Introduction
Osteosarcoma (OS) is the most common malignant bone
tumor, which typically develops in children and adolescents. The
estimated annual incidence worldwide is 10 patients per million
people, with the majority of cases occurring in patients under 20
years of age (1,2). OS has a high propensity for metastasis,
and ~80% of all metastases occur in the lungs (3). The 5-year survival rate for patients
with primary OS tumors has greatly improved from ~20 to 70% since
the introduction of chemotherapy in the 1970s; however, the
survival rate for patients with metastatic OS is estimated to be
<30%, based on the current therapeutic strategies (4). Conventional chemotherapy remains the
standard method for treating patients with OS; however, patients
with metastatic OS have previously demonstrated poor responses to
chemotherapeutic drugs (5).
Therefore, developing novel strategies to improve the treatment of
patients with metastatic OS will require elucidation of the
mechanisms underlying metastasis in patients with OS, and its
corresponding biological processes.
Whole genome expression and proteomic analyses are
able to detect the abnormal expression of genes in OS samples, and
thus permit the identification of various metastasis-associated
targets (6). It has previously been
reported that thatezrin, a member of the ezrin-radixin-moesin
family, is highly expressed in metastatic OS cell lines, and is
required for OS metastasis, due to its functional connection with
the actin cytoskeleton (7). In
addition, the expression of Fas has previously been associated with
OS metastasis, and an inhibitor of the Fas pathway (c-FLIP) has
been developed as a potential treatment for patients with lung
metastasis (8). Nagao-Kitamoto et
al (9) demonstrated that
knockdown of GLI family zinc finger 2 (GLI2) using RNA interference
was able to significantly attenuate the migration and invasion of
OS cells; thus suggesting that inhibition of GLI2 may be a
potential strategy for the treatment of patients with metastatic
OS. Furthermore, numerous microRNAs (miRNAs) have been implicated
in the OS metastatic process, including miRNA-20a, miRNA-143,
miRNA-202 and miRNA-9 (10–12).
In the present study, a high-throughput method was
used to identify factors associated with the OS metastatic process,
and potential novel targets that may be considered as biomarkers
for the treatment of patients with metastatic OS. The aims of the
present study were to identify metastasis-associated genes for OS
tumor and to extend our mechanistic understanding of metastatic
processes in OS cells. The results may provide new insight into
therapeutic strategy for OS patients.
Materials and methods
Data collection
The Gene Expression Omnibus (GEO) database
(http://www.ncbi.nlm.nih.gov/geo/) was
searched, and microarray expression data (GSE14359) from two groups
was obtained, which included five non-metastatic OS samples and
four OS lung metastases tumor samples. Each sample had two
replicates, and the data were analyzed using the Affymetrix Human
Genome U133A Array (Affymetrix, Inc., Santa Clara, CA, USA).
Unprocessed data sets (.cel files) were collected for further
analysis. The probe annotation files were downloaded for further
research.
Data processing and filtering
Numerous algorithms have been developed in order to
quantify microarray signals, and the present study applied Guanine
Cytosine Robust Multi-Array Analysis (13). The normalization process consisted of
three steps: i) Model-based background correction; ii) quantile
normalization; and iii) summarizing.
In order to filter out uninformative data, including
control probe sets and other internal controls, as well as genes
whose expression levels were uniformly close to the background
detection levels, the nsFilter function from the genefilter package
in R programming language was used (14). However, the filter was unable to
remove probe-sets without Entrez Gene identifiers or with identical
Entrez Gene identifiers.
Analysis of differentially expressed
genes (DEGs)
Statistical comparisons between the two groups were
conducted. Limma in the nsFilter function from the genefilter
package in R programming language version 3.1.1 was used. to
identify genes that were significantly differentially expressed
between the two groups (15). For
probes with identical Entrez Gene identifiers, only the probes
occupying the biggest variance were retained for further DEG
analysis. In addition, only DEGs with a log2 (fold
change) >1.5 and an adjusted P<0.01 were recognized as
statistically significant. The adjusted P-value was obtained by
applying Benjamini and Hochberg's false discovery rate correction
on the original P-value (16). The
fold change threshold was selected based on the requirement for
focusing on only genes that were significantly differentially
expressed.
Hierarchical clustering
Hierarchical clustering was conducted using the DEGs
in order to classify the samples according to their gene expression
profiles and observe global alterations in gene expression patterns
(17). The DEGs were classified into
specific biological processes (GO terms) and KEGG pathways, which
were represented in heat maps. To be specific, DEG expression
values were used in the hierarchical clustering analysis using
gplots software (18).
GO and KEGG pathway analysis
The R packages GO.db (19) and KEGGREST (20) were used to detect GO categories and
KEGG pathways that were significantly over represented in the DEGs
compared with the whole genome. P<0.01 indicated significantly
enriched GO terms, whereas P<0.05 indicated significantly
enriched KEGG pathways.
Construction of biological
networks
Protein-protein interaction (PPI) databases were
downloaded from the Human Protein Reference Database (HPRD;
http://www.hprd.org/) (21), the Biological General Repository for
Interaction Datasets (BioGRID; http://thebiogrid.org/) (22), and the Human Protein-Protein
Interaction Prediction database (PIPs; http://www.compbio.dundee.ac.uk/www-pips/) (23). The pair interactions, which were
included in all three databases, were selected to be included in
the curated PPI database. A total of 56,1405 pair interactions were
identified in the PPI database. Cytoscape version 3.1.1 (http://www.cytoscape.org/) was used to construct
interaction networks. Interacting gene pairs from the curated PPI
database were imported as a stored network. Following functional
enrichment analysis, the DEGs specified in significantly enriched
GO terms and KEGG pathways were mapped to corresponding networks in
order to analyze the interaction relationships.
Results
Analysis of DEGs
A comparison of the gene expression levels between
metastatic and non-metastatic OS tumor samples was conducted using
microarray analysis. A log2 fold-change >1.5 and an
adjusted P<0.01 indicated that a gene was significantly
differentially expressed. A total of 282 DEGs were obtained, of
which 212 were upregulated and 70 were downregulated (Table I). The top 50 upregulated and
downregulated DEGs are presented in Table II. Of the DEGs, at least eight genes
may have been associated with OS metastasis to the lungs, including
the homeobox only protein (HOPX), lysosomal-associated membrane
protein-3 (LAMP3), chemokine (C-C motif) ligand-18 (CCL18),
carcinoembryonic antigen-related cell adhesion molecule-6
(CEACAM6), keratin-19 (KRT19), prostaglandin-endoperoxide
synthase-2 (PTGS2), clusterin (CLU), and nucleoside diphosphate
kinase-1 (NME1).
 | Table I.Statistical distribution of
differentially expressed genes. |
Table I.
Statistical distribution of
differentially expressed genes.
| Probes and
genes | Probes | Genes |
|---|
| Alla | 10,348 | 7,323 |
| Differentially
expressedb | 347 | 282 |
|
Upregulated | 265 | 212 |
|
Downregulated | 82 | 70 |
 | Table II.The top 50 upregulated and
downregulated differentially expressed genes (DEGs) in the
metastatic osteosarcoma (OS) tissue samples, as compared with the
non-metastatic OS tissue samples. |
Table II.
The top 50 upregulated and
downregulated differentially expressed genes (DEGs) in the
metastatic osteosarcoma (OS) tissue samples, as compared with the
non-metastatic OS tissue samples.
| Gene symbol | Log2
(fold-change) | P-value | Adjusted
P-value |
|---|
| Upregulated |
|
|
|
|
SFTPC | 10.13 | 2.93E-22 | 3.26E-18 |
|
SFTPA2 | 9.46 | 1.58E-17 | 3.50E-14 |
|
SFTPB | 8.35 | 2.01E-16 | 3.72E-13 |
|
SCGB1A1 | 6.43 | 3.23E-07 | 6.93E-05 |
|
SFTPD |
6.27 | 1.63E-09 | 1.21E-06 |
|
CYP4B1 |
5.46 | 2.33E-08 | 8.91E-06 |
|
TACSTD2 |
5.44 | 4.06E-09 | 2.60E-06 |
|
HOPX |
5.22 | 4.46E-11 | 5.50E-08 |
|
LAMP3 |
5.16 | 6.76E-09 | 3.58E-06 |
|
SERPINA1 |
5.01 | 1.73E-10 | 1.60E-07 |
|
CLDN18 |
5.00 | 1.70E-10 | 1.60E-07 |
|
CCL18 |
4.83 | 2.48E-08 | 8.91E-06 |
|
C4BPA |
4.52 | 6.24E-07 | 1.05E-04 |
|
GPRC5A |
4.43 | 9.93E-08 | 2.56E-05 |
|
SLC34A2 |
4.32 | 1.43E-09 | 1.13E-06 |
|
ADH1B |
4.21 | 5.01E-06 | 4.19E-04 |
|
AGER |
4.19 | 6.63E-05 | 2.18E-03 |
|
CEACAM6 |
4.13 | 2.23E-07 | 5.17E-05 |
|
HPGD |
3.95 | 5.12E-07 | 9.01E-05 |
|
MUC1 |
3.87 | 2.49E-08 | 8.91E-06 |
|
CXCL2 |
3.74 | 1.76E-04 | 3.94E-03 |
|
CDH1 |
3.71 | 4.49E-09 | 2.62E-06 |
|
TMEM100 |
3.56 | 6.11E-05 | 2.09E-03 |
|
TNS1 |
3.52 | 3.32E-07 | 6.96E-05 |
|
SLPI |
3.43 | 4.91E-04 | 7.11E-03 |
|
FOS |
3.33 | 3.80E-05 | 1.59E-03 |
|
MFAP4 |
3.33 | 1.01E-08 | 4.47E-06 |
|
MARCO |
3.27 | 1.69E-04 | 3.85E-03 |
|
CAV1 |
3.18 | 3.83E-07 | 7.87E-05 |
|
KRT19 |
3.17 | 3.03E-06 | 3.01E-04 |
|
MEST |
3.15 | 1.95E-04 | 4.13E-03 |
|
IGJ |
3.11 | 3.34E-04 | 5.65E-03 |
|
AQP3 |
3.10 | 6.90E-07 | 1.09E-04 |
|
KRT7 |
3.09 | 4.65E-07 | 8.85E-05 |
|
EMP2 |
3.09 | 3.16E-06 | 3.06E-04 |
|
ICAM1 |
3.08 | 6.29E-08 | 1.84E-05 |
|
FCN3 |
3.05 | 3.34E-04 | 5.65E-03 |
|
C1orf116 |
3.05 | 4.00E-05 | 1.61E-03 |
|
PTGS2 |
3.02 | 2.48E-06 | 2.62E-04 |
|
CD52 |
3.01 | 8.12E-06 | 5.94E-04 |
|
CLIC5 |
3.00 | 1.37E-04 | 3.50E-03 |
|
CLIC3 |
2.99 | 8.67E-05 | 2.67E-03 |
|
VWF |
2.98 | 1.78E-04 | 3.95E-03 |
|
CAV2 |
2.97 | 1.92E-06 | 2.21E-04 |
|
CLU |
2.92 | 1.15E-04 | 3.13E-03 |
|
APOC1 |
2.89 | 1.98E-04 | 4.18E-03 |
|
CTSH |
2.88 | 3.47E-05 | 1.51E-03 |
|
INHBA |
2.87 | 2.96E-05 | 1.37E-03 |
|
TPSAB1 |
2.87 | 2.02E-05 | 1.09E-03 |
|
PTGDS |
2.87 | 1.17E-05 | 7.63E-04 |
| Downregulated |
|
|
|
|
MMP13 |
-4.21 | 1.61E-06 | 2.00E-04 |
|
PRAME | −3.72 | 5.97E-05 | 2.07E-03 |
|
UCHL1 | −3.68 | 1.85E-04 | 4.01E-03 |
|
RRM2 | −2.78 | 3.09E-06 | 3.01E-04 |
|
KIAA0101 | −2.72 | 3.62E-06 | 3.34E-04 |
|
ITGA4 | −2.71 | 3.65E-11 | 5.06E-08 |
|
ADAM12 | −2.60 | 2.71E-04 | 4.96E-03 |
|
CCNB1 | −2.57 | 4.13E-04 | 6.38E-03 |
|
GOLM1 | −2.55 | 5.02E-08 | 1.51E-05 |
|
ADAMTS5 | −2.44 | 2.66E-07 | 6.02E-05 |
|
SLC6A15 | −2.43 | 1.78E-04 | 3.95E-03 |
|
MAD2L1 | −2.39 | 2.95E-04 | 5.19E-03 |
|
NEK2 | −2.35 | 6.61E-05 | 2.18E-03 |
|
NCALD | −2.32 | 5.23E-04 | 7.38E-03 |
|
CDK1 | −2.28 | 5.58E-04 | 7.68E-03 |
|
BUB1 | −2.27 | 5.15E-07 | 9.01E-05 |
|
ACP1 | −2.15 | 2.27E-05 | 1.16E-03 |
|
TRIP13 | −2.15 | 3.25E-07 | 6.93E-05 |
|
BCAT1 | −2.14 | 5.19E-05 | 1.92E-03 |
|
RAD51AP1 | −2.09 | 2.95E-05 | 1.37E-03 |
|
IGF2BP3 | −2.07 | 7.05E-04 | 8.83E-03 |
|
CCNB2 | −2.05 | 6.36E-04 | 8.31E-03 |
|
RFC5 | −2.04 | 2.75E-08 | 9.24E-06 |
|
SRPK1 | −2.03 | 6.66E-04 | 8.53E-03 |
|
AURKA | −2.03 | 3.16E-05 | 1.44E-03 |
|
HIST3H2A | −2.00 | 1.02E-04 | 2.94E-03 |
|
TYMS | −1.99 | 2.26E-04 | 4.48E-03 |
|
PHB | −1.99 | 2.06E-07 | 4.97E-05 |
|
KIF20A | −1.91 | 7.55E-04 | 9.22E-03 |
|
RMDN1 | −1.88 | 2.41E-04 | 4.62E-03 |
|
NME1 | −1.87 | 5.56E-06 | 4.50E-04 |
|
MRPL42 | −1.86 | 7.13E-08 | 2.03E-05 |
|
DNAJC12 | −1.86 | 6.06E-04 | 8.07E-03 |
|
NPM3 | −1.86 | 1.08E-04 | 3.04E-03 |
|
MRPS16 | −1.85 | 1.21E-06 | 1.65E-04 |
|
PFKM | −1.85 | 2.62E-04 | 4.85E-03 |
|
NDUFS1 | −1.85 | 7.34E-08 | 2.04E-05 |
|
HSPE1 | −1.84 | 3.04E-05 | 1.39E-03 |
|
NUSAP1 | −1.83 | 4.10E-04 | 6.38E-03 |
|
RPP40 | −1.81 | 1.22E-04 | 3.21E-03 |
|
ALG13 | −1.80 | 8.54E-05 | 2.64E-03 |
|
EEF1E1 | −1.80 | 1.48E-05 | 8.69E-04 |
|
MRPL35 | −1.79 | 3.93E-08 | 1.26E-05 |
|
HMMR | −1.79 | 1.08E-06 | 1.51E-04 |
|
UQCRFS1 | −1.77 | 2.74E-04 | 4.97E-03 |
|
TWF1 | −1.77 | 2.42E-06 | 2.58E-04 |
|
GGH | −1.71 | 5.79E-05 | 2.06E-03 |
|
OIP5 | −1.70 | 4.83E-05 | 1.83E-03 |
|
MCTS1 | −1.69 | 8.91E-08 | 2.36E-05 |
|
CEP55 | −1.67 | 2.94E-06 | 2.96E-04 |
Construction of biological
networks
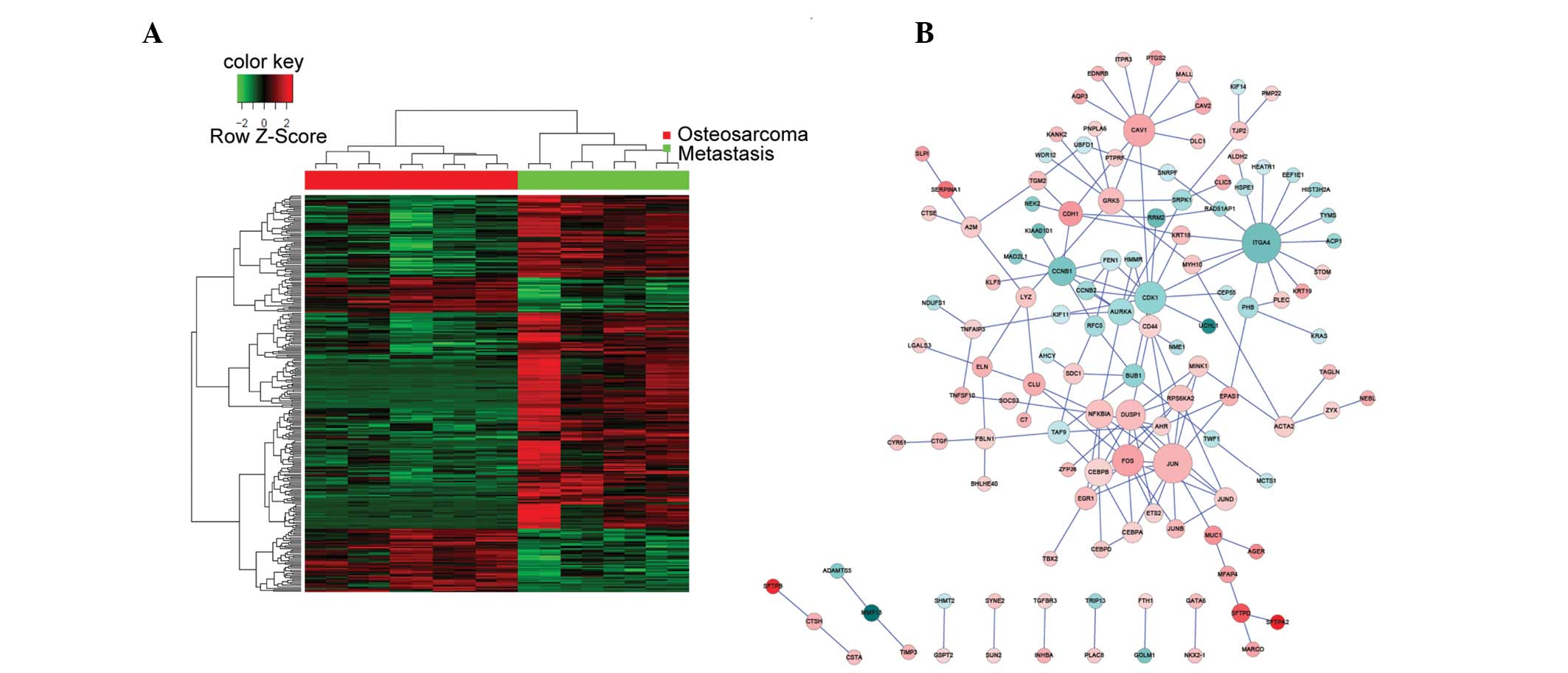
The non-metastatic and metastatic OS tumor samples
had different gene expression profiles, as demonstrated by the
heatmap representing the hierarchical clustering of all DEGs
(Fig. 1A). Biological networks for
upregulated and downregulated DEGs were constructed according to
the protein-protein interactions identified in the HPRD, BIOGRID,
and PIP databases (Fig. 1B). The
majority of proteins were involved in >1 sub-networks. A total
of four genes: JUN, CAV1, NFKB1A and ITGA4, were consistently
over-represented in the networks; thus suggesting that they may
contribute to the OS metastatic process. Of the four genes, JUN,
CAV1 and NFKBIA were upregulated in the metastatic OS tumor
samples, as compared with the non-metastatic OS tumor samples.
Conversely, ITGA4 was downregulated in the metastatic OS tumor
samples, as compared with the non-metastatic OS tumor samples. The
network of all the DEGs was too complex to allow elucidation of the
various functions of the sub-networks; thus requiring further
analysis.
GO and KEGG pathway analysis
A GO and KEGG pathway analysis was performed for the
DEGs. A total of 529 GO terms and 10 KEGG pathways were
over-represented in the metastatic OS tumor samples, as compared
with the non-metastatic OS tumor samples (Table III). P<0.01 indicated
significantly enriched GO terms, whereas P<0.05 indicated
significantly enriched KEGG pathways. The five most significantly
enriched GO terms included: Cell proliferation; response to
external stimulus; positive regulation of biological process; cell
migration; and cellular component organization or biogenesis
(Table IV). The most significantly
enriched KEGG pathways included: Extracellular matrix
(ECM)-receptor interaction; cell adhesion molecules; complement and
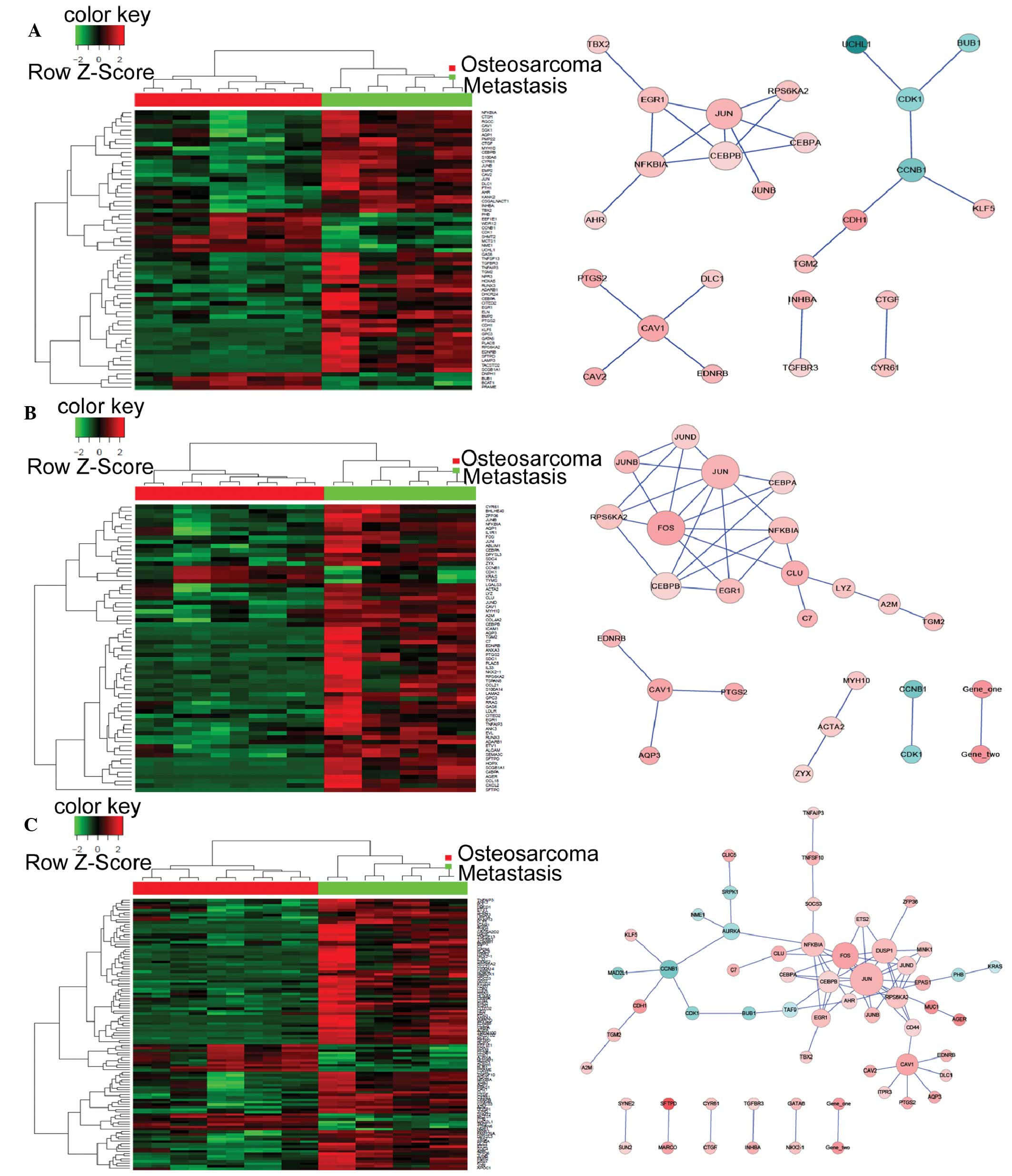
coagulation cascades; and osteoclast differentiation (Table V). Heat maps and biological networks
of the five GO terms and the top KEGG pathways are presented in
Figs. 2 and 3, respectively. The network analysis
identified four genes that may be involved in the molecular events
associated with the metastasis of OS cells to the lungs: JUN, CAV1,
NFKBIA and ITGA4; thus suggesting that these genes may be potential
targets for the treatment of patients with metastatic OS.
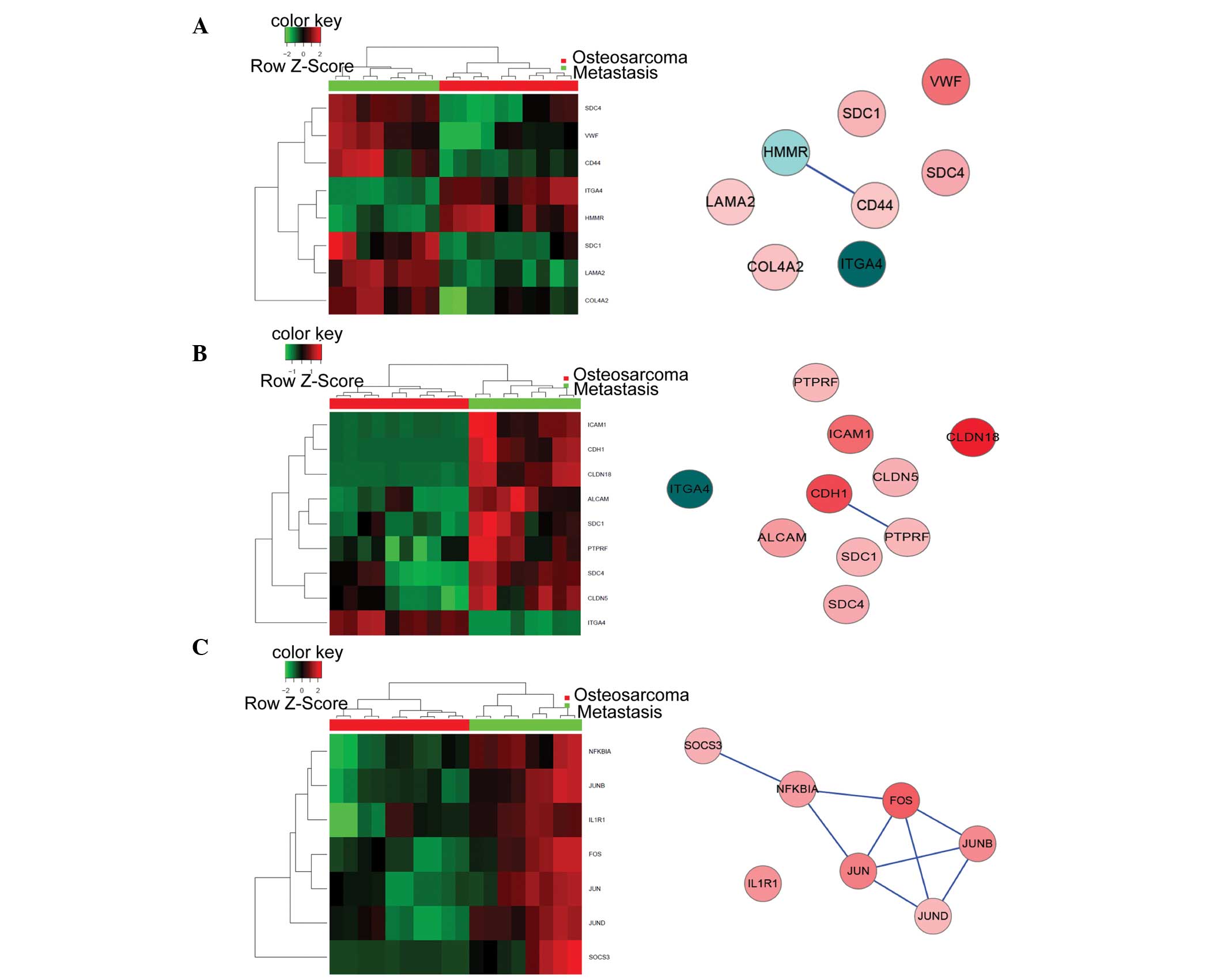
 | Figure 2.Heat maps (left) and corresponding
biological networks (right) for the five most significantly altered
GO biological processes. (A) Cell proliferation (GO:0008283), (B)
response to external stimulus (GO:0009605), and (C) positive
regulation of biological process (GO:0048518). Red and green in the
heat maps indicate high and low relative expression levels,
respectively. The red/pink and blue coloring in the networks
indicate upregulated and downregulated expression levels,
respectively. The darker the color, the greater the gene expression
levels were altered between the metastatic and non-metastatic
osteosarcoma tissue samples. GO, gene ontology. (D) Cell migration
(GO:0016477) and (E) cellular component organization or biogenesis
(GO:0071840). Red and green in the heat maps indicate high and low
relative expression levels, respectively. The red/pink and blue
coloring in the networks indicate upregulated and downregulated
expression levels, respectively. The darker the color, the greater
the gene expression levels were altered between the metastatic and
non-metastatic osteosarcoma tissue samples. GO, gene ontology. |
 | Table III.Over-represented GO biological
processes and KEGG pathways. |
Table III.
Over-represented GO biological
processes and KEGG pathways.
| Analysis | P-value | Number |
|---|
| GO BP | <0.01 | 529 |
| KEGG pathways | <0.05 | 10 |
 | Table IV.Significantly altered Gene Ontology
(GO) terms. |
Table IV.
Significantly altered Gene Ontology
(GO) terms.
| GO-ID | P-value | Count | Term |
|---|
| GO:0008283 | 2.74E-09 | 63 | Cell
proliferation |
| GO:0009605 | 6.13E-09 | 66 | Response to
external stimulus |
| GO:0048518 | 1.75E-08 | 110 | Positive regulation
of biological process |
| GO:0016477 | 7.80E-08 | 41 | Cell migration |
| GO:0071840 | 8.03E-08 | 125 | Cellular component
organization or biogenesis |
 | Table V.Significantly altered Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways. |
Table V.
Significantly altered Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathways.
| KEGG-ID | P-value | Count | Term |
|---|
| 04512 | 3.30E-04 | 8 | Extracellular
matrix-receptor interaction |
| 04514 | 1.59E-03 | 9 | Cell adhesion
molecules |
| 04610 | 2.82E-03 | 6 | Complement and
coagulation cascades |
| 04380 | 1.61E-02 | 7 | Osteoclast
differentiation |
Discussion
OS is the most common malignant bone tumor in
children and adolescents. The prognosis for patients with OS is
affected by whether or not metastasis to the lungs has occurred;
thus highlighting the importance of developing novel therapeutic
strategies for the treatment of patients with metastatic OS.
Numerous genes associated with metastasis have previously been
described for other types of cancer (24); however, the molecular mechanisms
underlying the metastasis of OS are currently not well understood.
The present study aimed to investigate the underlying metastatic
processes of OS in order to identify potential biomarkers for the
treatment of patients with metastatic OS.
The present study compared the gene expression
profiles of non-metastatic and metastatic OS tissue samples, using
microarray analysis. A total of 282 DEGs, including 212 upregulated
and 70 downregulated DEGs, were identified, all of which had
>1.5-fold change in gene expression levels. In addition,
significantly enriched GO terms and KEGG pathways were analyzed, in
order to identify significantly altered molecular events that were
associated with metastasis, and biological networks were
constructed to screen for candidate metastasis-associated genes,
which may provide potential therapeutic targets for the treatment
of patients with metastatic OS.
According to previous studies, a number of the DEGs
identified in the present study may be associated with OS
metastatic processes, including HOPX, LAMP3, CCL18, CEACAM6, KRT19,
PTGS2, CLU and NME1. The majority of these genes, with the
exception of NME1, were upregulated in the metastatic OS tissue
samples, as compared with the non-metastatic OS tissue samples.
HOPX contains a homeobox-like domain lacking DNA
binding properties due to the loss of required conserved residues.
HOPX is a core regulator of epigenetics, and its aberrant
expression has previously been associated with the progression of
cancer (25). In addition, HOPX has
been demonstrated to be involved in the metastatic process of
sarcoma cells, and its knockdown decreased cell motility and
metastasis formation in vitro and in vivo (26). The present study detected
upregulation of HOPX in metastatic OS samples, as compared with in
non-metastatic OS tissue samples; thus suggesting that upregulation
of HOPX may accelerate metastasis in patients with OS.
LAMP3 is a member of the LAMP family of proteins and
is recurrently upregulated in cancer cells (27). Previous studies have demonstrated an
association between cell migration and LAMP3 expression in some
solid tumors, including breast cancer (28) and cervical cancer (29). Furthermore, overexpression of LAMP3
has been associated with an enhanced metastatic potential in a
cervical xenograft model (29).
Alongside the findings of the present study, these results
indicated that LAMP3 may promote the mobility of OS tumor
cells.
It has previously been suggested that chemokines in
the tumor microenvironment have an important role in tumor
progression and metastasis (30).
CCL18 is a small cytokine predominantly produced by the innate
immune system. Li et al (31)
suggested that CCL18 was able to induce breast cancer metastasis
via phosphorylation of protein tyrosine kinase-2 and proto-oncogene
tyrosine-protein kinase, and concomitant downstream signaling
(31). In the present study, CCL18
was demonstrated to be overexpressed in the metastatic OS tumor
samples, as compared with the non-metastatic OS tumor samples; thus
suggesting that CCL18 may have a potential role in OS
metastasis.
CEACAM6 belongs to a family of carcinoembryonic
antigen cell adhesion molecules, and has functions in various
biological processes, including cancer progression, inflammation,
angiogenesis and metastasis (32).
Previous studies have detected an association between CEACAM6
expression and poor prognosis of patients with primary cancers. As
a marker of cancer progression and metastasis, the CEACAM protein
family is considered to have high therapeutic value. Furthermore,
specific monoclonal antibodies against CEACAM1 and CEACAM6 have
been developed and have demonstrated their potential in the
treatment of numerous types of cancer (33). The results of the present study
demonstrated that CEACAM6 was upregulated in the metastatic OS
samples, as compared with the non-metastatic OS samples; thus
suggesting that CEACAM6 may be considered a potential therapeutic
target for the treatment of patients with metastatic OS.
KRT-19 is a member of the keratin family, which is
responsible for maintaining the structural integrity of epithelial
cells. The expression of KRT-19 has previously been associated with
chemoresistance, and was demonstrated to confer an invasive
potential on human hepatocellular carcinoma cells (HCC) (34). In the present study, KRT-19 was
upregulated in the metastatic OS tissue samples, as compared with
the non-metastatic OS tissue samples; thus suggesting that KRT-19
may have conferred an invasive ability on OS tumor cells.
PTGS2, which is also known as cyclooxygenase 2,
catalyzes the conversion of arachidonic acid and O2 to
prostaglandin H2, which is an important precursor in prostanoid
biosynthesis. The overexpression of PTGS2 has been associated with
the pathogenesis and cell mobility of various tumors, including
Ewing sarcoma (35) and osteosarcoma
(36,37). The selective PTGS2 inhibitors,
celecoxib and meloxicam, have previously been demonstrated to
inhibit cell proliferation and invasion in vitro and in
vivo (35,38). In line with previous studies,
upregulation of PTGS2 in the present study may have been associated
with initiation of the OS metastatic process.
CLU is a 75–80 kDa heterodimeric protein, which is
involved in the clearance of cellular debris and apoptosis
(39). Furthermore, a role for CLU
in tumor invasion has previously been reported for various cancer
types; its downregulation via short hairpin RNA reduced migratory
ability, whereas upregulation of CLU was associated with increased
cell invasion in HCC (40). In the
present study, CLU expression levels were upregulated in the
metastatic OS tissue samples, as compared with the non-metastatic
OS tissues samples; thus suggesting that CLU may increase the
metastatic potential of OS.
NME1 exerts nucleoside diphosphate kinase and
histidine protein kinase activities. It was initially identified as
a suppressor of metastasis, and has been shown to be associated
with numerous biological processes, including cell migration,
proliferation and differentiation (41). Previous studies have suggested that
the anti-metastatic effects of NME1 occur due to inhibition of the
Ras/extracellular signal-regulated kinase signaling pathway in
numerous types of human cancer, including melanoma, breast and
stomach carcinomas (42–44). In addition, alterations in the
expression profiles of genes regulated by NME1 have been
demonstrated in melanoma and thyroid carcinomas (45). In the present study, NME1 expression
levels were decreased in the metastatic OS tissue samples, as
compared with the non-metastatic OS tissue samples; thus suggesting
that cell migration and invasion may result from the downregulation
of NME1 expression levels in the OS cells.
In the present study, the identified DEGs were
clustered according to their functions by performing GO term and
KEGG enrichment pathway analyses. GO term analysis was used to
identify metastasis-associated biological processes that were
over-represented in the OS metastatic tumor samples, as compared
with the non-metastatic samples. The most significant biological
processes included cell proliferation, response to external
stimulus, positive regulation of biological process, cell
migration, and cellular component organization or biogenesis.
Notably, the majority of the DEGs were associated with cell
proliferation and cell migration, which are key processes in
metastasis. In addition, cellular responses to the tumor
microenvironment were important upon the migration of the OS cells
to the lungs. Metastasis is a complex cascade of events, which
requires a precise gene regulatory network in order to overcome
barriers that exist within the tumor microenvironment. The present
GO analysis suggested that aberrant regulation of molecular events
may contribute to metastasis of OS.
KEGG enrichment analysis demonstrated that the DEGs
were involved in numerous pathways that have previously been
associated with metastatic processes. Furthermore, the
identification of pathways involving ECM-receptor interactions and
cell adhesion molecules corroborated the results obtained from the
GO analysis. It has previously been reported that cancer cell
invasion and migration involves the degradation of ECM proteins,
including matrix metalloproteinases and integrins (46). Based on the GO term and KEGG pathway
analyses, the present study obtained a comprehensive understanding
of the DEGs identified in the metastatic samples, including their
functions, and upstream and downstream relationships.
In the present study, a signal network was
constructed in order to identify the number of genes involved in
significant biological processes and pathways. A total of four
genes: JUN, CAV1, NFKBIA and ITGA4, were associated with numerous
enriched biological pathways and formed the center of the network.
All of the genes, with the exception of ITGA4, were upregulated in
the metastatic OS samples, as compared with the non-metastatic OS
samples. The JUN gene encodes the c-Jun protein, which may be
activated via double phosphorylation in the c-Jun N-terminal kinase
signaling pathway. It has previously been suggested that c-Jun may
have roles in cell proliferation, the cell cycle, apoptosis
prevention, and cancer progression (47); however, there is currently no direct
evidence that associates c-Jun with metastasis. Sze et al
(48) demonstrated that the
C-terminal truncation of the hepatitis B virus X protein increased
HCC cell migration via activation of c-Jun (48). Furthermore, activated c-Jun was
predominantly expressed in invasive breast cancer cells and
associated with proliferation and angiogenesis (49). CAV1 is a multi-functional scaffold
protein associated with cell surface caveolae, which has previously
been demonstrated to regulate numerous cancer-associated processes,
including tumor growth, cell death and survival, and cellular
transformation (50). In addition,
numerous studies have associated CAV1 with metastasis. For example,
upregulation of CAV1 has been associated with enhanced metastatic
potential and exacerbated prognosis in HCC cells (51) and Ewing's sarcoma (52). NFKBIA is a member of the inhibitor of
κB (IkB) proteins, which are able to inhibit the nuclear
localization of nuclear factor-κB (53). There is currently no report
supporting a role for NFKBIA in tumor metastasis; however, IkBg,
another member of the IkB proteins, was previously shown to promote
the metastatic progression of melanoma (54). The ITGA4 gene encodes the integrin
alpha4 protein, which is involved in cell-cell and cell-ECM
interactions (55). Previous studies
have detected that over-expression of ITGA4 is associated with a
reduction in the cell invasion of numerous types of cancer
(56–58). In the present study, a network
analysis enabled the identification of the most significantly
enriched genes with the highest repetition frequency, which may be
associated with OS metastasis.
In conclusion, the present study identified a total
of 282 DEGs in the metastatic OS tissue samples, as compared with
the non-metastatic OS issue samples, of which 212 were upregulated
and 70 were downregulated. GO term, KEGG pathway and network
analyses identified numerous genes that may have a role in the
metastasis of OS cells, and these may be considered as potential
therapeutic targets in the treatment of patients with OS.
References
|
1
|
Whelan J, McTiernan A, Cooper N, Wong YK,
Francis M, Vernon S and Strauss SJ: Incidence and survival of
malignant bone sarcomas in England 1979-2007. Int J Cancer.
131:E508–E517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bielack SS, Carrle D, Hardes J, Schuck A
and Paulussen M: Bone tumors in adolescents and young adults. Curr
Treat Options Oncol. 9:67–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hegyi M, Semsei AF, Jakab Z, Antal I, Kiss
J, Szendroi M, Csoka M and Kovacs G: Good prognosis of localized
osteosarcoma in young patients treated with limb-salvage surgery
and chemotherapy. Pediatr Blood Cancer. 57:415–422. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mialou V, Philip T, Kalifa C, Perol D,
Gentet JC, Marec-Berard P, Pacquement H, Chastagner P, Defaschelles
AS and Hartmann O: Metastatic osteosarcoma at diagnosis: Prognostic
factors and long-term outcome-the French pediatric experience.
Cancer. 104:1100–1109. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Flores RJ, Li Y, Yu A, Shen J, Rao PH, Lau
SS, Vannucci M, Lau CC and Man TK: A systems biology approach
reveals common metastatic pathways in osteosarcoma. BMC Syst Biol.
28:50–67. 2012. View Article : Google Scholar
|
|
7
|
Zhang Y, Zhang L, Zhang G, Li S, Duan J,
Cheng J, Ding G, Zhou C, Zhang J, Luo P, et al: Osteosarcoma
metastasis: Prospective role of ezrin. Tumour Biol. 35:5055–5059.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rao-Bindal K, Rao CK, Yu L and Kleinerman
ES: Expression of c-FLIP in pulmonary metastases in osteosarcoma
patients and human xenografts. Pediatr Blood Cancer. 60:575–579.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagao-Kitamoto H, Nagata M, Nagano S,
Kitamoto S, Ishidou Y, Yamamoto T, Nakamura S, Tsuru A, Abematsu M,
Fujimoto Y, et al: GLI2 is a novel therapeutic target for
metastasis of osteosarcoma. Int J Cancer. 136:1276–1284. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang G, Nishimoto K, Zhou Z, Hughes D and
Kleinerman ES: miR-20a encoded by the miR-17-92 cluster increases
the metastatic potential of osteosarcoma cells by regulating Fas
expression. Cancer Res. 72:908–916. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang H, Cai X, Wang Y, Tang H, Tong D and
Ji F: MicroRNA-143, down-regulated in osteosarcoma, promotes
apoptosis and suppresses tumorigenicity by targeting Bcl-2. Oncol
Rep. 24:1363–1369. 2010.PubMed/NCBI
|
|
12
|
Diao CY, Guo HB, Ouyang YR, Zhang HC, Liu
LH, Bu J, Wang ZH and Xiao T: Screening for metastatic osteosarcoma
biomarkers with a DNA microarray. Asian Pac J Cancer Prev.
15:1817–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu J, Irizarry R, MacDonald J and Gentry
J: Gcrma: Background adjustment using sequence information. R
package. version 2.36.0.
|
|
14
|
Gentleman R, Carey V, Huber W and Hahne F:
Genefilter: Methods for filtering genes from microarray
experiments. R package. version 1.46.1.
|
|
15
|
Smyth GK: Limma: Linear models for
microarray data. Bioinformatics and Computational Biology Solutions
Using R and Bioconductor. 397–420. 2005. View Article : Google Scholar
|
|
16
|
Benjamini Y and Hochberg Y: Controlling
the false discovery rate: A practical and powerful approach to
multiple testing. J R Stat Soc Series B Stat Methodol. 57:289–300.
1995.
|
|
17
|
Tavazoie S, Hughes JD, Campbell MJ, Cho RJ
and Church GM: Systematic determination of genetic network
architecture. Nat Genet. 22:281–285. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Warnes GR, Bolker B, Bonebakker L, et al:
gplots: Various R programming tools for plotting data. R package
version 2. 2009.
|
|
19
|
Carlson M: GOdb: A set of annotation maps
describing the entire Gene Ontology. R package. version 2.14.0.
|
|
20
|
Tenenbaum D: KEGGREST: Client-side REST
access to KEGG. R package. version 1.4.0.
|
|
21
|
Prasad Keshava TS, Goel R, Kandasamy K,
Keerthikumar S, Kumar S, Mathivanan S, Telikicherla D, Raju R,
Shafreen B, Venugopal A, et al: Human protein reference
database-2009 update. Nucleic Acids Res. 37:D767–D772. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatr-Aryamontri A, Breitkreutz BJ,
Heinicke S, Boucher L, Winter A, Stark C, Nixon J, Ramage L, Kolas
N, O'Donnell L, et al: The BioGRID interaction database: 2013
update. Nucleic Acids Res. 41:D816–D823. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McDowall MD, Scott MS and Barton GJ: PIPs:
Human protein-protein interaction prediction database. Nucleic
Acids Res. 37:D651–D656. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun Y and Ma L: The emerging molecular
machinery and therapeutic targets of metastasis. Trends Pharmacol
Sci. 36:349–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yamashita K, Katoh H and Watanabe M: The
homeobox only protein homeobox (HOPX) and colorectal cancer. Int J
Mol Sci. 14:23231–23243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kovárová D, Plachy J, Kosla J, Trejbalová
K, Čermák V and Hejnar J: Downregulation of HOPX controls
metastatic behavior in sarcoma cells and identifies genes
associated with metastasis. Mol Cancer Res. 11:1235–1247. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ozaki K, Nagata M, Suzuki M, Fujiwara T,
Ueda K, Miyoshi Y, Takahashi E and Nakamura Y: Isolation and
characterization of a novel human lung-specific gene homologous to
lysosomal membrane glycoproteins 1 and 2: Significantly increased
expression in cancers of various tissues. Cancer Res. 58:3499–3503.
1998.PubMed/NCBI
|
|
28
|
Nagelkerke A, Bussink J, Mujcic H, Wouters
BG, Lehmann S, Sweep FC and Span PN: Hypoxia stimulates migration
of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded
protein response. Breast Cancer Res. 15:R22013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kanao H, Enomoto T, Kimura T, Fujita M,
Nakashima R, Ueda Y, Ueno Y, Miyatake T, Yoshizaki T, Buzard GS, et
al: Overexpression of LAMP3/TSC403/DC-LAMP promotes metastasis in
uterine cervical cancer. Cancer Res. 65:8640–8645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen J, Yao Y, Gong C, Yu F, Su S, Liu B,
Deng H, Wang F, Lin L, et al: CCL18 from tumor-associated
macrophages promotes breast cancer metastasis via PITPNM3. Cancer
Cell. 19:541–555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li HY, Cui XY, Wu W, Yu FY, Yao HR, Liu Q,
Song EW and Chen JQ: Pyk2 and Src mediate signaling to
CCL18-induced breast cancer metastasis. J Cell Biochem.
115:596–603. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kuespert K, Pils S and Hauck CR: CEACAMs:
Their role in physiology and pathophysiology. Curr Opin Cell Biol.
18:565–571. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Beauchemin N and Arabzadeh A:
Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs)
in cancer progression and metastasis. Cancer Metastasis Rev.
32:643–671. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Govaere O, Komuta M, Berkers J, Spee B,
Janssen C, de Luca F, Katoonizadeh A, Wouters J, van Kempen LC,
Durnez A, et al: Keratin 19: A key role player in the invasion of
human hepatocellular carcinomas. Gut. 63:674–685. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Barlow M, Edelman M, Glick RD, Steinberg
BM and Soffer SZ: Celecoxib inhibits invasion and metastasis via a
cyclooxygenase 2-independent mechanism in an in vitro model of
Ewing sarcoma. J Pediatr Surg. 47:1223–1227. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu X, Cai M, Ji F and Lou LM: The impact
of COX-2 on invasion of osteosarcoma cell and its mechanism of
regulation. Cancer Cell Int. 14:272014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee EJ, Choi EM, Kim SR, Park JH, Kim H,
Ha KS, Kim YM, Kim SS, Choe M, Kim JI and Han JA: Cyclooxygenase-2
promotes cell proliferation, migration and invasion in U2OS human
osteosarcoma cells. Exp Mol Med. 39:469–476. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Naruse T, Nishida Y, Hosono K and Ishiguro
N: Meloxicam inhibits osteosarcoma growth, invasiveness and
metastasis by COX-2-dependent and independent routes.
Carcinogenesis. 27:584–592. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pucci S, Mazzarelli P, Nucci C, Ricci F
and Spagnoli LG: CLU “in and out”: Looking for a link. Adv Cancer
Res. 105:93–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang C, Jiang K, Kang X, Gao D, Sun C, Li
Y, Sun L, Zhang S, Liu X, Wu W, et al: Tumor-derived secretory
clusterin induces epithelial-mesenchymal transition and facilitates
hepatocellular carcinoma metastasis. Int J Biochem Cell Biol.
44:2308–2320. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Marshall JC, Collins J, Marino N and Steeg
P: The Nm23-H1 metastasis suppressor as a translational target. Eur
J Cancer. 46:1278–82. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takács-Vellai K: The metastasis suppressor
Nm23 as a modulator of Ras/ERK signaling. J Mol Signal. 9:42014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jarrett SG, Novak M, Dabernat S, Daniel
JY, Mellon I, Zhang Q, Harris N, Ciesielski MJ, Fenstermaker RA,
Kovacic D, et al: Metastasis suppressor NM23-H1 promotes repair of
UV-induced DNA damage and suppresses UV-induced melanomagenesis.
Cancer Res. 72:133–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ouatas T, Salerno M, Palmieri D and Steeg
PS: Basic and translational advances in cancer metastasis: Nm23. J
Bioenerg Biomembr. 35:73–79. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
McCorkle JR, Leonard MK, Kraner SD,
Blalock EM, Ma D, Zimmer SG and Kaetzel DM: The metastasis
suppressor NME1 regulates expression of genes linked to metastasis
and patient outcome in melanoma and breast carcinoma. Cancer
Genomics Proteomics. 11:175–194. 2014.PubMed/NCBI
|
|
46
|
Price JT and Thompson EW: Mechanisms of
tumour invasion and metastasis: Emerging targets for therapy.
Expert Opin Ther Targets. 6:217–233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vogt PK: Fortuitous convergences: The
beginnings of JUN. Nat Rev Cancer. 2:465–469. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Sze KM, Chu GK, Lee JM and Ng IO:
C-terminal truncated hepatitis B virus x protein is associated with
metastasis and enhances invasiveness by C-Jun/matrix
metalloproteinase protein 10 activation in hepatocellular
carcinoma. Hepatology. 57:131–139. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vleugel MM, Greijer AE, Bos R, van der
Wall E and van Diest PJ: c-Jun activation is associated with
proliferation and angiogenesis in invasive breast cancer. Hum
Pathol. 37:668–674. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Burgermeister E, Liscovitch M, Röcken C,
Schmid RM and Ebert MP: Caveats of caveolin-1 in cancer
progression. Cancer Lett. 268:187–201. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yu H, Shen H, Zhang Y, Zhong F, Liu Y, Qin
L and Yang P: CAV1 promotes HCC cell progression and metastasis
through Wnt/β-catenin pathway. PLoS One. 9:e1064512014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sáinz-Jaspeado M, Lagares-Tena L, Lasheras
J, Navid F, Rodriguez-Galindo C, Mateo-Lozano S, Notario V, Sanjuan
X, Del Garcia Muro X, Fabra A and Tirado OM: Caveolin-1 modulates
the ability of Ewing's sarcoma to metastasize. Mol Cancer Res.
8:1489–1500. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Crépieux P, Kwon H, Leclerc N, Spencer W,
Richard S, Lin R and Hiscott J: I kappaB alpha physically interacts
with a cytoskeleton-associated protein through its signal response
domain. Mol Cell Biol. 17:7375–7385. 1997.PubMed/NCBI
|
|
54
|
Torabian SZ, de Semir D, Nosrati M,
Bagheri S, Dar AA, Fong S, Liu Y, Federman S, Simko J, Haqq C, et
al: Ribozyme-mediated targeting of IkappaBgamma inhibits melanoma
invasion and metastasis. Am J Pathol. 174:1009–1016. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kummer C and Ginsberg MH: New approaches
to blockade of alpha4-integrins, proven therapeutic targets in
chronic inflammation. Biochem Pharmacol. 72:1460–1468. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Park J, Song SH, Kim TY, Choi MC, Jong HS,
Kim TY, Lee JW, Kim NK, Kim WH and Bang YJ: Aberrant methylation of
integrin alpha4 gene in human gastric cancer cells. Oncogene.
23:3474–3480. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Qian F, Vaux DL and Weissman IL:
Expression of the integrin alpha 4 beta 1 on melanoma cells can
inhibit the invasive stage of metastasis formation. Cell.
77:335–347. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gosslar U, Jonas P, Luz A, Lifka A, Naor
D, Hamann A and Holzmann B: Predominant role of alpha 4-integrins
for distinct steps of lymphoma metastasis. Proc Natl Acad Sci USA.
93:4821–4826. 1996. View Article : Google Scholar : PubMed/NCBI
|