7 expression
was used for all subsequent experiments.
Construction of recombinant
CXCR7-short hairpin RNA lentiviral vector (LV-CXCR7-shRNA)
LV-CXCR7-shRNA was constructed as previously
described (10). The small
interfering RNA (siRNA) sequence against CXCR7 (GenBank, NM_020311)
was designed using BLOCK-iT™ RNAi Designer (https://rnaidesigner.invitrogen.com/rnaiexpress/design.do)
as follows: gcagccggaagatcatcttct. Double-stranded CXCR7-shRNA
hairpins (forward,
5′-accacgcgtggcagccggaagatcatcttctctctcttgaattc-3′; reverse,
5′-aaaatcgataaaaaagcagccggaagatcatcttctgaattcaagagag-3′) were
synthesized and cloned into the plVTHM shuttle plasmid (Telebio
Biomedical Co., Ltd., Shanghai, China) with T4 DNA ligase (New
England Biolabs, Ipswich, MA, USA). 293T cells (Cell Bank of the
Shanghai Institutes for Biological Sciences, Shanghai, China) were
co-transfected with plVTHM-CXCR7-shRNA and lentiviral packaging
plasmids. The viral supernatant was collected 72 h after
transfection. The negative control was simultaneously generated,
and the standard negative control recombinant plasmid (interference
sequence: ttctccgaacgtgtcacgt) was provided by Telebio Biomedical
Co., Ltd.
Transfection
The human colon cancer cells were divided into three
groups: Experimental (treated with LV-CXCR7-shRNA), negative
control (treated with LV-shRNA negative control) and blank control
(no treatment). Each group of cells was inoculated in six-well
culture plates and incubated overnight prior to transfection. As
the cells reached 60–75% confluence, CXCR7 shRNA or negative
control virus vectors were inoculated [multiplicity of infection
(MOI) = virus number/cell number = 20]. The medium was changed
after 24 h to remove any remaining viral vector. Cells were
cultured for 72 h, and green fluorescent protein (GFP) expression
was examined using fluorescence microscopy (BA410; Motic, Xiamen,
China).
Immunofluorescence (IF) analysis of
CXCR7 protein
Cells were seeded and grown on coverslips in
six-well culture plates for 24 h and fixed in a mixture of 6 parts
absolute ethanol:3 parts chloroform:1 part glacial acetic acid for
20 min. The cells were then washed three times in
phosphate-buffered saline (0.01 M, pH 7.2) and blocked with goat
serum albumin for 20 min. The coverslips were incubated with
primary polyclonal rabbit anti-CXCR7 antibody (1:50; ab12870;
Abcam, Cambridge, UK) overnight at 4°C, and then incubated with
goat anti-rabbit IgG fluorescein isothiocyanate (FITC)-conjugated
secondary antibody (ZF-0311; ZSGB-Bio, Beijing, China) at 37°C for
40 min. IF images were visualized using a fluorescence microscope
(BA410; Motic).
MTT assay
Each group of cells was seeded in 96-well plates at
a density of ~1×104 cells (100 µl) per well 76 h after
transfection. After 1, 2, 3, 4 and 5 days, MTT (Sigma, St. Louis,
MO, USA) was added to the cultures at a final concentration of 0.2
mg/ml and incubated for 4 h at 37°C. Dimethylsulfoxide (Sigma) (150
µl/well) was added to each well following the removal of the
supernatant. The optical density (OD) was measured at 490 nm using
a spectrophotometer. Cell survival rates (CSRs) were calculated
according to the following formula: CSR (%) = (ODdosing
cell/ODblank control cell) ×100.
Cell migration assay
A 200-µl cell suspension containing 1×104
cells was seeded into the upper chamber of a Transwell® system
(6.5-mm Transwell filter with 8.0-µm pore polycarbonate membrane),
and 500 µl chemotactic factor from NIH/3T3 cell supernatant (Cell
Bank of the Shanghai Institutes for Biological Sciences) was added
to the lower chamber. Following incubation at 37°C for 24 h, cells
that had migrated to the external surface of the membrane were
fixed with 4% paraformaldehyde and stained with hematoxylin and
eosin.
Statistical analysis
Statistical analysis was performed using SPSS 19.0
statistical software (IBM SPSS, Armonk, NY, USA). The data are
presented as the mean ± standard deviation. Differences among
multiple groups were analyzed using one-way analysis of variance.
The differences between two groups were determined using the
Student-Newman-Keuls method. P<0.05 was considered to indicate a
statistically significant difference.
Results
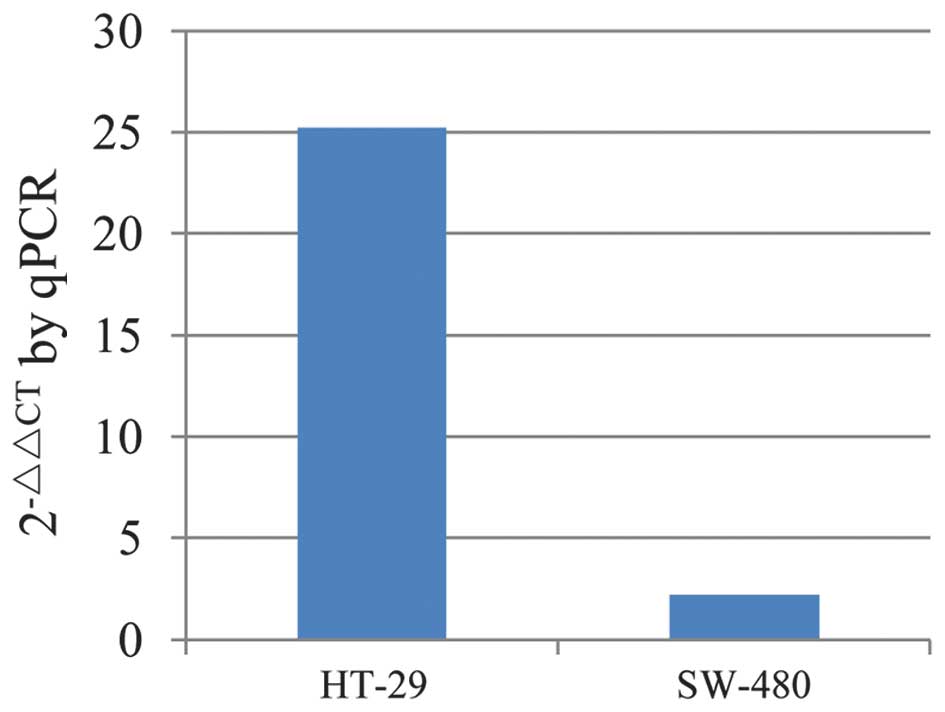
CXCR7 mRNA expression in HT-29 and
SW-480 human colon cancer cell lines
CXCR7mRNA expression was evaluated in HT-29 and
SW-480 cells using qPCR (Fig. 1).
The results indicated that CXCR7 mRNA was expressed in both cell
lines, although the expression in the HT-29 cells was notably
higher than that in the SW-480 cells. HT-29 cells were therefore
used for further analysis.
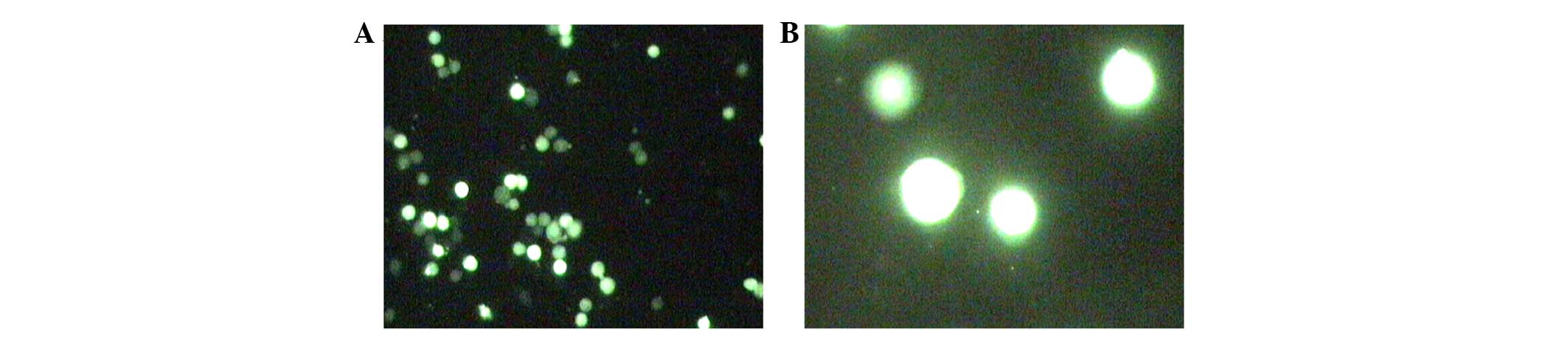
Transfection efficiency of lentiviral
vector
HT-29 cells were transfected with LV-CXCR7-shRNA and
LV-shRNA negative control, according to the grouping, for 96 h
(MOI=20), and GFP expression was examined using fluorescence
microscopy. An intense green signal (Fig. 2) was observed in ~80% of the cells.
These results showed that the cells could be stably transfected
with high efficiency.
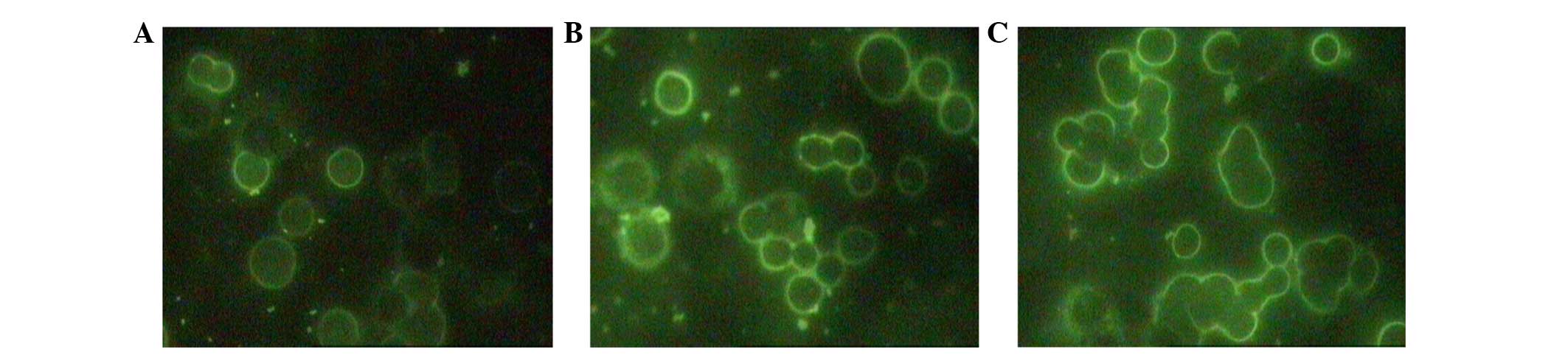
CXCR7 protein localizes to the plasma
membrane
The localization of CXCR7 protein was examined using
IF, and it was found that the protein localized to the membrane and
was not intracellular (Fig. 3).
CXCR7-knockdown was confirmed to decrease protein expression, as
the experimental group of cells showed little to weak expression
whereas both the negative control and the blank control group cells
showed abundant, intense expression. No obvious differences could
be detected between the negative control and blank control groups.
The IF analysis suggested that LV-CXCR7-shRNA effectively decreased
the expression of CXCR7 protein in HT-29 cells.
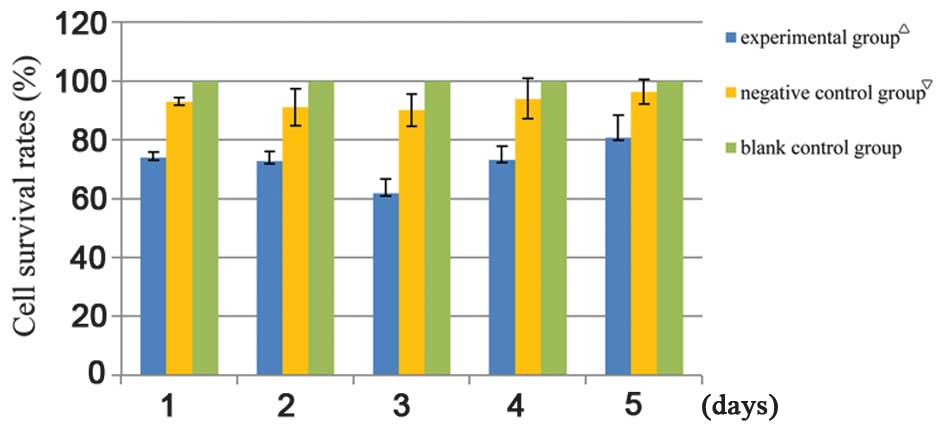
CXCR7-knockdown decreases cell
survival
Cell survival following CXCR7-knockdown was measured
using an MTT assay. As shown in Table
I and Fig. 4, the CSRs of cells
with knocked down CXCR7 were significantly decreased and the
survival curve was lower than that in the negative control and
blank control groups (P<0.05). The results suggested that CXCR7
inhibition significantly reduced the colon cancer CSR; however, the
cell survival of the negative control group cells was also
significantly decreased compared with that of the blank control
group cells (Table I and Fig. 4, P<0.05). Furthermore, cells in
both the experimental and the negative control group showed
decreased survival for the first 3 days after lentiviral
transfection. Survival rates reached their lowest value on the
third day, and then began to gradually increase. These data
suggested that the lentiviral vector itself was cytotoxic, leading
to decreased cell survival.
 | Table I.Cell survival rates. |
Table I.
Cell survival rates.
| Group | Day 1 (%) | Day 2 (%) | Day 3 (%) | Day 4 (%) | Day 5 (%) |
|---|
| Experimental
groupa | 74.16±1.63 | 72.97±3.05 | 62.01±4.78 | 73.23±4.71 | 80.78±7.54 |
| Negative control
groupb | 93.06±1.30 | 91.14±6.22 | 90.12±5.47 | 94.08±6.87 | 96.35±4.16 |
| Blank control
group | 100 | 100 | 100 | 100 | 100 |
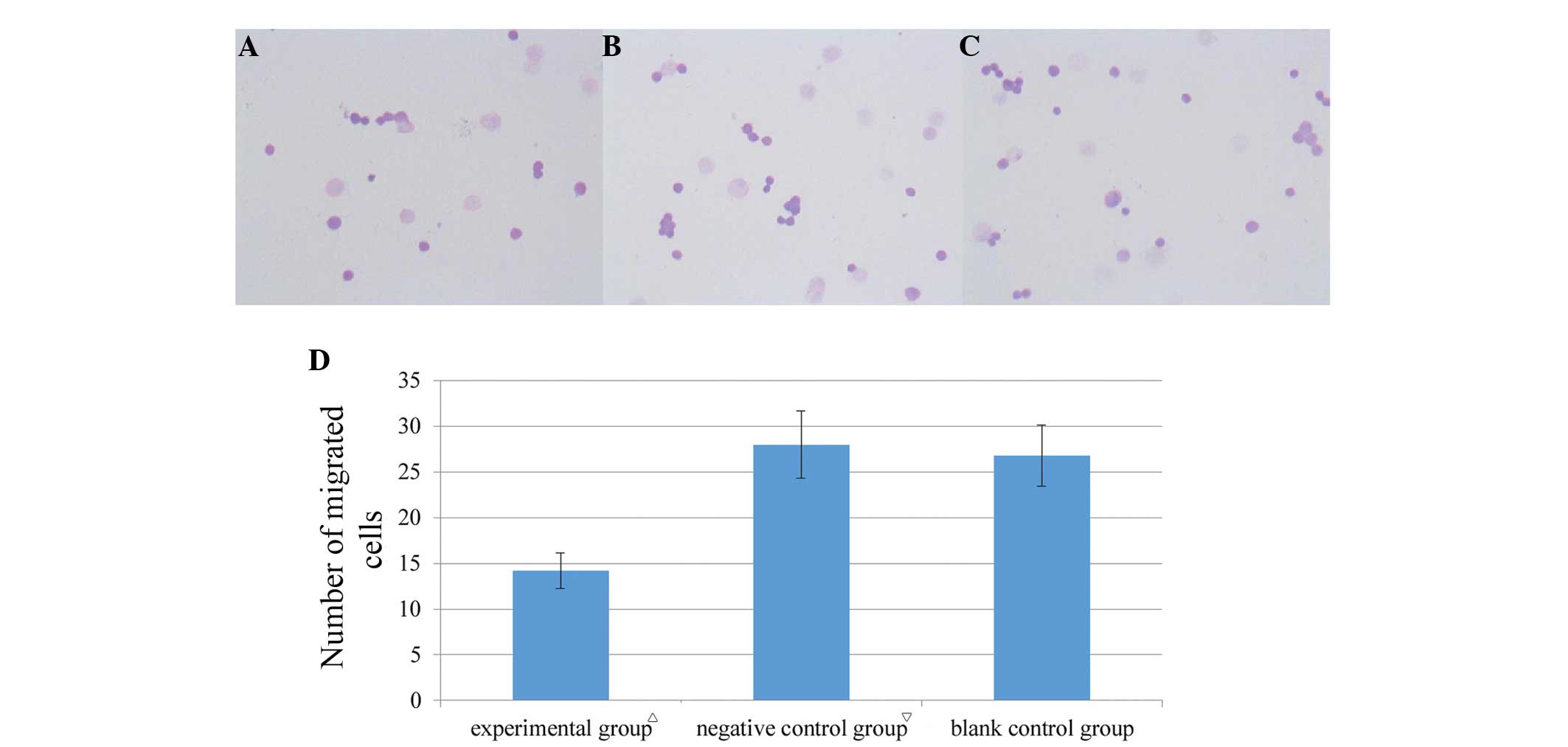
CXCR7-knockdown inhibits cell
migration
Transwell migration assays were performed to
evaluate whether CXCR7 plays a role in HT-29 cell migration. Upon
CXCR7-knockdown, cell migration significantly decreased compared
with the negative control and blank control groups (Fig. 5, P<0.05), but did not
significantly change between the two control groups (Fig. 5, P>0.05). The results indicated
that CXCR7-knockdown inhibited colon cancer cell migration.
Discussion
The CXCL12-CXCR4 axis has been shown to regulate
cancer growth and metastasis (11,12).
Increasing evidence has suggested that CXCR7 acts as an alternative
CXCL12 receptor and is expressed on embryonic and neoplastic
transformed cells, but is absent or weakly expressed in normal
tissues (1,7). Studies have also shown that CXCR7 is
involved in various biological functions, including cancer cell
proliferation, invasion and migration (13,14).
A previous study found that CXCR7 was co-expressed
with epidermal growth factor receptor and/or CXCR4 (double- or
triple-positive) to contribute to cervical cancer progression
(15). Other studies of prostate and
hepatocellular carcinomas have shown that CXCR7 promotes vascular
endothelial growth factor secretion, resulting in tumor
angiogenesis (16,17). The CXCR7 transduction pathway
involves β-arrestin 2 recruitment and enhanced extracellular
signal-regulated kinase and p38 signaling in response to CXCL12
stimulation (8,18); however, it does not involve increased
K-Ras activity (4).
Although CXCR7 has been widely implicated in various
types of cancer, its function in colon cancer remains unclear. We
hypothesized that CXCR7 may contribute to colon cancer progression
through the promotion of cell survival and migration; therefore,
the localization of CXCR7 in human colon cancer cells in
vitro and the role of CXCR7 in cell survival and migration were
evaluated in the present study.
In this study, it was found that the HT-29 human
colon cancer cell line expressed higher CXCR7 levels than SW-480
cells (Fig. 1), and HT-29 cells were
therefore used for further analyses. Recombinant CXCR7-siRNA
lentivirus was constructed and transfected into HT-29 cells with
~80% transfection efficiency (Fig.
2). Consistent with several studies that CXCR7 provides a
survival advantage for cancer cells (1,7,16), the present data demonstrated that
CXCR7-depleted HT-29 cells had significantly decreased CSRs
(Table I and Fig. 4), indicating that CXCR7 facilitates
HT-29 cell survival in vitro; however, HT-29 cells
transfected with LV-shRNA negative control lentiviral vector also
showed a significant reduction in cell survival, suggesting that
the lentivirus itself was cytotoxic.
Transwell migration assays revealed that knocking
down CXCR7 significantly inhibited cell migration (Fig. 5), suggesting that CXCR7 facilitates
cell migration, which may contribute to tumor invasion or
metastasis. These data are consistent with those of previous
studies, showing that decreased CXCR7 expression in cancer cells
resulted in decreased cell migration (19,20).
Since invasion and metastasis are the primary causes
of cancer-related morbidity and mortality, small-molecule CXCR7
inhibitors could be highly beneficial cancer therapies. The CXCR7
antagonist CCX771 has been shown to reduce extravasation and
attenuate metastasis in breast cancer (9,21),
providing an attractive therapeutic modality.
Using IF microscopy, it was found in the present
study that CXCR7 localized to the cytomembrane; however, the
literature describes conflicting results. A study by Berahovich
et al (22) did not detect
any surface CXCR7 signal in the PC-3 prostate cancer cell line;
however, the present results are consistent with the findings of
Singh and Lokeshwar (23), which
showed that CXCR7 localized to the membrane and did not emit a
strong intracellular signal (Fig.
3). Furthermore, CXCR7 membrane expression was detected in all
HT-29 colon cancer cells in the present study. Membrane
localization of CXCR7 is beneficial for its use as a cancer
target.
In conclusion, the present study provides insight
into the function of CXCR7 in colon cancer cells. CXCR7-knockdown
negatively affected cell survival and migration in vitro,
suggesting that CXCR7 functions in tumor aggravation. The present
study also supports the previous finding that CXCR7 is expressed on
the cytomembrane rather than being found intracellularly, which may
be of benefit for the therapeutic targeting of CXCR7. Further
research will focus on the molecular mechanisms of CXCR7 in colon
cancer and its biological effects in vivo and the
development of CXCR7-targeted therapies to improve survival.
Acknowledgements
The authors would like to acknowledge Professor
Hui-Qing Mao for statistical assistance and Xiao-Hua Li for
technical assistance. This study was also supported by Professor
Guang-Hua Yang from Telebio Biomedical Co., Ltd.
References
|
1
|
Burns JM, Summers BC, Wang Y, Melikian A,
Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ,
et al: A novel chemokine receptor for SDF-1 and I-TAC involved in
cell survival, cell adhesion and tumor development. J Exp Med.
203:2201–2213. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hartmann TN, Grabovsky V, Pasvolsky R,
Shulman Z, Buss EC, Spiegel A, Nagler A, Lapidot T, Thelen M and
Alon R: A crosstalk between intracellular CXCR7 and CXCR4 involved
in rapid CXCL12-triggered integrin activation but not in
chemokine-triggered motility of human T lymphocytes and CD34+
cells. J Leukoc Biol. 84:1130–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Levoye A, Balabanian K, Baleux F,
Bachelerie F and Lagane B: CXCR7 heterodimerizes with CXCR4 and
regulates CXCL12-mediated G protein signaling. Blood.
113:6085–6093. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heinrich EL, Lee W, Lu J, Lowy AM and Kim
J: Chemokine CXCL12 activates dual CXCR4 and CXCR7-mediated
signaling pathways in pancreatic cancer cells. J Transl Med.
10:682012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Odemis V, Boosmann K, Heinen A, Küry P and
Engele J: CXCR7 is an active component of SDF-1 signalling in
astrocytes and Schwann cells. J Cell Sci. 123:1081–1088. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Infantino S, Moepps B and Thelen M:
Expression and regulation of the orphan receptor RDC1 and its
putative ligand in human dendritic and B cells. J Immunol.
176:2197–2207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miao Z, Luker KE, Summers BC, Berahovich
R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A,
Luker GD, et al: CXCR7 (RDC1) promotes breast and lung tumor growth
in vivo and is expressed on tumor-associated vasculature. Proc Natl
Acad Sci USA. 104:15735–15740. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Y, Li G, Stanco A, Long JE, Crawford
D, Potter GB, Pleasure SJ, Behrens T and Rubenstein JL: CXCR4 and
CXCR7 have distinct functions in regulating interneuron migration.
Neuron. 69:61–76. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zabel BA, Lewén S, Berahovich RD, Jaén JC
and Schall TJ: The novel chemokine receptor CXCR7 regulates
trans-endothelial migration of cancer cells. Mol Cancer. 10:732011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HX, Chen DJ, Hu G, Cheng W and Yang
KY: The effect of lentiviral vector of shRNA on CXCR7 expression in
human colon cancer. Zhong Hua Pu Tong Wai Ke Za Zhi. 18:156–161.
2009.(In Chinese).
|
|
11
|
Muller A, Homey B, Soto H, Ge N, Catron D,
Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al:
Involvement of chemokine receptors in breast cancer metastasis.
Nature. 410:50–56. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vandercappellen J, Van Damme J and Struyf
S: The role of CXC chemokines and their receptors in cancer. Cancer
Lett. 267:226–244. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao M, Zheng J, Hou K and Wang J, Chen X,
Lu X, Bo J, Xu C, Shen K and Wang J: Role of chemokine receptor
CXCR7 in bladder cancer progression. Biochem Pharmacol. 84:204–214.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yates TJ, Knapp J, Gosalbez M, Lokeshwar
SD, Gomez CS, Benitez A, Ekwenna OO, Young EE, Manoharan M and
Lokeshwar VB: C-X-C chemokine receptor 7: A functionally associated
molecular marker for bladder cancer. Cancer. 119:61–71. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schrevel M, Karim R, ter Haar NT, van der
Burg SH, Trimbos JB, Fleuren GJ, Gorter A and Jordanova ES: CXCR7
expression is associated with disease-free and disease-specific
survival in cervical cancer patients. Br J Cancer. 106:1520–1525.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Shiozawa Y, Wang J, Wang Y, Jung
Y, Pienta KJ, Mehra R, Loberg R and Taichman RS: The role of
CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate
cancer. J Biol Chem. 283:4283–4294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng K, Li HY, Su XL, Wang XY, Tian T, Li
F and Ren GS: Chemokine receptor CXCR7 regulates the invasion,
angiogenesis and tumor growth of human hepatocellular carcinoma
cells. J Exp Clin Cancer Res. 29:312010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Décaillot FM, Kazmi MA, Lin Y, Ray-Saha S,
Sakmar TP and Sachdev P: CXCR7/CXCR4 heterodimer constitutively
recruits beta-arrestin to enhance cell migration. J Biol Chem.
286:32188–32197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YH, Burdick MD, Strieter BA, Mehrad B
and Strieter RM: CXCR4, but not CXCR7, discriminates metastatic
behavior in non-small cell lung cancer cells. Mol Cancer Res.
12:38–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Zhao X, Zhu X, Zhong Z, Xu R, Wang
Z, Cao J and Hou Y: Decreased expression of miR-430 promotes the
development of bladder cancer via the upregulation of CXCR7. Mol
Med Rep. 8:140–146. 2013.PubMed/NCBI
|
|
21
|
Hawkins OE and Richmond A: The dynamic
yin-yang interaction of CXCR4 and CXCR7 in breast cancer
metastasis. Breast Cancer Res. 14:1032012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berahovich RD, Penfold ME and Schall TJ:
Nonspecific CXCR7 antibodies. Immunol Lett. 133:112–114. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh RK and Lokeshwar BL: The
IL-8-regulated chemokine receptor CXCR7 stimulates EGFR signaling
to promote prostate cancer growth. Cancer Res. 71:3268–3277. 2011.
View Article : Google Scholar : PubMed/NCBI
|