Introduction
In recent years, with the rapid development of
radiotherapy technologies, three-dimensional conformal and
intensity-modulated radiotherapy has been widely used for the
treatment of primary hepatocellular carcinoma due to its efficacy
to induce apoptosos or lose its infinite proliferation ability, so
as to control tumor growth and make surrounding normal tissues and
organs less affected by irradiation (1). However, radiation-induced liver injury
following radiotherapy has become the major factor influencing the
radiation tolerance dose (1). Liver
is a type of late-responding tissue, and most of the earlier acute
radiation-induced liver injuries can be repaired. Nonetheless,
acute radiation-induced liver injuries may develop into sub-acute
radiation-induced liver injury should clinically appropriate
treatment not be administered, a phenomenon known as
radiation-induced liver disease (RILD) (2). This disease is difficult to detect due
to its occult clinical manifestations, but progresses until the
failure of liver functions (2).
Thus, earlier detection and diagnosis of acute radiation-induced
liver injury is of great significance for restorative treatment.
The importance of adjustment of the tumor radiotherapy dose has
been previously reported (2). In the
present study, we established a rat model of acute
radiation-induced liver injury, and detected a relationship between
quantitative analysis of contrast-enhanced ultrasound parameters
and pathological parameters indicating severity of liver
injury.
Materials and methods
Animals
Sixty female SD rats, weighing 180–200 g, aged 6
weeks were provided by the Experimental Animal Center of the
Zhejiang Academy of Medical Sciences (Zhejiang, China). The rats
were randomly divided into the control (10 rats) and experimental
(50 rats) groups, and kept on the same normal diet throughout the
experiment.
Acute radiation-induced liver injury
model
The animals were initially anesthetized with 10%
chloral hydrate (300 mg/kg) by intraperitoneal injection and were
fixed in the supine position. The right lobe of the liver was
located with B ultrasound and the radiation field was determined:
the upper boundary was diaphragm, the lower boundary was 3 cm from
the upper one, the medial boundary was right of the spine, but
there was no lateral boundary. The radiation field was ~3×3 cm and
was drawn on the surface. The Siemens PrimusM linear accelerator
and 6 MV-X-ray source skin distance irradiation technique were used
with a source skin distance (SSD) of 80 cm; irradiation depth, 2
cm; irradiation dose rate, 200 cGy/min; total dose of single
radiation, 20 Gy. Ten rats from the injury group and 2 rats from
the control group were randomly selected on days 3, 7, 14, 21 and
28, and underwent liver contrast-enhanced ultrasound (CEUS). The
animals were then sacrificed by decapitation and liver tissue was
removed and dissected for the pathological examination.
Ultrasonography examination
Preparations prior to the examination
After fasting for 8 h, the rats were anesthetized
with 10% chloral hydrate (300 mg/kg) by intraperitoneal injection.
Limbs were fixed in the supine position. The experimenter clamped
the tail of the rat with two fingers to lead to caudal vein
distention. Venipuncture was performed with a scalp acupuncture of
5 and a half. A venous channel was established and maintained.
Instruments and methods
CEUS examination was performed using S2000 color
ultrasonic diagnostic apparatus (Siemens Ultrasonography, Mountain
View, CA, USA). The 9L4 probe was used for fundamental imaging at
7–9 Mhz and for the contrast-enhanced ultrasound imaging at 4 Mhz.
The contrast-tuned imaging (cnTI) was built-in and quantitative
analysis of contrast-enhanced ultrasound imaging software (Contrast
Dynamics) was used. Ultrasonography contrast agent SonoVue (Bracco,
Milan, Italy) was used. The diameter of ultrasonography contrast
agent is far less than the untrasonic wavelength. It can form a new
acoustic interface after entering the blood vessel with the help of
the scattered signal of the micro bubbles, so as to produce a
contrast effect. SonoVue was supplied as a lyophilized powder and
reconstituted with 5 ml of saline to form a homogeneous microbubble
suspension with sulfur hexafluoride encapsulated in phospholipids.
The mechanical index was 0.10 (the mechanical parameters of the
experimental conditions showing consistency and repeatability) and
the volume of contrast agent injected was 0.06 ml. Microbubble
suspension was injected through the caudal vein. Each injection was
followed by a 5 ml saline flush. Liver transverse section showing
liver parenchyma, the inferior vena cava, the abdominal aorta, the
portal vein and other large vessels was considered. CEUS was
performed immediately after contrast medium injection and lasted
for 90 sec. The results were stored in a hard disk for subsequent
offline analysis.
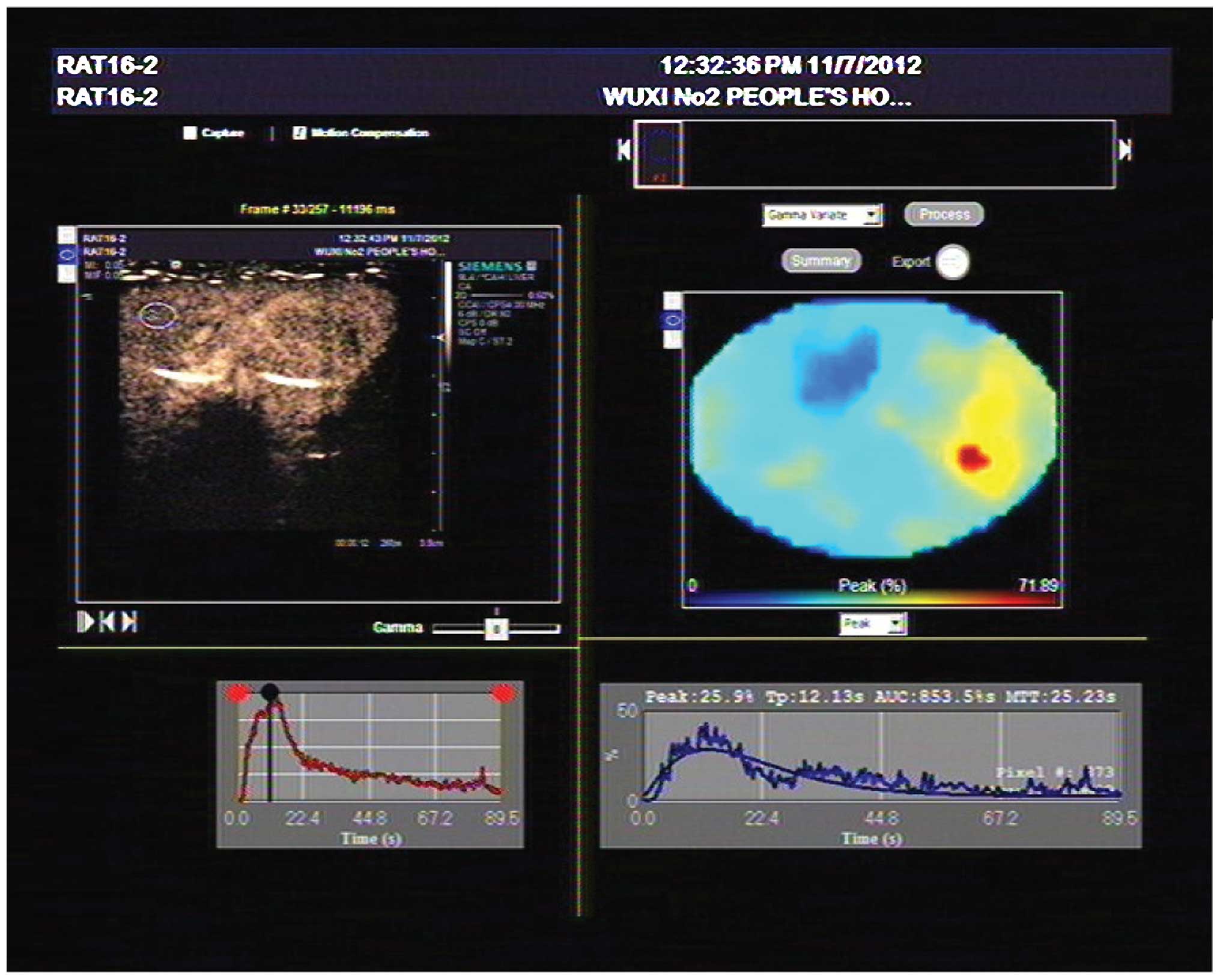
Parameter measurement
Hepatic parenchyma of region of interest (ROI) with
the principle of consistent depth and avoidance of large vessels
were selected. The time-intensity curve (TIC) of liver parenchyma
was drawn automatically. The time to peak (TTP) and peak intensity
(PI) were also analyzed with quantitative analysis of
contrast-enhanced ultrasound imaging software (Fig. 1). Arriving time (AT) was defined as
the time of the emergence of the first contrast agent microbubble
in the vessel. Hepatic artery arriving time (HAAT) and hepatic vein
arriving time (HVAT) were recorded, and hepatic artery to vein
transit time (HA-HVTT) was the difference of the two times.
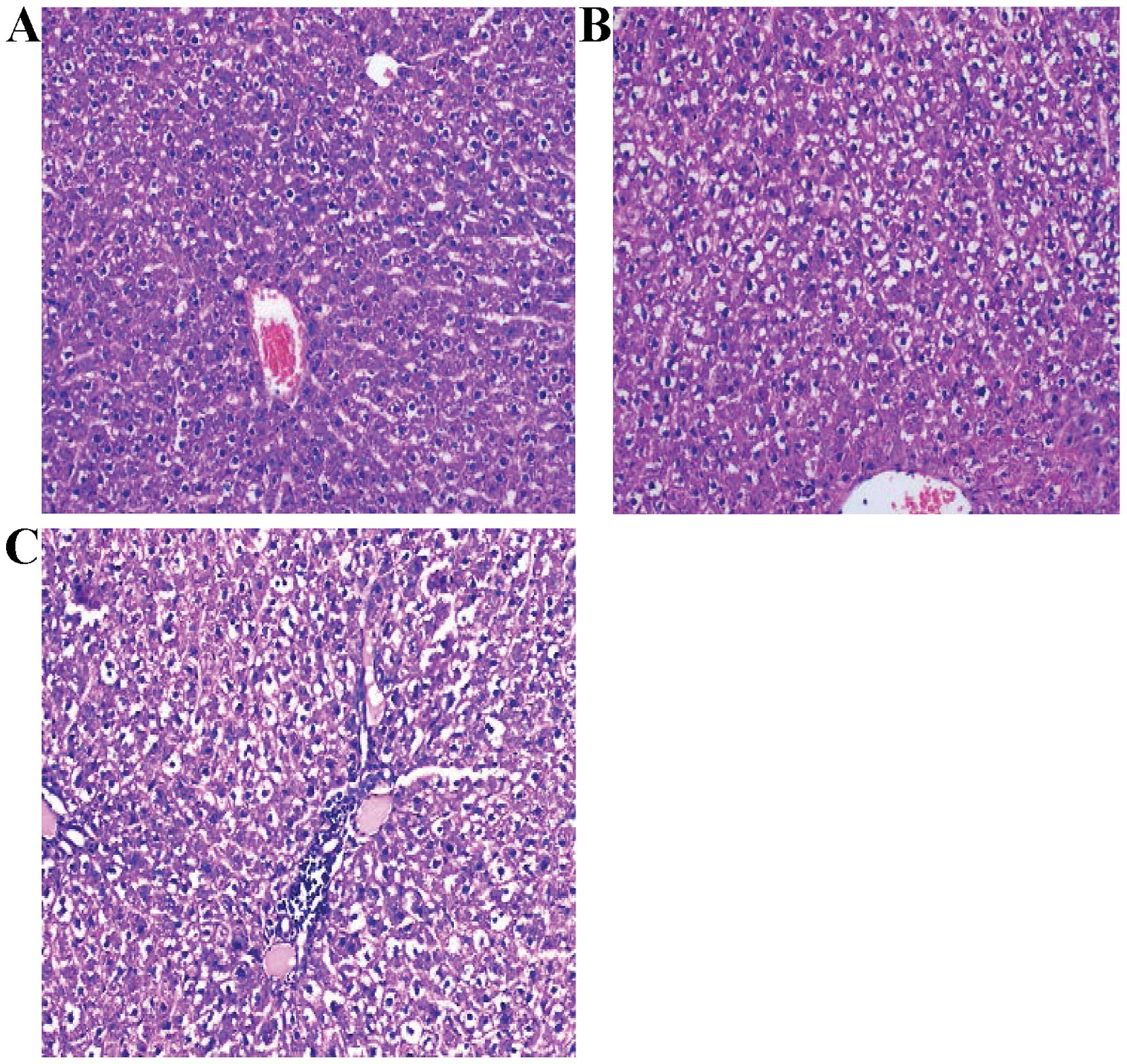
Pathological examination
The pathological sections were prepared with liver
tissue of irradiation region in the right lobe of the liver in rats
and were read using the double-blind method. Pathology grouping
criteria adhered to were: i) Normal control group: normal liver
cells, hepatic lobule structure integrity, and hepatic cord
arranged in neat rows; ii) mild liver injury group: slight swelling
of liver cells, fatty and/or hydropic degeneration, no liver cell
necrosis and hemorrhage, and mild hepatic sinus dilation; iii)
moderate liver injury group: moderate swelling of liver cells,
fatty and/or hydropic degeneration, punctate necrosis of liver
cells or scattered bleeding points, inflammatory cell infiltration
in the portal area, and centrilobular vein and hepatic sinus
dilation; and iv) severe liver injury group: diffused swelling and
degeneration of liver cells, liver cell necrosis and hemorrhage, an
apparent increase in inflammatory cells in the portal area, and
veno-occlusive disease (VOD) of hepatic sinus and centrilobular
veins (Fig. 2).
Statistical analysis
Continuous variables relevant to the normal
distribution are shown as mean ± SD. The univariate analysis of
variance was conducted to compare differences between groups. The
LSD test was used to compare differences between any two groups.
The data were analyzed using SPSS, version 18.0 (IBM, Armonk, NY,
USA). α=0.05 was used for the statistical comparisons. P<0.05
was considered to indicate a statistically significant result.
Results
Pathological results
An acute radiation-induced liver injury model was
successfully established in rats and complete information from 60
animals (including 10 controls) was obtained. Liver biopsy results
showed 10 cases of control, and 50 cases of radioactive liver
injury (16 cases of mild, 22 cases of moderate, and 12 cases of
severe liver injury).
Ultrasonography results
i) Significant differences were observed for PI
(P<0.05) between the control and liver injury groups. Compared
to the mild and moderate liver injury groups, PI was apparently
shorter in the severe liver injury group (P<0.05), with no
significant difference being observed between the mild and moderate
groups.
ii) TTP was significantly different between the
liver injury and control groups (P<0.05). TTP was much longer in
the severe liver injury group compared to the mild and moderate
liver injury groups (P<0.05), while no significant differences
were observed between the mild and moderate groups.
iii) Compared to the control group, HA-HVTT in the
moderate and severe liver injury groups was significantly higher
(P<0.05), whereas no significant differences were detected
between the mild and control groups.
There were significant differences among the
indicators between groups (Tables I
and II). The aggravation of
radiation-induced liver injury was accompanied by the trends of
decreased peak strength, prolonged peak time and reduced
HA-HVTT.
 | Table I.Comparison of contrast-enhanced
ultrasound parameter of the liver radiation-induced injury in
different groups. |
Table I.
Comparison of contrast-enhanced
ultrasound parameter of the liver radiation-induced injury in
different groups.
| Groups | Cases | PI (dB) | TPP (sec) |
|---|
| Normal control
group | 10 | 57.98±8.55 | 17.26±7.43 |
| Mild injury
group | 16 |
41.82±8.69a |
26.49±6.8a |
| Moderate injury
group | 22 |
35.27±14.28a |
28.64±10.77a |
| Severe injury
group | 12 |
26.35±5.52a–c |
41.51±11.23a–c |
| F-value | 17.355 | 12.488 |
|
| P-value | <0.001 | <0.001 |
|
 | Table II.Comparison of HA-HVTT of
radiation-induced liver injury in each group. |
Table II.
Comparison of HA-HVTT of
radiation-induced liver injury in each group.
| Group | Cases | HA-HVTT (sec) |
|---|
| Normal control
group | 10 | 8.12±0.75 |
| Mild injury
group | 16 | 7.5±0.94 |
| Moderate injury
group | 22 |
7.11±0.89a |
| Severe injury
group | 12 | 5.78±0.5a–c |
| F-value | 16.757 |
|
| P-value | <0.001 |
|
Discussion
Since the liver is highly sensitive to radiation,
the radiation-induced liver injury caused by radiotherapy is
difficult to avoid, but has a high degree of reversibility. RILD
occurred 4 weeks after radiotherapy and based on the sub-acute
damage of liver function is known as local radiation-induced
hepatic injury. Previous findings performed on acute
radiation-induced liver injury in animal models differ slightly in
the radiation range, radiation dose, and inspection method
(3). Preliminary results led to
selection of the right lobe of the liver as the irradiation target
area, and was determined as: the upper boundary was diaphragm, the
lower boundary was 3 cm from the upper one, the medial boundary was
right of the spine, and there was no lateral boundary. The
radiation field was ~3×3 cm and was drawn on the surface. Single
dose X-ray stereotactic irradiation was 20 Gy. The observation time
was 1–28 days following irradiation.
VOD is a vein occlusion type of disease which is the
pathological manifestation of radiation-induced liver injury,
characterized by congestion in the central area of the hepatic
lobule and necrosis of the central area in impaired hepatic sinus
under a microscope. The pathological change is a dynamic process,
initially involving a cell dysfunction phase, followed by a
radioactive hepatitis phase and subsequently a liver fibrosis and
cirrhosis phase (3,4). It has been previously shown that the
specific functional abnormality occurs at a very early stage after
irradiation (mostly in the first month) and is known as acute
radiation-induced liver injury (5).
The main manifestations include dilatation of the central venous
and sinus in hepatic lobules as congestion is the main response to
irradiation, and endothelial cell injury throughout the process,
which is the basis of hepatic veno-occlusive lesions. Hepatic sinus
congestion after endothelial cell injury and inflammatory cell
infiltration is the main lesion of early radiation-induced hepatic
injury. The degeneration and necrosis of liver cells are observed
two weeks after irradiation, mainly including eosinophilic necrosis
and punctate necrosis.
The blood supply to normal liver is mainly derived
from the hepatic artery and portal vein, and the terminal branches
of hepatic arterioles and portal vein converge with hepatic vein in
sinus hepaticus (6). The
arteriovenous direct path is closed under normal circumstances;
however, it opens in some liver diseases manifested as hepatic
hemodynamic changes (6). The
microbubble ultrasound contrast agent currently used possesses
hemodynamic characteristics that are similar to red blood cells,
and can filter through pulmonary circulation to reach microvascular
organs and lesions allowing images to be captured (6). The ultrasound contrast agent can
evaluate the perfusion of the organs and tumor from the perspective
of microcirculation, and reflect the changes of organic
hemodynamics and eventually contribute to the understanding of
organ function (7).
The present study indicates that the hepatic
hemodynamic changes have important effects on the liver blood
perfusion in the process of the formation and development of acute
radiation-induced liver injury. The TIC curve of the liver
parenchyma contrast-enhanced ultrasound showed that with the
aggravation of the liver injury, PI continued to decrease and TP
was gradually extended with statistically significant differences.
Possible reasons for this finding include, fat degeneration and
swelling of liver cells occurring in the early phase of acute
radiation-induced liver injury may lead to compression and
degeneration of the hepatic duct structure and an increase in the
resistance, resulting in the reduction of liver parenchyma blood
perfusion (6,7). Additionally, with the development of
hepatic injury, liver cell necrosis, inflammatory cell infiltration
and hepatic sinusoid capillarization occurred, which eventually
leads to the formation of blood stasis in the hepatic sinusoids,
the increase of sinus pressure and further formation of
intrahepatic shunt, resulting in a blood flow reduction in the
liver parenchyma (8). Furthermore,
formation of fiber spacing due to injury of the oppressed
sub-lobular vein, central vein and hepatic venous sinus leads to
disturbance of the recovery of the portal vein, an increase in
portal vein pressure, and reduced liver perfusion, resulting in a
decrease in the extension of PI and TP. The abovementioned three
changes are increased with aggravation of liver injury.
In the present study, HA-HVTT was gradually reduced
along with the severity of liver damage, and these differences were
statistically significant in the inter-group comparison. Possible
reasons for this finding include the fact that in the early phase
of acute liver injury, liver sinusoidal endothelial cells are
impaired; endothelial fenestrae are reduced or gradually dissipate;
and a continuous structure-like capillary endothelium.is formed
beneath the endothelial, which leads to the formation of hepatic
sinusoid capillarization. These changes form the exchange barrier
between substances in liver cells and oxygen and eventually lead to
liver cell atrophy and hepatic sinusoidal collapse. Blood flow in
hepatic sinusoid gradually bypasses the damaged liver tissue and
enters the hepatic venous system directly. As a result, blood
circulation is accelerated in the liver and HA-HVTT is shortened
(9–11). In the process of liver injury, a
variety of neurotransmitters responding to the course such as
histamine, serotonin, and vasoactive factors, including vascular
endothelium-derived relaxing factor, cause damage to the hepatic
sinusoids as well as hepatic vein system and the disturbance of
liver microcirculation. Therefore, the hepatic blood flow at a high
level of circulation, which accelerates the distribution of
contrast agent microbubbles in vivo (12–14).
Additionally, with the aggravation of liver injury, the formation
of cytokines containing transforming growth factor-β1 (TGF-β1),
platelet-derived growth factor (PDGF), and connective tissue growth
factor (CTGF), promotes collagen synthesis by hepatic stellate
cells (HSCs) and accelerates the transformation from HSCs to
fibroblast cells. Proliferous fibroblasts and their connection with
fiber bundles around them form a fibrous septum, in which the blood
vessels form rami communicantes among hepatic arteries, portal
veins and hepatic veins. Blood bypasses due to rami communicantes,
resulting in enhancement of HVAT and shortening of HA-HVTT
(8). However, radiation-induced
liver injury is a gradual process, in which the expression of
cytokines is not obvious in the early stages and gradually
increases after 2 weeks. No significant differences were observed
in the mild and control groups (P>0.05). Thus, HA-HVTT as a
sensitive index to evaluate the severity of liver fibrosis is
important in the early diagnosis of moderate and severe acute
radiation-induced liver injury.
Radiation-induced liver injury is a progressive
process, and early detection and early intervention in the acute
period is of great significance to the recovery treatment of the
liver. Contrast-enhanced ultrasound as a non-invasive, simple and
highly repetitive examination can more intuitively reflect the
changes of the microvascular circulation dynamics. The circulatory
physiology of the contrast agent in liver is analyzed to identify
the sensitive parameters applied in the quantitative diagnosis of
acute radiation-induced liver injury. The present findings have
shown that in the quantitative analysis of contrast-enhanced
ultrasonography, PI, TP and HA-HVTT may be used as important
reference indices for the early diagnosis of acute
radiation-induced liver injury.
Acknowledgements
This study was supported by the Science and
Technology Development Foundation of Nanjing Medical University
(no. 2011NJMU1234) and the Medical Science and Technology
Development Fund Management Center of Wuxi Hospital (no.
YGM1126).
References
|
1
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bujold A and Dawson LA: Stereotactic
radiation therapy and selective internal radiation therapy for
hepatocellular carcinoma. Cancer Radiother. 15:54–63. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng RY, Wang DW, Xu ZH, et al: Dynamic
observation of the process of radiation-induced liver fibrosis.
Chin J Radiol Med Prot. 14:243–245. 1994.(In Chinese).
|
|
4
|
Fajardo LF: The pathology of ionizing
radiation as defined by morphologic patterns. Acta Oncol. 44:13–22.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang SX, Zhu XD, Lu HJ, Pan CY, Li FX,
Huang QF, Wang AY, Chen L, Fu XL and Jiang GL: Hypofractionated
three-dimensional conformal radiation therapy for primary liver
carcinoma. Cancer. 103:2181–2188. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li N, Ding H, Lin XY, Fan PL, Xu C and
Wang WP: Correlations between quantitative parameters of
contrast-enhanced ultrasonography and histopathologic stage of
liver fibrosis. Chin J Ultrason. 18:942–945. 2009.(In Chinese).
|
|
7
|
Zhang L, Duan YY, Zhang Y, et al: Primary
research on diagnosis of liver fibrosis stage by perfusion dynamics
of ultrasound contrast agent. Chin J Ultrason. 16:616–619. 2007.(In
Chinese).
|
|
8
|
Jie LM, Guo QY, Liu X, et al: The
experimental study on the evaluation of liver fibrosis using
contrast enhanced sonography. Chin J Ultrason. 17:809–812. 2008.(In
Chinese).
|
|
9
|
Shen JK, Jiang Z, Zhou J, et al:
Experimental study on the early effects of radiation induced liver
injury: the number and the function evaluation of Kupffer cells.
Chin J Radiol Med Prot. 6:5532004.(In Chinese).
|
|
10
|
Zhou YX: The Modern Diagnosis and
Treatment of Liver Cirrhosis. Beijing: People's Medical Publishing
House. 142000.
|
|
11
|
Lu X, Xu GF, Chen WH, et al: The dynamic
changes of hepatic sinusoid capillarization during the formation of
hepatic fibrosis in rats. Shi Jie Hua Ren Xiao Hua Za Zhi.
8:1415–1416. 2000.(In Chinese).
|
|
12
|
Li R and Hua X: Experimental study on the
diagnostic value of the appearing time of ultrasound contrast agent
in hepatic vein in early liver cirrhosis. Chongqing Med J.
33:1684–1685. 2004.
|
|
13
|
Wang CP and Han J: Cellular and molecular
mechanism of portal hypertension. Infect Dis Info. 18:117–119.
2005.
|
|
14
|
Tan XQ, Qian LX, Zhang XL, et al:
Contrast-enhanced ultrasound in diagnosis of early hepatic
cirrhosis. Chin J Ultrason. 17:1048–1050. 2008.(In Chinese).
|