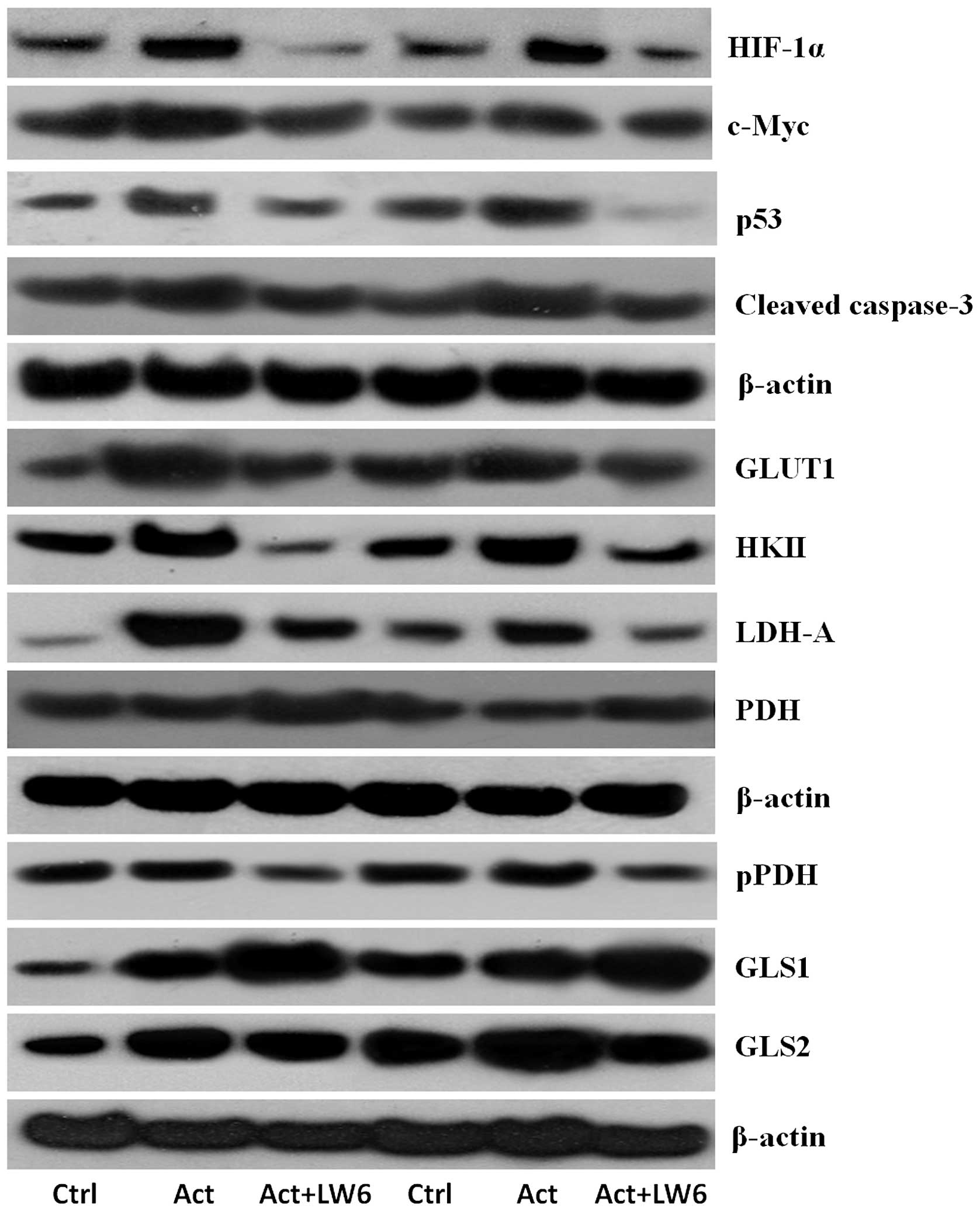
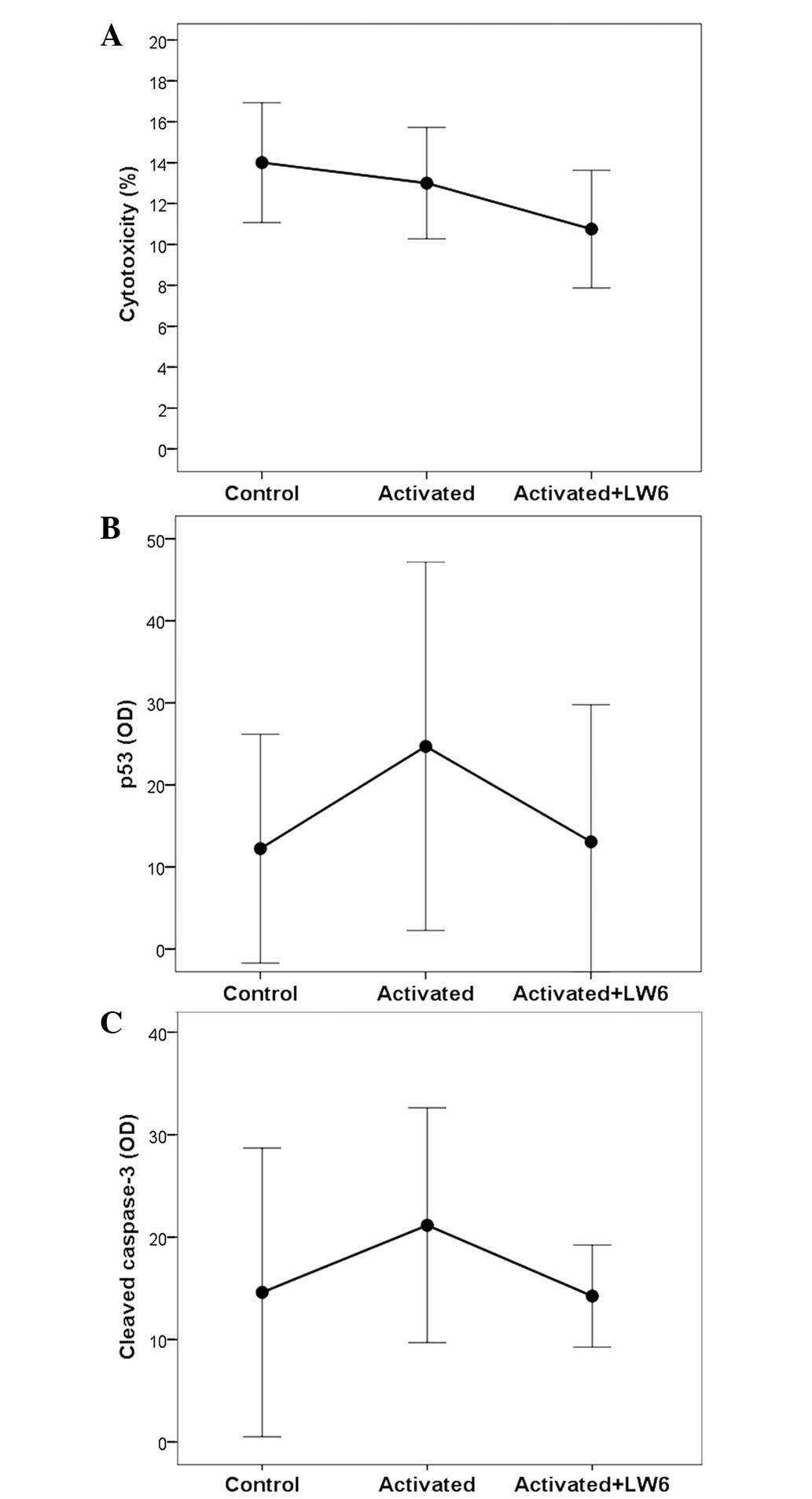
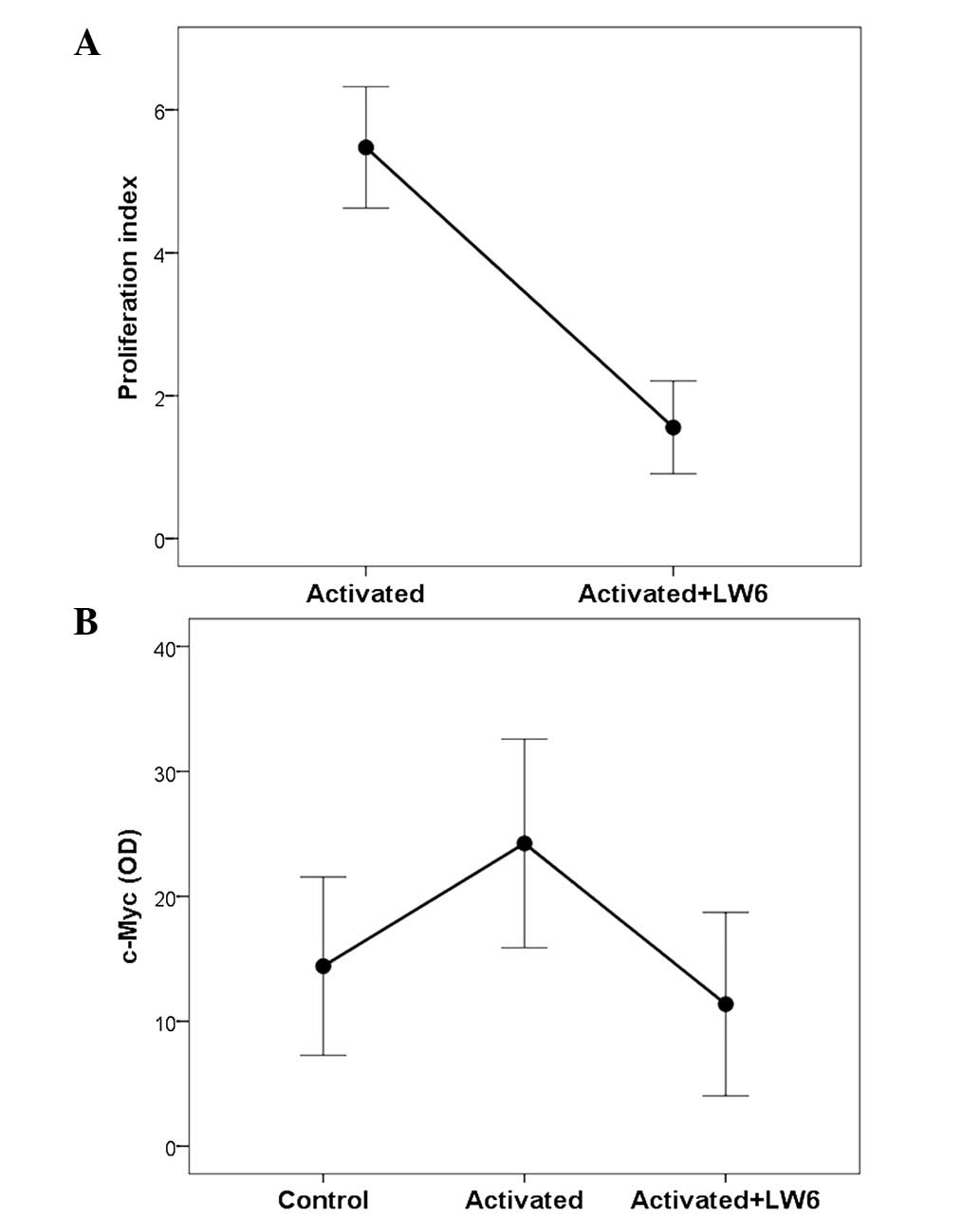
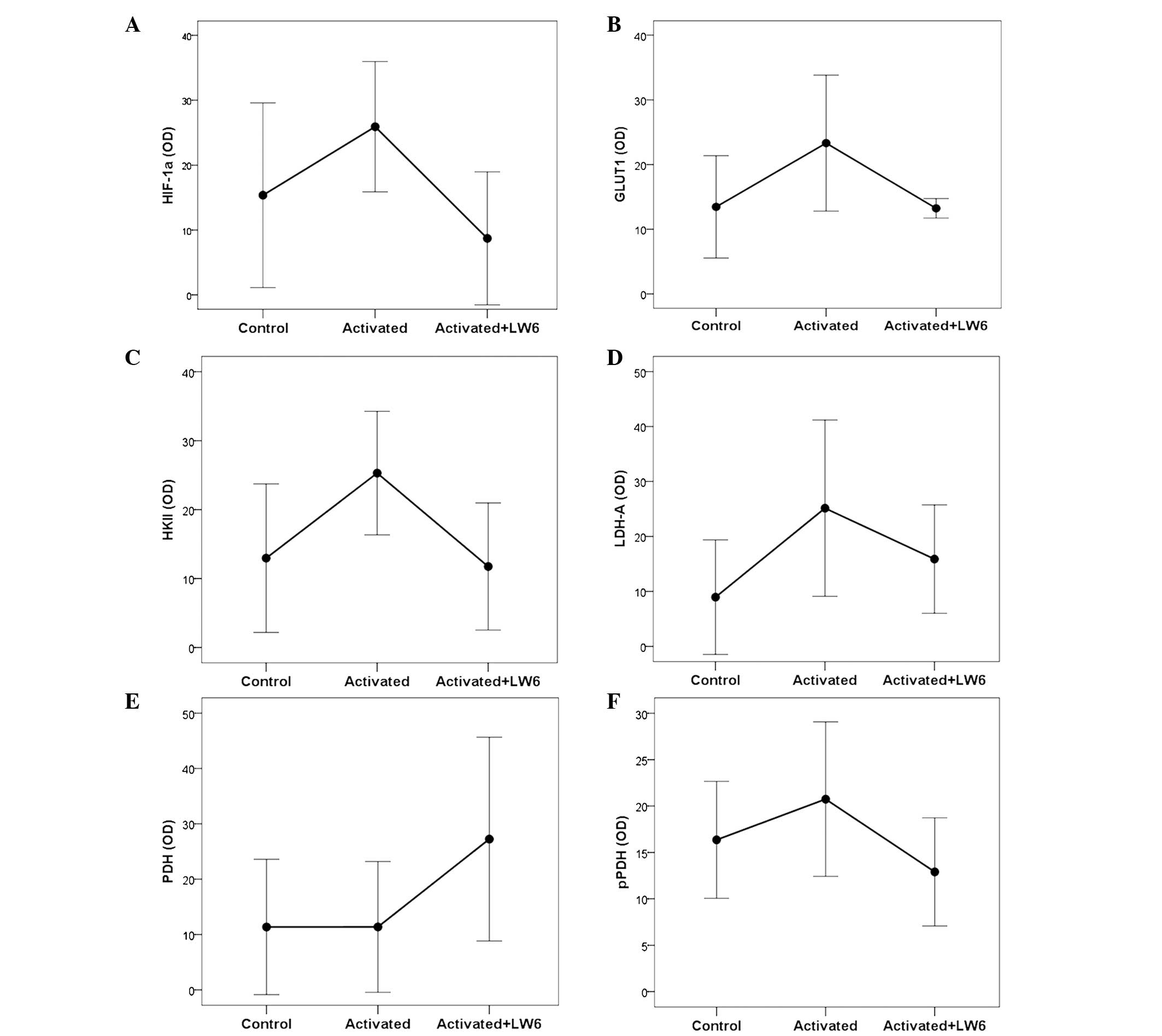
|
1
|
Wang R, Dillon CP, Shi LZ, Milasta S,
Carter R, Finkelstein D, McCormick LL, Fitzgerald P, Chi H, Munger
J and Green DR: The transcription factor Myc controls metabolic
reprogramming upon T lymphocyte activation. Immunity. 35:871–882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang R and Green DR: Metabolic
reprogramming and metabolic dependency in T cells. Immunol Rev.
249:14–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ostroukhova M, Goplen N, Karim MZ,
Michalec L, Guo L, Liang Q and Alam R: The role of low-level
lactate production in airway inflammation in asthma. Am J Physiol
Lung Cell Mol Physiol. 302:L300–L307. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shi LZ, Wang R, Huang G, Vogel P, Neale G,
Green DR and Chi H: HIF1alpha-dependent glycolytic pathway
orchestrates a metabolic checkpoint for the differentiation of TH17
and Treg cells. J Exp Med. 208:1367–1376. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eleftheriadis T, Pissas G, Karioti A,
Antoniadi G, Antoniadis N, Liakopoulos V and Stefanidis I:
Dichloroacetate at therapeutic concentration alters glucose
metabolism and induces regulatory T-cell differentiation in
alloreactive human lymphocytes. J Basic Clin Physiol Pharmacol.
24:271–276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eleftheriadis T, Pissas G, Karioti A,
Antoniadi G, Liakopoulos V, Dafopoulou K, Pournaras S, Koukoulis G
and Stefanidis I: The indoleamine 2,3-dioxygenase inhibitor
1-methyl-tryptophan suppresses mitochondrial function, induces
aerobic glycolysis and decreases interleukin-10 production in human
lymphocytes. Immunol Invest. 41:507–520. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eleftheriadis T, Pissas G, Yiannaki E,
Markala D, Arampatzis S, Antoniadi G, Liakopoulos V and Stefanidis
I: Inhibition of indoleamine 2,3-dioxygenase in mixed lymphocyte
reaction affects glucose influx and enzymes involved in aerobic
glycolysis and glutaminolysis in alloreactive T-cells. Hum Immunol.
74:1501–1509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eleftheriadis T, Pissas G, Antoniadi G,
Spanoulis A, Liakopoulos V and Stefanidis I: Indoleamine
2,3-dioxygenase increases p53 levels in alloreactive human T cells
and both indoleamine 2,3-dioxygenase and p53 suppress glucose
uptake, glycolysis and proliferation. Int Immunol. 26:673–684.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee K, Kang JE, Park SK, Jin Y, Chung KS,
Kim HM, Lee K, Kang MR, Lee MK, Song KB, et al: LW6, a novel HIF-1
inhibitor, promotes proteasomal degradation of HIF-1alpha via
upregulation of VHL in a colon cancer cell line. Biochem Pharmacol.
80:982–989. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee K, Ban HS, Naik R, Hong YS, Son S, Kim
BK, Xia Y, Song KB, Lee HS and Won M: Identification of malate
dehydrogenase 2 as a target protein of the HIF-1 inhibitor LW6
using chemical probes. Angew Chem Int Ed Engl. 52:10286–10289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Friedman B, Goodman EH Jr, Saunders HL,
Kostos V and Weinhouse S: Estimation of pyruvate recycling during
gluconeogenesis in perfused rat liver. Metabolism. 20:2–12. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Berridge MV, Herst PM and Tan AS:
Tetrazolium dyes as tools in cell biology: New insights into their
cellular reduction. Biotechnol Annu Rev. 11:127–152. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dang EV, Barbi J, Yang HY, Jinasena D, Yu
H, Zheng Y, Bordman Z, Fu J, Kim Y and Yen HR: Control of
T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell.
146:772–784. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guy CS, Vignali KM, Temirov J, Bettini ML,
Overacre AE, Smeltzer M, Zhang H, Huppa JB, Tsai YH, Lobry C, et
al: Distinct TCR signaling pathways drive proliferation and
cytokine production in T cells. Nat Immunol. 14:262–270. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Doe MR, Ascano JM, Kaur M and Cole MD: Myc
posttranscriptionally induces HIF1 protein and target gene
expression in normal and cancer cells. Cancer Res. 72:949–957.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen C, Cai S, Wang G, Cao X, Yang X, Luo
X, Feng Y and Hu J: c-Myc enhances colon cancer cell-mediated
angiogenesis through the regulation of HIF-1α. Biochem Biophys Res
Commun. 430:505–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Podar K and Anderson KC: A therapeutic
role for targeting c-Myc/Hif-1-dependent signaling pathways. Cell
Cycle. 9:1722–1728. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong KS, Park JI, Kim MJ, Kim HB, Lee JW,
Dao TT, Oh WK, Kang CD and Kim SH: Involvement of SIRT1 in hypoxic
down-regulation of c-Myc and β-catenin and hypoxic preconditioning
effect of polyphenols. Toxicol Appl Pharmacol. 259:210–218. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang LE: Carrot and stick: HIF-alpha
engages c-Myc in hypoxic adaptation. Cell Death Differ. 15:672–677.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Q, Kluz T, Sun H and Costa M:
Mechanisms of c-myc degradation by nickel compounds and hypoxia.
PloS One. 4:e85312009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sermeus A and Michiels C: Reciprocal
influence of the p53 and the hypoxic pathways. Cell Death Dis.
2:e1642011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eischen CM, Weber JD, Roussel MF, Sherr CJ
and Cleveland JL: Disruption of the ARF-Mdm2-p53 tumor suppressor
pathway in Myc-induced lymphomagenesis. Genes Dev. 13:2658–2669.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brady CA and Attardi LD: p53 at a glance.
J Cell Sci. 123:2527–2532. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Green DR and Kroemer G: Cytoplasmic
functions of the tumour suppressor p53. Nature. 458:1127–1130.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iyer NV, Kotch LE, Agani F, Leung SW,
Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY
and Semenza GL: Cellular and developmental control of O2
homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev.
12:149–162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Osthus RC, Shim H, Kim S, Li Q, Reddy R,
Mukherjee M, Xu Y, Wonsey D, Lee LA and Dang CV: Deregulation of
glucose transporter 1 and glycolytic gene expression by c-Myc. J
Biol Chem. 275:21797–21800. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gordan JD, Thompson CB and Simon MC: HIF
and c-Myc: Sibling rivals for control of cancer cell metabolism and
proliferation. Cancer Cell. 12:108–113. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Valera A, Pujol A, Gregori X, Riu E, Visa
J and Bosch F: Evidence from transgenic mice that myc regulates
hepatic glycolysis. FASEB J. 9:1067–1078. 1995.PubMed/NCBI
|
|
29
|
Shim H, Dolde C, Lewis BC, Wu CS, Dang G,
Jungmann RA, Dalla-Favera R and Dang CV: c-Myc transactivation of
LDH-A: Implications for tumor metabolism and growth. Proc Natl Acad
Sci USA. 94:6658–6663. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Korotchkina LG and Patel MS: Site
specificity of four pyruvate dehydrogenase kinase isoenzymes toward
the three phosphorylation sites of human pyruvate dehydrogenase. J
Biol Chem. 276:37223–37229. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JW, Tchernyshyov I, Semenza GL and
Dang CV: HIF-1-mediated expression of pyruvate dehydrogenase
kinase: A metabolic switch required for cellular adaptation to
hypoxia. Cell Metab. 3:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Munger J, Bajad SU, Coller HA, Shenk T and
Rabinowitz JD: Dynamics of the cellular metabolome during human
cytomegalovirus infection. PLoS Pathog. 2:e1322006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Le A, Lane AN, Hamaker M, Bose S, Gouw A,
Barbi J, Tsukamoto T, Rojas CJ, Slusher BS, Zhang H, et al:
Glucose-independent glutamine metabolism via TCA cycling for
proliferation and survival in B cells. Cell Metab. 15:110–121.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang C, Ko B, Hensley CT, Jiang L, Wasti
AT, Kim J, Sudderth J, Calvaruso MA, Lumata L, Mitsche M, et al:
Glutamine oxidation maintains the TCA cycle and cell survival
during impaired mitochondrial pyruvate transport. Mol Cell.
56:414–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mates JM, Segura JA, Martin-Rufián M,
Campos-Sandoval JA, Alonso FJ and Marquez J: Glutaminase isoenzymes
as key regulators in metabolic and oxidative stress against cancer.
Curr Mol Med. 13:514–534. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Colombo SL, Palacios-Callender M, Frakich
N, De Leon J, Schmitt CA, Boorn L, Davis N and Moncada S:
Anaphase-promoting complex/cyclosome-Cdh1 coordinates glycolysis
and glutaminolysis with transition to S phase in human T
lymphocytes. Proc Natl Acad Sci USA. 107:18868–18873. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gao P, Tchernyshyov I, Chang TC, Lee YS,
Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT and
Dang CV: c-Myc suppression of miR-23a/b enhances mitochondrial
glutaminase expression and glutamine metabolism. Nature.
458:762–765. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Suzuki S, Tanaka T, Poyurovsky MV, Nagano
H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y,
et al: Phosphate-activated glutaminase (GLS2), a p53-inducible
regulator of glutamine metabolism and reactive oxygen species. Proc
Natl Acad Sci USA. 107:7461–7466. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu W, Zhang C, Wu R, Sun Y, Levine A and
Feng Z: Glutaminase 2, a novel p53 target gene regulating energy
metabolism and antioxidant function. Proc Natl Acad Sci USA.
107:7455–7460. 2010. View Article : Google Scholar : PubMed/NCBI
|