Introduction
Cadmium (Cd) is a heavy metal and one of the major
environmental toxicants. The general population is exposed to Cd by
ingestion or inhalation (1). The Cd
content in the air is ~0.04 µg/m3; in drinking water, it
is <1 µg/l, which is not of particular concern. Much of the Cd
entering the body comes from terrestrial foods (2,3). For the
average individual, 1–3 µg Cd is absorbed via food every day
(4). In addition, Cd, having a long
biological half-life in humans, particularly accumulates in the
kidneys and liver (1). More
significantly, Cd has been identified as a severely adverse element
for the mammalian reproductive system and causes considerable
damage to the ovaries and testes (5). Consequently, it can affect the
offspring to a certain extent.
Cd can increase lipid peroxidation through the
generation of noxious radicals, such as superoxide anion radicals,
hydroxyl radicals, nitric oxide and hydrogen peroxide (6). Findings from numerous studies have
confirmed the proposal that oxidative stress can play a significant
role in the etiology of defective sperm formation, function and
count profile, as well as male infertility (7–9). Germ
cells are more subject to oxidative stress than somatic cells, thus
contributing to the alteration of enzyme activities and various
important signal transduction pathways and, in turn, affecting
fertility (10,11).
The effects of maternal Cd exposure on offspring
have been well-documented (12–14).
Maternal Cd exposure during pregnancy was shown to markedly reduce
serum testosterone levels, while the placenta could deter most of
the Cd from passing from dams to fetuses (13); however, few studies that have
investigated the effects of paternal Cd exposure on offspring are
rare. Consequently, the aim of the present study was to investigate
whether paternal Cd exposure could affect the offspring and to
explore how any effects were expressed. Since humans are
chronically exposed to Cd via ingestion or absorption from food and
drinking water, the gavage method was adopted in this study to
simulate the real Cd exposure pathway, and the effects of paternal
Cd exposure on the sperm quality of male rats and the
neurobehavioral system development of their offspring were
investigated.
Materials and methods
Chemicals
Cadmium chloride (CdCl2), methanol,
sodium hydrogen phosphate and sodium dihydrogen phosphate were of
analytical grade and were purchased from Sinopharm Chemical Reagent
Co., Ltd. (Beijing, China). Considering the quantitative exposure
and tissue retention capacity of humans, a dose of 22.15 mg/kg body
weight was selected for the study.
Animals and treatments
Sprague Dawley rats (5–6 weeks old; male rats,
180–220 g; female rats, 60–80 g) were obtained from Weitonglihua
Experimental Animal Technical Co., Ltd. (Beijing, China). Rats were
housed in cages and acclimated to laboratory conditions
(temperature, 23±2°C; 12-h light/dark cycle; humidity, 50±5%) and
fed a balanced diet and water ad libitum for 1 week prior to
the experiments. Twelve male rats were selected and randomly
divided into a control and a Cd-treated group (n=6/group). In
addition, 12 female rats were administered distilled water and
randomly divided into two groups (n=6/group). The control and
Cd-treated groups of male rats were gavaged distilled water and
CdCl2, respectively, every 2 days for 9 weeks in total.
For mating purposes, the control group was housed in the same cage
as one group of female rats for 1 week, while the Cd-treated group
was housed with the remaining female rats. During the mating
process, measures were taken to avoid the cross-contamination of Cd
between male and female rats. The presence of a vaginal plug was
designated as gestational day (GD) 0. After GD 0, the male rats
were separated from the female rats. All experimental protocols and
procedures were approved by the Animal Ethics Committee of China
Agricultural University (R2012072; Beijing, China).
Sperm motility, viability and
malformation rate
Sperm motility and viability were detected using an
automatic semen analyzer (Songjing Tianlun Biological Science and
Technology, Co., Ltd., Nanning, China). The spermatozoa were
classified as motile or immotile. Sperm motility was expressed as
the percentage of motile sperm. Sperm viability was assessed via
eosin-nigrosin staining. Unstained, live sperm were differentiated
from pink-stained, dead sperm, and their numbers were calculated. A
50-µl aliquot of sperm suspension was diluted 20 times in
phosphate-buffered saline at 37°C, smeared gently on a glass slide
with methanol and dyed using 1% eosin Y for 1 h. The number of
malformed sperm was recorded under a light microscope at high
magnification, and the sperm malformation rate was calculated.
Malformed sperm included an abnormality of the head, rump and
body.
Neuromotor maturation assessment of
the offspring
Righting reflex
In this study, the righting reflex included the
surface-righting reflex and the air-righting reflex. The
surface-righting reflex was evaluated on postnatal days (PNDs) 3–9.
The latency time for a pup placed on its back to turn over and
place all four paws on the floor was recorded. The air-righting
reflex was measured on PNDs 10–15. A pup was placed in the air on
its back and dropped 25 cm to a pad on the floor. Success was
defined by the four paws touching the floor smoothly, and the number
of successful trials was recorded. Every pup was tested with three
consecutive trials. A maximum time of 30 sec was allowed.
Cliff avoidance
Cliff avoidance was evaluated on PNDs 4, 5, 7 and 9.
Pups were placed with the forepaws and face on the edge of a table
top. The latency to return the body 1.5 cm from the ‘cliff’ was
recorded. Subjects were given a maximum time of 30 sec per
trial.
Negative geotaxis
The negative geotaxis reflex test was conducted every
other day between PNDs 2 and 10. A pup was oriented toward the top
when placed in a head-down position on a board inclined at 25°, and
the latency to rotate 180° was measured. The trail was considered
to be a success when the pup turned 180° within the allotted time
of 3 min. The number of successful trials and the time taken to
turn the 180° were recorded.
Forelimb grip strength
On PNDs 10–15, forelimb grip strength was measured.
Each pup was suspended by its forefeet from a fixed wire, and the
duration that each pup held on to the wire was recorded.
Cd accumulation in different organs
The liver, kidney, heart, brain and left testis of
the newborns were incinerated according to the method described by
Wickliff et al (15). Cd
accumulation was determined by inductively coupled plasma atomic
emission spectrometry (Varian Medical Systems, Inc., Palo Alto, CA,
USA) with a detection limit of 15 ng/ml for blood and 30 ng/g for
solid samples.
Biochemical assays
The glutathione (GSH), superoxide dismutase (T-SOD)
and malondialdehyde (MDA) levels of the tissues (liver, brain,
heart, kidney and testis) of the newborns were determined.
According to the method described by Kuo et al (16), GSH was measured in deproteinized
supernatant fractions from 10% tissue homogenates, using 0.04%
5,5′-dithiobis-(2-nitrobenzoic acid) in 10% sodium citrate
(Sigma-Aldrich, St. Louis, MO, USA). The absorption at 412 nm was
recorded using a spectrophotometer (Agilent Technologies, Inc.,
Palo Alto, CA, USA). SOD in the tissues was determined according to
the method of Kakkar et al (17). Lipid peroxidation was measured by the
MDA formed. The 10% tissue homogenate was mixed with 150 mM KCl for
30 min at 37°C, and the MDA was then determined using the
thiobarbituric acid reaction (Sigma-Aldrich) (18).
Statistical analysis
Results are presented as the mean ± standard error.
The data were statistically analyzed using one-way analysis of
variance followed by Duncan's test. The statistical analysis was
conducted using SPSS software, version 11.5 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effect of Cd exposure on sperm
motility, viability and malformation rate
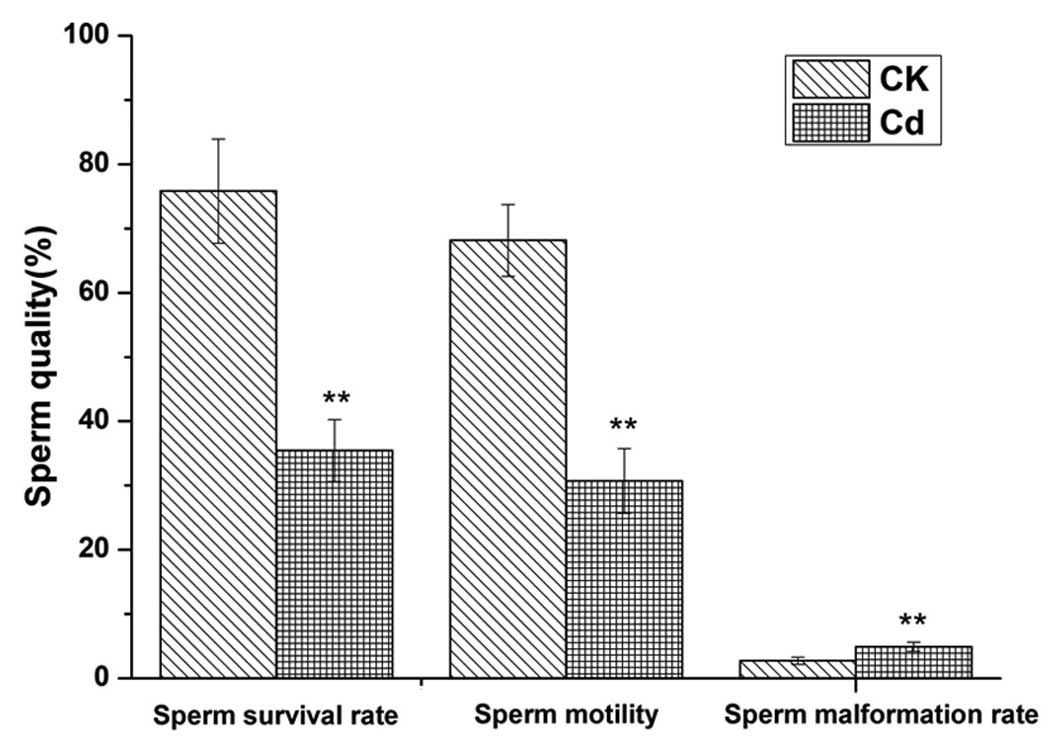
Fig. 1 shows the
effect of Cd exposure on epididymal sperm motility and viability,
as well as the malformation rate. The Cd-treated group showed
significantly lower sperm motility and viability when compared with
the control group (P<0.01). In addition, the sperm malformation
rate of the Cd-treated group was significantly higher than that of
the control group (P<0.01). The abnormalities of the malformed
sperm included large heads, decollation, and folding and broken
tails. The sperm quality of the adult male rats decreased markedly
following Cd exposure.
Effect of Cd exposure on neuromotor
maturation of the offspring
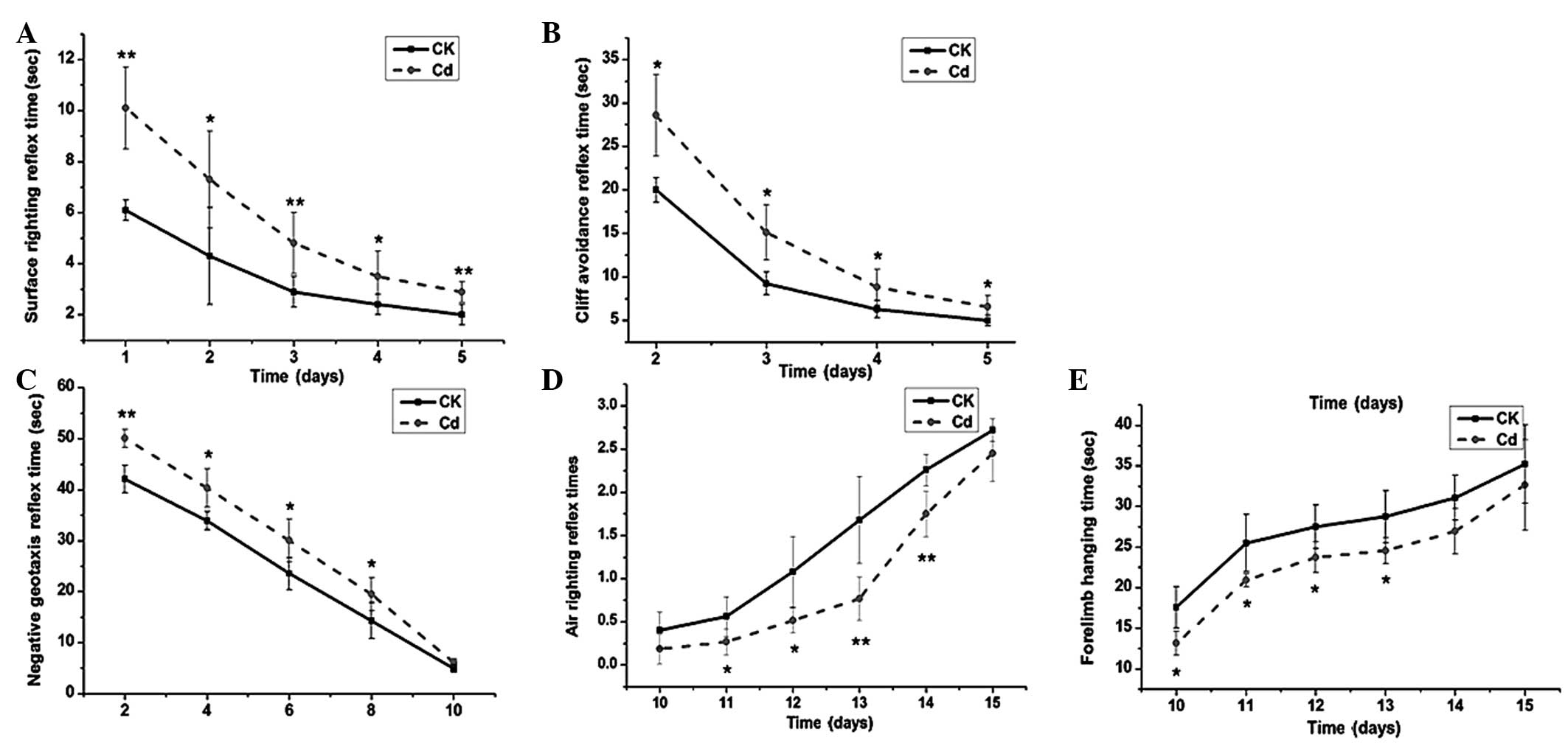
The neuromotor maturation of the offspring of the
Cd-treated group, assessed via surface- and air-righting, cliff
avoidance and negative geotaxis reflexes, as well as forelimb grip
strength, was found to be significantly suppressed throughout the
test period (Fig. 2). Compared with
the control group, the surface-righting (Fig. 2A), cliff avoidance (Fig. 2B) and negative geotaxis (Fig. 2C) reflex times of the Cd-treated
group were significantly longer than those of the control group
(P<0.05), while the number of successful air-righting reflex
trials (Fig. 2D) and the forelimb
hanging times (Fig. 2E) of the
Cd-treated group were significantly lower than those of the control
group (P<0.05).
Cd accumulation in different organs of
the offspring
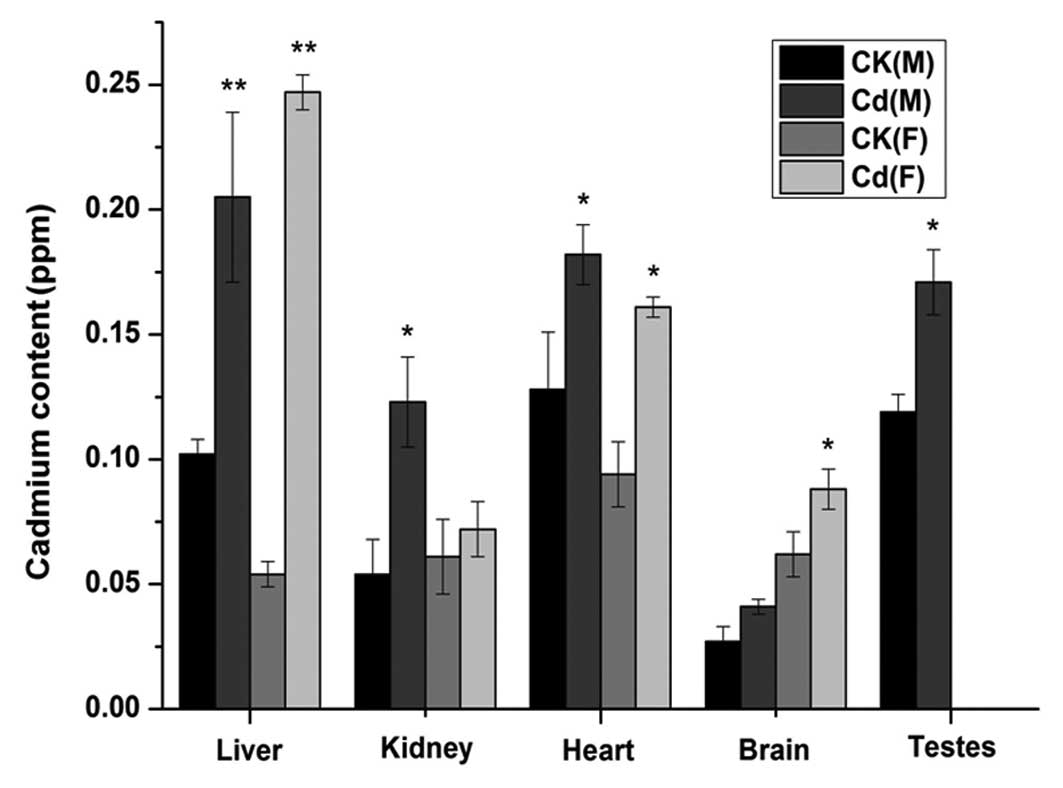
For the offspring on PND 21, the Cd levels in the
liver and heart of male and female newborns in the Cd-treated group
were significantly higher than those of the control group (liver,
P<0.01; heart, P<0.05) (Fig.
3). Furthermore, the Cd content in the kidney was significantly
higher in the Cd-treated male newborns than that in the control
male newborns (P<0.05); however, the difference between the
Cd-treated female newborns and control female newborns was not
significant (P>0.05). The opposite phenomenon was exhibited in
the brain. In addition, the testis Cd concentration was
significantly lower in the male newborns of the Cd-treated group
than that in the control group (P<0.05). The data for the
Cd-treated group could be summarized as follows: Cd accumulation
was highest in the liver for male and female newborns; Cd
accumulation was lowest in the brain and kidney for male and female
newborns, respectively.
Biochemical assays
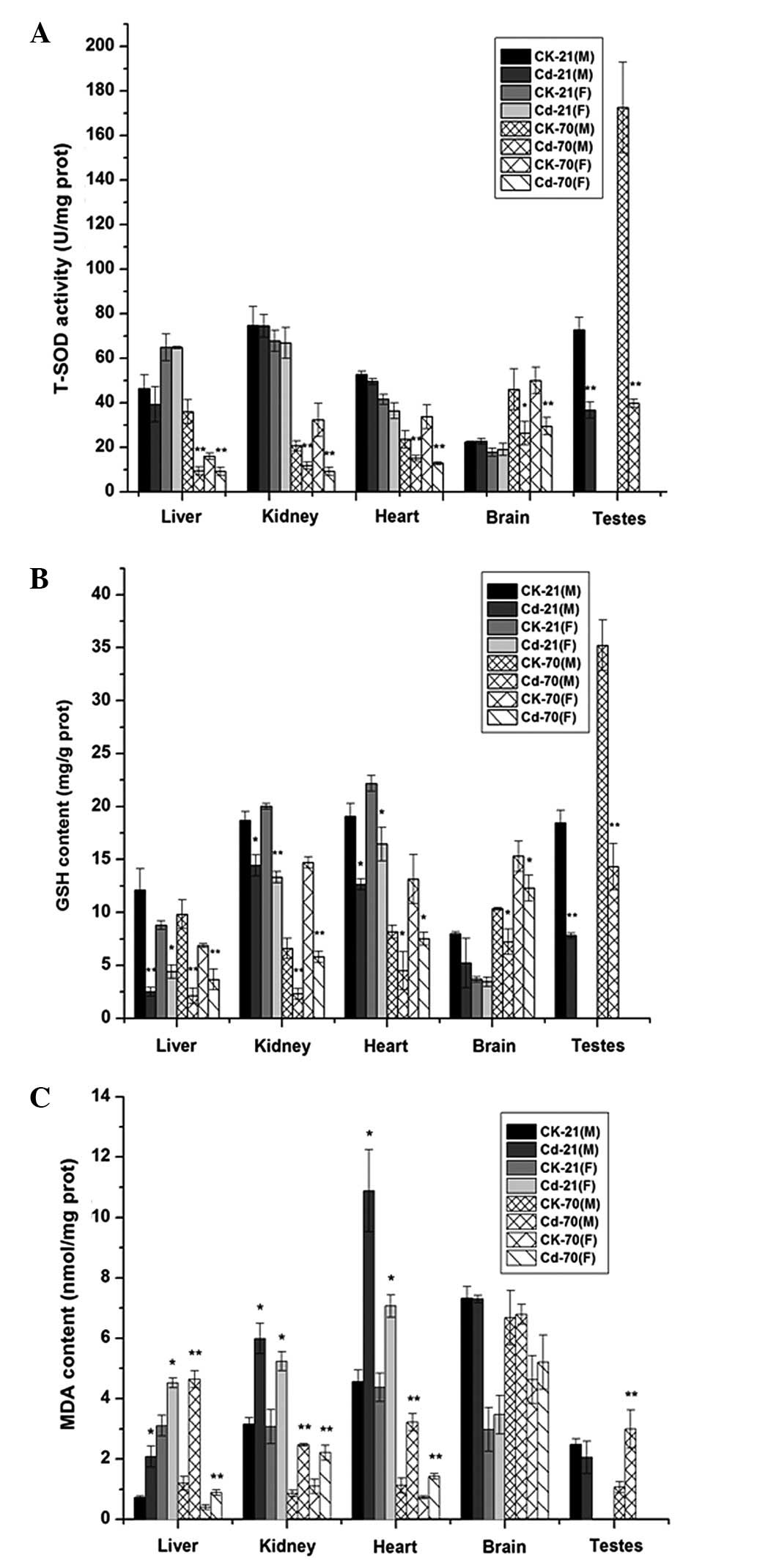
At PND 21, no significant differences were found
between the Cd-treated and control groups in terms of the T-SOD
levels in the liver, kidney, heart and brain of the male and female
newborns (P>0.05); however, the difference in the testes was
statistically significant (P<0.01). At PND 70, however, the
T-SOD levels in all tissues of the Cd-treated male and female
offspring were significantly decreased compared with those of the
control group (P<0.05) (Fig. 4A).
As shown in Fig. 4B, at PNDs 21 and
70, the GSH levels in all tissues of the Cd-treated newborns, with
the exception of the brain at PND 21, were significantly depleted
compared with those of the newborns of the control group. The
results of the MDA content are shown in Fig. 4C. At PND 21, significant differences
were found between the offspring of the Cd-treated group and the
control group in terms of the MDA content of the liver, kidney and
heart in both males and females (P<0.05); however, the brain and
testis MDA levels in the offspring of the Cd-treated group were not
significantly higher than those of the control group offspring
(P>0.05). For the offspring at PND 70, the MDA content in all
tissues of the Cd-treated group, with the exception of the brain,
was significantly increased compared with that of the control
group.
Discussion
The present study showed that, for adult male rats,
sperm quality was significantly affected by the administration of
CdCl2. Sperm motility, which is mainly a post-testicular
factor, is vulnerable to reproductive toxicants (19,20) and
relies on a maturation process in the epididymis. When the rats
were exposed to Cd, it is likely that the synthesis of epididymal
proteins and other substances associated with sperm maturation was
affected and structural and biochemical changes in the spermatozoa
were produced. Thus, Cd exposure contributed to a decrease in sperm
motility and an increase in the sperm malformation rate.
For the newborns of the Cd-treated group at PND 21,
the Cd concentration in the liver, kidney, brain, testes and heart
was significantly higher than that observed in the control group
tissues, particularly in the liver. Neonate rats exhibit
significantly increased Cd absorption and storage compared with
adult rats (21). The observed
result was therefore likely due to paternal Cd exposure affecting
the ability of the offspring to absorb and store Cd; however, the
specific mechanism is unclear.
Cd exposure can generate neurobehavioral
disturbances, including reductions in attention, psychomotor speed
and memory (22). It has been
suggested that Cd is more toxic in newborn than in adult rats due
to its ability to diffuse across all biological membranes, thus
allowing Cd penetration through the blood-brain barrier (BBB)
(23). The Cd administration
initially affects the integrity and permeability of the vascular
endothelium, and the necrotic changes in nerve cells are only
secondary to this effect, which results in edema and interference
with oxygen and nutrient uptake into the brain (24,25). In
the present study, significant suppression of all reflexes, including
surface righting, air righting, cliff avoidance, forelimb grip
strength and negative geotaxis, clearly suggested a direct effect
of Cd on the neuromotor maturation of the pups.
The effect of Cd has been demonstrated by increased
lipid peroxidation and enzyme inhibition (26). Treatment with Cd can induce decreased
T-SOD activity and GSH content and an increase in the MDA content,
which is consistent with the Cd burden of different tissues
(27). These changes appear to be
due to the generation of reactive oxygen species (ROS) (28). GSH is a major component of the
oxidant defense system, which acts to scavenge free radicals
generated during Cd intoxication. It has been well-documented that
reactive intermediates can react with GSH and undergo
transformation into oxidized GSH, either through a direct chemical
reaction or through a glutathione transferase-mediated reaction
(29). In the present study, the
Cd-treated group at PNDs 21 and 70 exhibited significant changes in
the T-SOD activity and GSH and MDA levels of the tested tissues,
with the exception of the brain; these findings most likely
resulted from the difference in the Cd absorption and storage
abilities between different tissues.
It is believed that brain tissue is particularly
sensitive to oxidative stress. Neurons are abundant in mitochondria
and have a highly aerobic metabolism system; nearly 20% of total
oxygen may be used by the brain. During mitochondrial respiration,
≤2% of the consumed oxygen may be converted to ROS, and this
proportion increases with oxygen consumption (30). Thus, ROS content in the brain may be
higher than that in any other organ. In addition, due to the
increase in Cd content in the brain of newborns, the oxidant system
is damaged, as demonstrated by a decrease in T-SOD activity and GSH
content. Thus, the above effects may combine to make the brain a
preferential target for oxidative stress-related degeneration
(31). It has been reported that
numerous neurodegenerative illnesses, such as Parkinson's and
Alzheimer's disease and amyotrophic lateral sclerosis, result from
the increment of ROS and oxidative stress (32). Furthermore, it has been found that
iron accumulation in the brain induces neurodegenerative disorders
via oxidative stress mechanisms (33). It is possible that Cd accumulation is
closely associated with neurobehavioral function.
In the present study, it was concluded that Cd
exposure in adult male rats could significantly affect the sperm
quality. In addition, paternal Cd exposure could exert significant
effects on the neurobehavioral system of the offspring. These
findings may be explained by the fact that Cd is more toxic in
newborn than in adult rats due to its ability to diffuse across all
biological membranes, thus allowing Cd penetration through the BBB.
Furthermore, Cd accumulation in the brain can damage the oxidative
system, decreasing T-SOD activity and GSH content, which results in
neurodegenerative disorders or the damage of the neurobehavioral
system.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 31101263). The authors
gratefully acknowledge the financial support.
References
|
1
|
World Health Organization: Inorganic
pollutants. Air Quality Guidelines. WHO Regional Publications,
European Series. (91)(2nd). (Copenhagen, Denmark). World Health
Organization. 136–138. 2000.
|
|
2
|
Gummuluru K, MacArthur DF, Wang M and
Kozak L: Biogeochemistry of soil cadmium and the impact on
terrestrial food chain contamination. Biogeochemistry of Trace
Elements in the Rhizosphere. Huang P and Gobran G: (Amsterdam).
Elsevier. 1972011.
|
|
3
|
Calhôa CF, Monteiro MS, Soares AM and Mann
RM: The influence of metal speciation on the bioavailability and
sub-cellular distribution of cadmium to the terrestrial isopod,
Porcellio dilatatus. Chemosphere. 83:531–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Waalkes MP: Cadmium carcinogenesis in
review. J Inorg Biochem. 79:241–244. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thompson J and Bannigan J: Cadmium: Toxic
effects on the reproductive system and the embryo. Reprod Toxicol.
25:304–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stohs SJ, Bagchi D, Hassoun E and Bagchi
M: Oxidative mechanisms in the toxicity of chromium and cadmium
ions. J Environ Pathol Toxicol Oncol. 19:201–213. 2001.
|
|
7
|
Tremellen K: Oxidative stress and male
infertility: A clinical perspective. Studies on Men's Health and
Fertility. Agarwal A, Aitken RJ and Alvarez JG: (New York, NY).
Humana Press. 325–353. 2012. View Article : Google Scholar
|
|
8
|
Gharagozloo P and Aitken RJ: The role of
sperm oxidative stress in male infertility and the significance of
oral antioxidant therapy. Hum Reprod. 26:1628–1640. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ong CN, Shen HM and Chia SE: Biomarkers
for male reproductive health hazards: Are they available? Toxicol
Lett. 134:17–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaur P, Kaur G and Bansal MP:
Tertiary-butyl hydroperoxide induced oxidative stress and male
reproductive activity in mice: Role of transcription factor
NF-kappaB and testicular antioxidant enzymes. Reprod Toxicol.
22:479–484. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Agarwal A and Saleh RA: Role of oxidants
in male infertility: Rationale, significance and treatment. Urol
Clin North Am. 29:817–827. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ronco AM, Montenegro M, Castillo P,
Urrutia M, Saez D, Hirsch S, Zepeda R and Llanos MN: Maternal
exposure to cadmium during gestation perturbs the vascular system
of the adult rat offspring. Toxicol Applied Pharmacol. 251:137–145.
2011. View Article : Google Scholar
|
|
13
|
Ji Y-L, Wang H, Liu P, Zhao XF, Zhang Y,
Wang Q, Zhang H, Zhang C, Duan ZH, Meng C and Xu DX: Effects of
maternal cadmium exposure during late pregnant period on testicular
steroidogenesis in male offspring. Toxicol Lett. 205:69–78. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brako EE, Wilson AK, Jonah MM, Blum CA,
Cerny EA, Williams KL and Bhattacharyya MH: Cadmium pathways during
gestation and lactation in control versus metallothoinein
1,2-knockout mice. Toxicol Sci. 71:154–163. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wickliff C, Evans HJ, Carter KR and
Russell SA: Cadmium effects on the nitrogen fixation system of red
alder. J Environ Qual. 9:180–184. 1980. View Article : Google Scholar
|
|
16
|
Kuo CH, Maita K, Sleight SD and Hook JB:
Lipid peroxidation: A possible mechanism of cephaloridine-induced
nephrotoxicity. Toxicol Appl Pharmacol. 67:78–88. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kakkar P, Das B and Viswanathan P: A
modified spectrophotometric assay of superoxide dismutase. Indian J
Biochem Biophys. 21:130–132. 1984.PubMed/NCBI
|
|
18
|
Wilbur K, Bernheim F and Shapiro OW: The
thiobarbituric acid reagent as a test for the oxidation of
unsaturated fatty acids by various agents. Arch Biochem.
24:305–313. 1949.PubMed/NCBI
|
|
19
|
Wong EW and Cheng CY: Impacts of
environmental toxicants on male reproductive dysfunction. Trends
Pharmacological Sci. 32:290–299. 2011. View Article : Google Scholar
|
|
20
|
Delbès G, Hales BF and Robaire B:
Toxicants and human sperm chromatin integrity. Mol Hum Reprod.
16:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lockitch G: Perspectives on lead toxicity.
Clin Biochem. 26:371–381. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terçariol SG, Almeida AA and Godinho AF:
Cadmium and exposure to stress increase aggressive behavior.
Environ Toxicol Pharmacol. 32:40–45. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gonçalves JF, Fiorenza AM, Spanevello RM,
Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler
VL, Morsch VM and Schetinger MR: N-acetylcysteine prevents memory
deficits, the decrease in acetylcholinesterase activity and
oxidative stress in rats exposed to cadmium. Chem Biol Interact.
186:53–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shukla A, Shukla GS and Srimal R:
Cadmium-induced alterations in blood-brain barrier permeability and
its possible correlation with decreased microvessel antioxidant
potential in rat. Hum Exp Toxicol. 15:400–405. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gabbiani G, Baic D and Déziel C: Toxicity
of cadmium for the central nervous system. Exp Neurol. 18:154–160.
1967. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ognjanović BI, Marković SD, Ethordević NZ,
Trbojević IS, Stajn AS and Saicić ZS: Cadmium-induced lipid
peroxidation and changes in antioxidant defense system in the rat
testes: Protective role of coenzyme Q(10) and vitamin E. Reprod
Toxicol. 29:191–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tandon S, Singh S, Prasad S, Khandekar K,
Dwivedi VK, Chatterjee M and Mathur N: Reversal of cadmium induced
oxidative stress by chelating agent, antioxidant or their
combination in rat. Toxicol Lett. 145:211–217. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bray TM and Taylor CG: Tissue glutathione,
nutrition and oxidative stress. Can J Physiol Pharmacol.
71:746–751. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hao JR, Wang Y, Zhao WW, Xu WT, Luo YB,
Yang ZJ, Wu WH, Liang ZH and Huang KL: Effect of ochratoxin A and
buthioninesulfoximine on proteome and ascorbate-glutathione cycle
enzymes in Arabidopsis thaliana. Biologia Plantarum. 59:331–340.
2015. View Article : Google Scholar
|
|
30
|
Maaroufi K, Save E, Poucet B, Sakly M,
Abdelmelek H and Had-Aissouni L: Oxidative stress and prevention of
the adaptive response to chronic iron overload in the brain of
young adult rats exposed to a 150 kilohertz electromagnetic field.
Neuroscience. 186:39–47. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Halliwell B: Oxidative stress and
neurodegeneration: Where are we now? J Neurochem. 97:1634–1658.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rao BS, Gupta KK, Karanam P and Peruri A:
Alzheimer disease: An interactome of many diseases. Ann Indian Acad
Neurol. 17:48–54. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levi S and Finazzi D: Neurodegeneration
with brain iron accumulation: Update on pathogenic mechanisms.
Front Pharmacol. 5:992014. View Article : Google Scholar : PubMed/NCBI
|