Introduction
Ischemic heart disease (IHD) is the most common
cause of morbidity and mortality worldwide. Timely effective
reperfusion therapy is the major therapeutic strategy to salvage
the myocardium from tissue injury following prolonged ischemia;
however, the beneficial effects of this treatment can be
compromised by ischemia/reperfusion (I/R) injury (1). Previous studies have demonstrated that
I/R injury can be suppressed through the application of various
mechanical and pharmacological strategies (2–6). For
example, brief episodes of sublethal ischemia and reperfusion
before sustained ischemia, or at the onset of reperfusion, render
the heart resistant to I/R injury. These ischemic conditioning
phenomena are termed ischemic preconditioning (IPC) and ischemic
postconditioning (IPost), respectively (3,4).
Following identification of this phenomena, it became clear that
when administered prior to the onset of sustained myocardial
ischemia, or at the initial onset of reperfusion, certain
pharmacological agents, such as adenosine, opioid agonists and
bradykinin, could mimic the cardioprotective phenomena exhibited by
IPC and IPost. Such treatment was termed ‘pharmacological
preconditioning’ and ‘pharmacological postconditioning’ (5,6). This
was significant, as pharmacological agents can be more readily
applied in clinical practice as a means of protecting the heart
against I/R injury, rather than inducing ischemia directly.
Although IPC, IPost and their mimetic agents have
been shown to reduce I/R injury in animal models, a variety of
pharmacological agents have failed to demonstrate cardioprotective
effects in human clinical trials (7–9). There
may be various reasons for this discrepancy. One important factor
is that the animal studies were performed on healthy animals,
whereas humans who are treated with cardioprotective agents tend to
have various co-morbidities, such as hypercholesterolemia,
diabetes, obesity and aging, which may modify the myocardial
responses to I/R and cardioprotective agents (7–9).
Hypercholesterolemia is commonly found in patients with
cardiovascular disease and is considered to be a risk factor
(10), as previous studies have
shown that patients with a hypercholesterolemic myocardium are
vulnerable to I/R injury (10–15).
Previous studies have demonstrated that this vulnerability may be
associated with the following: Increased expression of proapoptotic
proteins, decreased expression of prosurvival proteins, increased
myocardial inflammatory responses and oxidative/nitrative stress,
inhibition of nitric oxide (NO) synthesis, and the impaired opening
of mitochondrial adenosine triphosphate-sensitive potassium
(mitoKATP) channels, in response to myocardial I/R (10–15).
Furthermore, hypercholesterolemia abrogates the cardioprotection
afforded by IPC, IPost and specific pharmacological agents, such as
sevoflurane (16–18), however the exact underlying
mechanisms are yet to be fully elucidated.
Nicorandil, a hybrid KATP channel opener and nitrate
compound, is used clinically for the treatment of angina pectoris
(19). A previous randomized and
placebo-controlled trial, termed the ‘Impact Of Nicorandil in
Angina’ (20), demonstrated that
nicorandil reduced the incidence of major cardiovascular events in
patients with stable angina. Nicorandil is not only an antianginal;
previous studies have demonstrated that it may also exert
potentially cardioprotective effects on I/R myocardium, some of
which are likely due to its ability to mimic IPC by opening
mitoKATP channels (21–23). However, previous studies have shown
that nicorandil, as a NO donor, may inhibit oxidative stress- or
hypoxia-induced apoptosis in cardiomyocytes, through the activation
of mitoKATP channels and a NO/soluble guanylyl cyclase
(sGC)-dependent mechanism (24,25).
Furthermore, nicorandil has been shown to be associated with the
regulation of apoptosis-related proteins (25,26).
Although nicorandil has been demonstrated to reduce
I/R injury in healthy animals and cardiomyocytes, whether
nicorandil has a cardioprotective effect on hypercholesterolemic
animals during I/R remains unknown. Therefore, the aim of the
present study was to determine whether pharmacological
preconditioning and postconditioning with nicorandil could
attenuate myocardial necrosis and apoptosis induced by I/R in the
isolated hypercholesterolemic hearts of rats, and, if so, to
explore the possible protective mechanisms involved.
Materials and methods
Ethics statement
The present study conformed to the Guide for the
Care and Use of Laboratory Animals published by the National
Institutes of Health (NIH publication no. 85–23, revised 1996), and
the protocol was approved by the China Medical University
(Liaoning, China) institutional Ethics Committee.
Induction of experimental
hypercholesterolemia
A total of 160 healthy male Wistar rats (6 weeks
old, ~100–120 g) were obtained from the Department of Experimental
Animals, China Medical University). The rats were housed in
polypropylene cages with a 12-h light-dark cycle at 22±1°C. The
rats were divided into two groups, the normocholesterolemic group
(n=10), fed on a normal diet for 8 weeks, and the
hypercholesterolemic group (n=150), fed on a high-cholesterol diet
for 8 weeks. The high-cholesterol diet consisted of 1.5%
cholesterol, 5% egg yolk powder, 10% lard, 0.5% sodium cholate, 3%
sugar and 80% normal feed (Beijing Keao Xieli Feed Co., Ltd.,
Beijing, China). Wistar rats were selected for the present study
because they had previously demonstrated a moderate increase in
serum cholesterol level after receiving a high-cholesterol diet,
without developing substantial atherosclerosis (18). The normocholesterolemic group
contained fewer animals as it was set up to determine that the
hypercholesterolemia model was successfully established. Following
the 8-week feeding period, blood samples were collected via the
caudal vein for serum lipid analysis in order to determine the
success of the hypercholesterolemic models.
Chemicals and therapeutic agents
Nicorandil, 5-hydroxydecanoic acid sodium salt
(5-HD), 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) and
triphenyltetrazolium chloride (Sigma-Aldrich, St. Louis, MO, USA);
in situ cell death detection kit (Roche Diagnostics GmbH,
Mannheim, Germany); rabbit monoclonal caspase-3 (ab32351), rabbit
polyclonal B-cell lymphoma-2 (Bcl-2; ab7973), and rabbit polyclonal
Bcl-2-associated X protein (Bax; ab7977) antibodies (Abcam,
Cambridge, MA, USA); mouse monoclonal β-actin (TA-09), goat
anti-rabbit IgG (ZB-2301) and goat anti-mouse IgG (ZB-2305)
antibodies (ZSGB-BIO, Beijing, China).
Isolated perfused heart
preparation
The rats were anesthetized immediately following the
8-week feeding period by intraperitoneal injection with 10% chloral
hydrate (4 ml/kg; Sinopharm Chemical Reagent Co., Ltd., Shenyang,
China) and 1,500 U/kg heparin was intravenously administered in
order to prevent the formation of intracoronary clots. The hearts
were rapidly excised and immediately immersed in ice-cold
heparinized, modified Krebs-Henseleit (K-H) solution (NaCl, 127
mmol/l; NaHCO3, 17.7 mmol/l; KCl, 5.1 mmol/l;
CaCl2, 1.5 mmol/l; MgCl2, 1.26 mmol/l;
D-glucose, 11 mmol/l; pH 7.4) at 4°C for trimming. The hearts were
subsequently mounted on Langendorff perfusion apparatus and
retrogradely perfused, via the aorta, with recirculating K-H
solution saturated with 95% O2 and 5% CO2 at
37°C. The hearts were maintained in a thermostatic chamber at 37°C,
with perfusion maintained at a constant pressure of 75 mmHg. In
order to measure cardiac pressure changes, a fluid-filled latex
balloon was connected to a pressure transducer, inserted into the
left ventricle (LV) via the left atrium, and inflated to an initial
LV end-diastolic pressure of 8–10 mmHg.
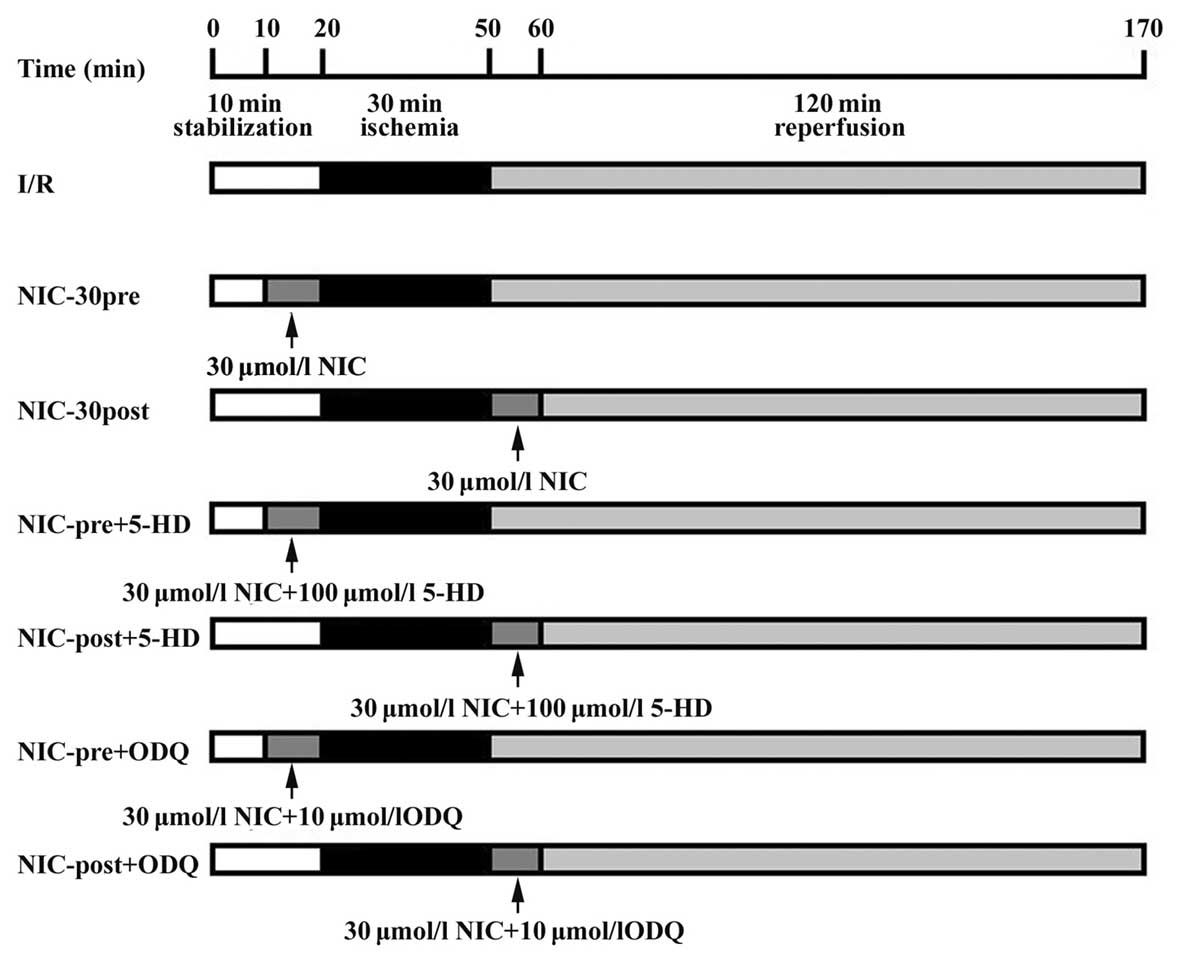
Experimental protocol
As outlined in Fig.
1, all hypercholesterolemic rats were randomized into seven
study groups. In all groups, the isolated hearts were perfused with
K-H solution and stabilized for 10 min. The I/R control group
(n=10) underwent 30 min global ischemia, followed by 120 min
reperfusion with no pharmacological intervention. To determine the
optimal concentration for pharmacological preconditioning, the
nicorandil preconditioning group (NIC-pre, n=50) were perfused with
five different concentrations of nicorandil (1, 3, 10, 30, 100
µmol/l; n=10 per subgroup) prior to global ischemia for 10 min. The
nicorandil postconditioning group (NIC-post, n=50) were perfused
with five different concentrations of nicorandil (1, 3, 10, 30, 100
µmol/l; n=10 per subgroup) for 10 min at the onset of reperfusion,
in order to determine the optimal concentration for pharmacological
postconditioning. To further examine the pharmacological mechanisms
of nicorandil in hypercholesterolemic hearts, four additional
groups underwent cotreatment with 100 µmol/l of the mitoKATP
channel blocker, 5-HD (NIC-pre + 5-HD, n=10; NIC-post + 5-HD,
n=10), or 10 µmol/l of the sGC blocker, ODQ (NIC-pre + ODQ, n=10;
NIC-post + ODQ, n=10). The control I/R group were perfused with K-H
solution prior to global ischemia for 10 min and at the onset of
reperfusion for 10 min to match the corresponding time in the other
groups.
Measurement of infarct size
The infarct size was determined as previously
described (17). Briefly, after 120
min of reperfusion, the hearts were harvested. The hearts were
partially frozen for 60 min at −20°C, sectioned from apex to base
into 3 mm sections, incubated in 1% TTC solution (TTC dissolved in
NaH2PO4/NaHPO4 buffer, pH 7.4) for
5 min at 37°C, and unstained tissue was subsequently separated from
stained tissue by an independent observer. Successful staining of
the tissue indicated that the cells were still viable, whereas the
unstained tissue contained the dead cells. Therefore, the unstained
mass was expressed as a percentage of the total left ventricular
mass, which was defined as the risk area since a global ischemia
was induced.
Terminal deoxynucleotidyl transferase
dUTP nick-end labeling (TUNEL) assay for apoptosis
Apoptotic cardiomyocytes were detected using an
in situ cell death detection kit (Roche Diagnostics GmbH),
according to the manufacturer's instructions. Following 30 min of
reperfusion, the hearts were removed and cut into 4 µm thick,
formalin-fixed, paraffin-embedded sections. The sections were
subsequently deparaffinized with xylene and rehydrated with graded
alcohol (Sinopharm Chemical Reagent Co., Ltd.). A total of 20 mg/l
proteinase K (Roche Diagnostics GmbH) was applied to the section
for 10 min to ensure optimal proteolysis, prior to supplementation
with 3% hydrogen peroxide in methanol for 30 min to inhibit the
endogenous peroxidase. The tissue sections were incubated with
terminal deoxynucleotidyl transferase enzyme in a humidified
chamber at 37°C for 60 min. Finally, streptavidin horseradish
peroxidase was bound to the biotinylated nucleotides and peroxidase
activity was revealed in each section by the application of a
stable chromogen diaminobenzidine. This technique caused the
apoptotic nuclei to be stained dark brown, whereas the total nuclei
were counterstained with hematoxylin. Three sections from each
myocardial sample were randomly selected and 10 microscopic fields
(magnification, ×400; BX51 microscope, Olympus Tokyo, Japan) per
section were evaluated by two independent blind observers. The
percentage of cardiomyocyte apoptosis was calculated as follows:
(Number of apoptotic cardiomyocytes / total number of
cardiomyocytes counted) × 100%.
Western blot analysis
Following 30 min of reperfusion, the hearts were
homogenized in radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Shanghai, China) prior to
protein quantification using the BCA method (11). Equal quantities of protein from each
sample were then separated by SDS-PAGE and transferred onto
polyvinylidene difluoride-plus membranes (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). After blocking with 5% bovine serum
albumin, the membranes were incubated overnight at 4°C with the
following primary antibodies: Caspase-3 (1:5,000), Bax (1:1,500),
Bcl-2 (1:1,500) and β-actin (1:2,000). The membranes were
subsequently washed three times with Tris-buffered saline and
Tween-20 (TBST; Beyotime Institute of Biotechnology) and incubated
with the corresponding goat anti-rabbit IgG and goat anti-mouse IgG
(1:5,000), prior to conjugation to horseradish peroxidase at room
temperature for 2 h. The membranes were once again washed three
times with TBST. Signals were detected using an enhanced
chemiluminescence kit (Beyotime institute of biotechnology) and
relative densitometry was performed using a computerized software
package (Image J, version 1.63; National Institutes of Health,
Bethesda, MD, USA).
Statistical analysis
The quantitative data are expressed as the mean ±
standard deviation. One-way analysis of variance was applied to
analyze the differences between the groups. If the difference was
deemed statistically significant, a Student-Newman-Keuls post hoc
test was applied in a further pairwise comparison. Statistical
analyses were performed using SPSS statistical software (version
17.0; SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of a high-cholesterol diet on
serum lipid levels
As outlined in Table
I, the levels of total cholesterol (TC; 2.11±0.30 vs. 1.21±0.31
mmol/l, P<0.05) and low density lipoprotein cholesterol (LDL-C;
0.84±0.28 vs. 0.34±0.14 mmol/l, P<0.05) were significantly
increased in rats fed with high-cholesterol diet, compared with
those fed a normal diet; whereas the levels of high density
lipoprotein cholesterol (HDL-C; 0.99±0.23 vs. 1.02±0.19, P=0.715)
were not significantly different between the two groups.
 | Table I.Effects of a high-cholesterol diet on
serum lipid level. |
Table I.
Effects of a high-cholesterol diet on
serum lipid level.
| Diet | TC (mmol/l) | LDL-C (mmol/l) | HDL-C (mmol/l) |
|---|
| Normal | 1.21±0.31 | 0.34±0.14 | 1.02±0.19 |
|
High-cholesterol |
2.11±0.30a |
0.84±0.28a | 0.99±0.23 |
Effects of preconditioning and
postconditioning with nicorandil on myocardial infarct size
Staining with TTC revealed the infarct size of the
hearts 120 min after reperfusion. As demonstrated in Fig. 2A, preconditioning with nicorandil (1,
3, 10, 30, 100 µmol/l) reduced the infarct size in a
concentration-dependent manner in the hypercholesterolemic hearts.
In particular, preconditioning with 30 µmol/l nicorandil
significantly decreased the infarct size to 14.88±3.25% compared
with 44.04±2.70% in the I/R group (P<0.05), whereas 100 µmol/l
nicorandil preconditioning did not reduce the infarct size further.
Similarly, as shown in Fig. 2B,
nicorandil (1, 3, 10, 30, 100 µmol/l) postconditioning also reduced
the infarct size in a concentration-dependent manner in the
hypercholesterolemic hearts, and postconditioning with 30 µmol/l
nicorandil significantly reduced the infarct size to 15.96±3.29%,
with 100 µmol/l nicorandil unable to further reduce the infarct
size. Therefore, these results suggested that the optimal
preconditioning and postconditioning concentration of nicorandil to
reduce infarct size is 30 µmol/l.
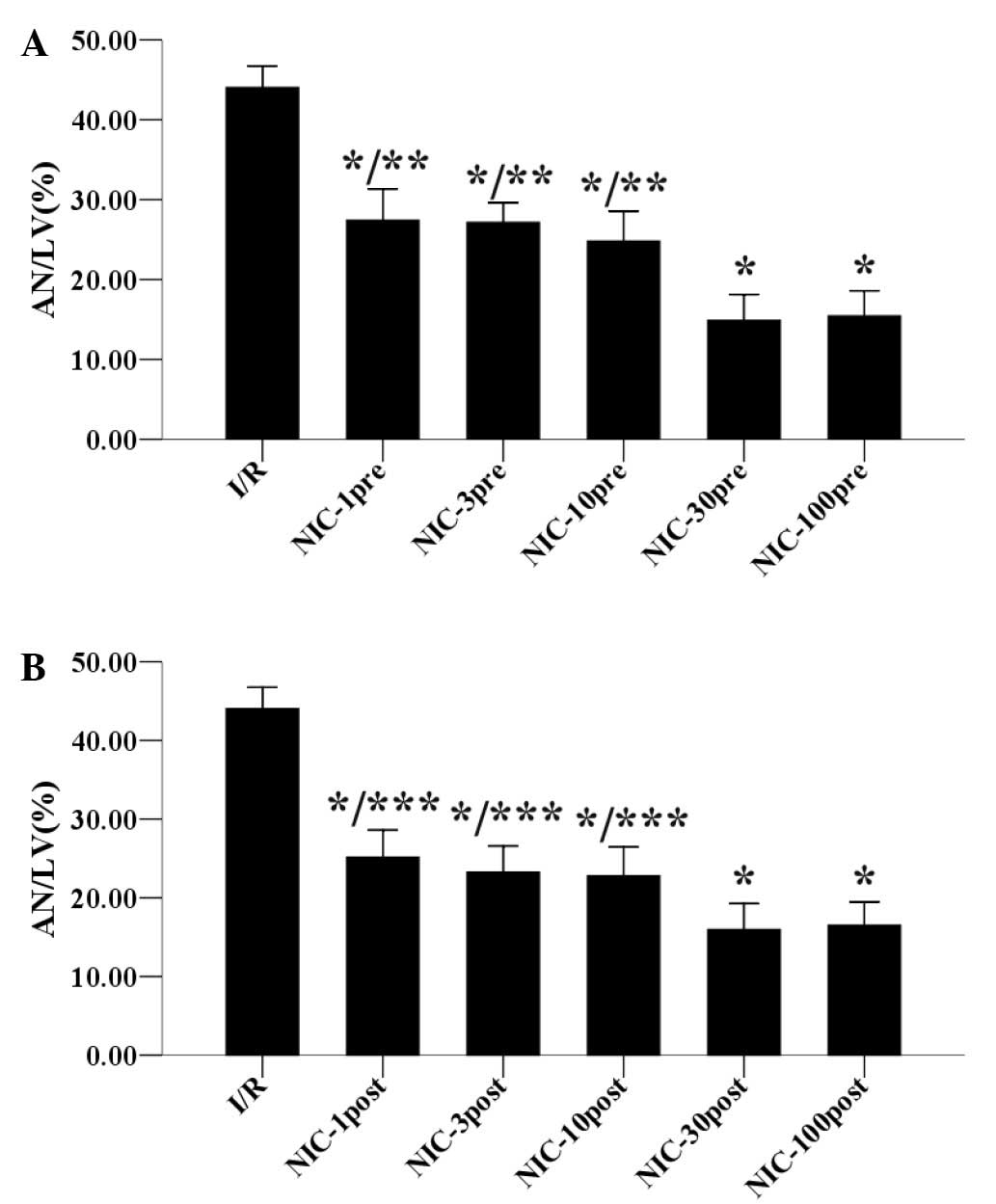 | Figure 2.Concentration-response relationships
for (A) preconditioning and (B) postconditioning with different
concentrations of NIC (1, 3, 10, 30, 100 µmol/l) on anti-infarct
effects in hypercholesterolemic rat hearts. Data are expressed as
the mean ± standard deviation, n=5 for each. *P<0.05 vs. I/R
group; **P<0.05 vs. NIC-30pre group; ***P<0.05 vs. NIC-30post
group. AN/LV, area of necrosis/left ventricle; I/R,
ischemia/reperfusion; NIC, nicorandil; pre, preconditioning; post,
postconditioning. |
Effects of preconditioning and
postconditioning with nicorandil on cardiomyocyte apoptosis
TUNEL staining was used to measure the cardiomyocyte
apoptosis, another form of I/R injury, by revealing apoptotic
cardiomyocytes 30 min after reperfusion. As demonstrated in
Fig. 3A, in the hypercholesterolemic
hearts, nicorandil (1, 3, 10, 30, 100 µmol/l) preconditioning
decreased the percentage of apoptotic cardiomyocytes in a
concentration-dependent manner. Furthermore, preconditioning with
30 µmol/l nicorandil significantly reduced the percentage of
apoptotic cardiomyocytes to 25.20±3.93%, and 100 µmol/l nicorandil
preconditioning did not reduce the percentage of apoptotic
cardiomyocytes any further. Similarly, as outlined in Fig. 3B, postconditioning with nicorandil
(1, 3, 10, 30, 100 µmol/l) also reduced the percentage of apoptotic
cardiomyocytes in a concentration-dependent manner in the
hypercholesterolemic hearts. Nicorandil (30 µmol/l)
postconditioning significantly decreased the percentage of
apoptotic cardiomyocytes to 26.18±4.82%, whereas 100 µmol/l
nicorandil postconditioning was unable to reduce this percentage
any further. Therefore, these results suggest that 30 µmol/l may be
the optimal concentration of preconditioning and postconditioning
with nicorandil to cause an anti-apoptotic effect.
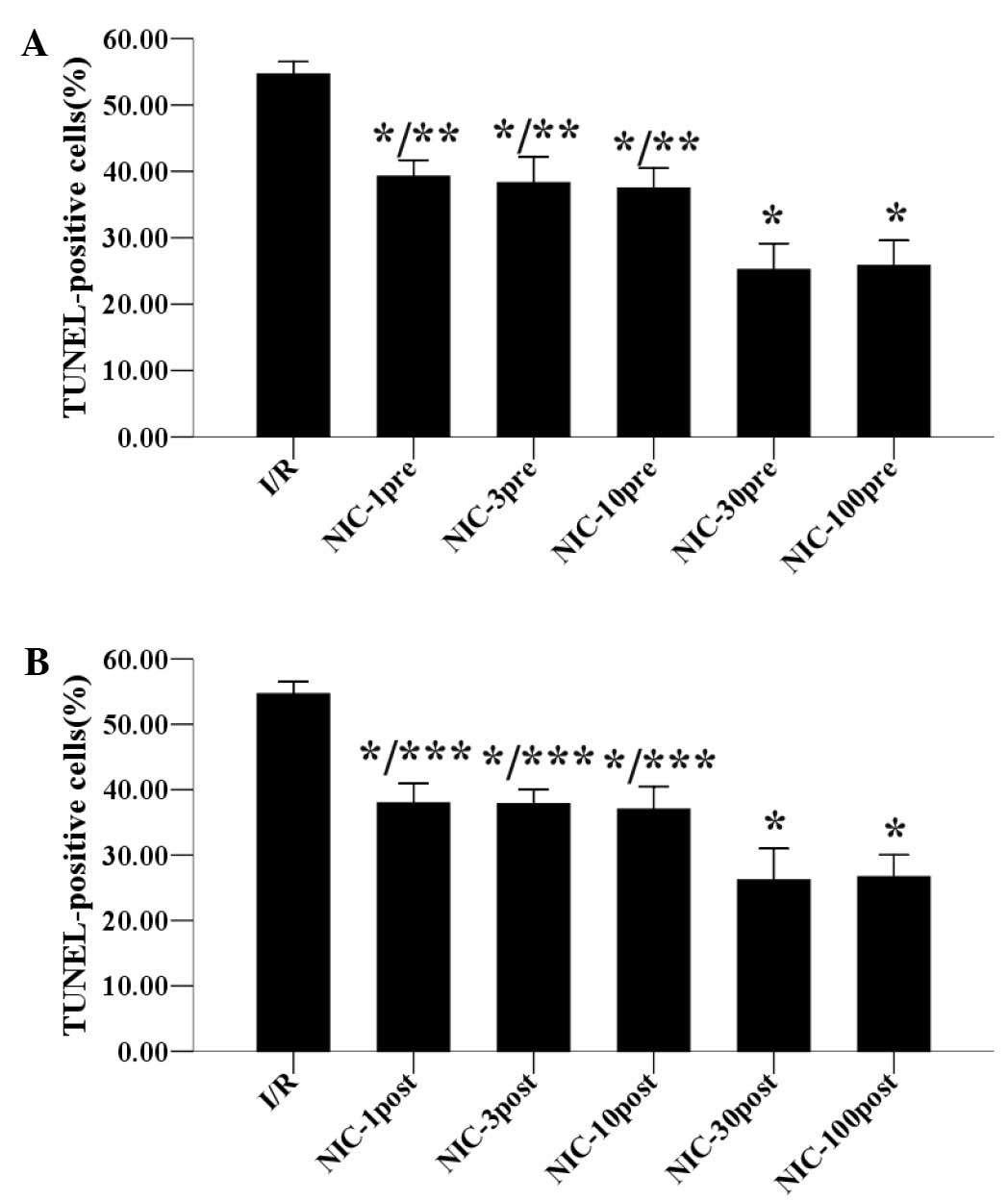 | Figure 3.Concentration-response relationships
for (A) preconditioning and (B) postconditioning with different
concentrations of NIC (1, 3, 10, 30, 100 µmol/l) on anti-apoptotic
effects in hypercholesterolemic hearts. Data are expressed as the
mean ± standard deviation, n=5. *P<0.05 vs. I/R group;
**P<0.05 vs. the NIC-30pre group; ***P<0.05 vs. the
NIC-30post group. TUNEL, terminal deoxynucleotidyl transferase dUTP
nick-end labeling; I/R, ischemia/reperfusion; NIC, nicorandil; pre,
preconditioning; post, postconditioning. |
Effects of 5-HD and ODQ on
nicorandil-induced inhibition of infarct size and apoptosis
As shown in Fig. 4,
the anti-infarct effects of preconditioning and postconditioning
with nicorandil (30 µmol/l) were significantly inhibited by
cotreatment with 100 µmol/l 5-HD (32.68±2.90 vs. 14.88±3.25%; and
33.00±2.57 vs. 15.96±3.29%) or 10 µmol/l ODQ (33.54±1.56 vs.
14.88±3.25%; and 34.04±2.89 vs. 15.96±3.29%) respectively, as
compared with untreated samples. Furthermore, as outlined in
Fig. 5, the anti-apoptotic effects
of respective preconditioning and postconditioning with nicorandil
(30 µmol/l) were also significantly inhibited by cotreatment with
100 µmol/l 5-HD (42.22±2.13 vs. 25.20±3.93%; and 41.80±3.05 vs.
26.18±4.82%) or 10 µmol/l ODQ (43.94±3.17 vs. 25.20±3.93%; and
43.20±1.78% vs. 26.18±4.82%) respectively, as compared with
untreated samples. Together, these results suggested that
nicorandil preconditioning and postconditioning may protect
hypercholesterolemic hearts against I/R-induced infarction and
apoptosis, which are both stimulated by the opening of mitoKATP
channels and a NO/sGC dependent mechanism.
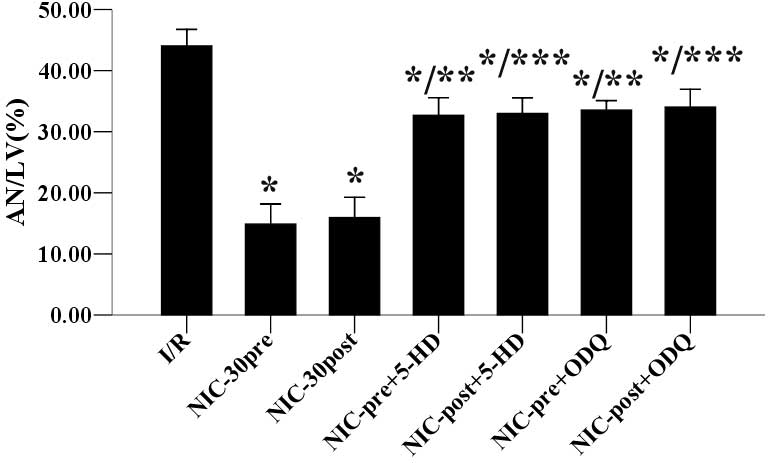 | Figure 4.Effects of 5-HD and ODQ on
NIC-induced (30 µmol/l) inhibition of infarct size in
hypercholesterolemic hearts. Data are expressed as the mean ±
standard deviation, n=5. *P<0.05 vs. I/R group; **P<0.05 vs.
NIC-30pre group; ***P<0.05 vs. NIC-30post group. AN/LV, area of
necrosis/left ventricle; I/R, ischemia/reperfusion; NIC,
nicorandil; pre, preconditioning; post, postconditioning; HD,
5-hydroxydecanoic acid sodium salt; ODQ,
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. |
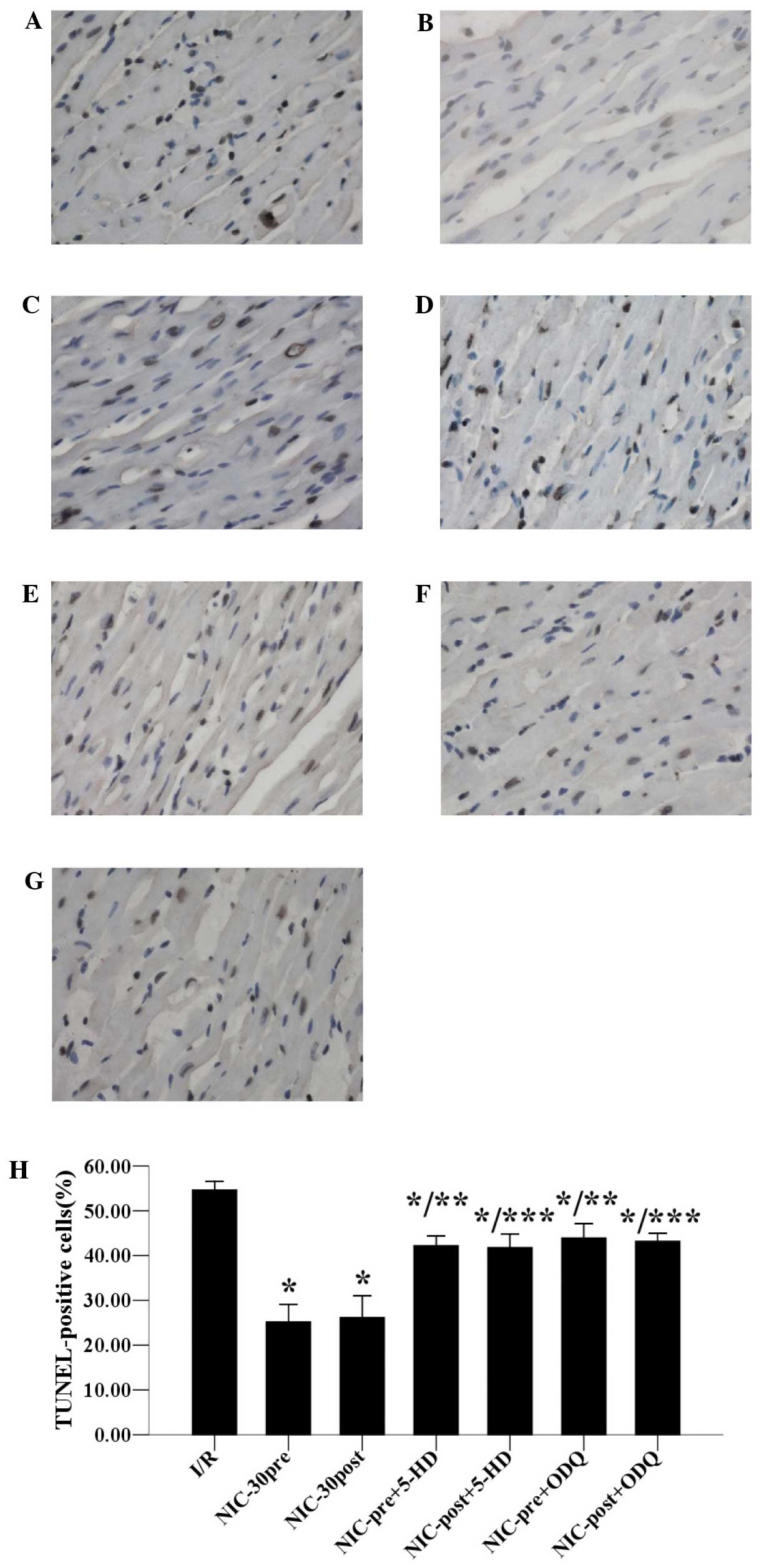 | Figure 5.Effects of 5-HD and ODQ on
NIC-induced inhibition of apoptosis in hypercholesterolemic hearts
(diaminobenzidine and hematoxylin staining; magnification, ×400).
Data are expressed as the mean ± standard deviation, n=5 for each.
*P<0.05 vs. I/R group;**P<0.05 vs. NIC-30pre group;
***P<0.05 vs. NIC-30post group. (A) I/R; (B) NIC-30pre; (C)
NIC-30post; (D) NIC-pre+5-HD; (E) NIC-post+5-HD; (F) NIC-pre+ODQ;
and (G) NIC-post+ODQ groups; (H) Statistical histogram of apoptosis
in the seven groups. TUNEL, terminal deoxynucleotidyl transferase
dUTP nick-end labeling; I/R, ischemia/reperfusion; NIC, nicorandil;
pre, preconditioning; post, postconditioning; HD, 5-hydroxydecanoic
acid sodium salt; ODQ,
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. |
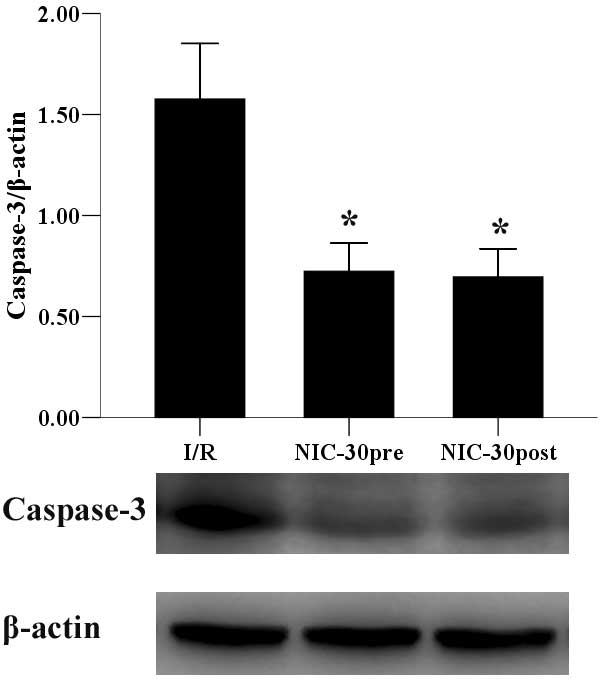
Preconditioning and postconditioning
with nicorandil inhibits expression of caspase-3 protein
As a common factor in caspase-dependent apoptosis,
the protein expression levels of caspase-3 were examined. As
outlined in Fig. 6, respective pre-
and post-conditioning with 30 µmol/l nicorandil, significantly
inhibited the expression of caspase-3, as compared with the I/R
group (0.72±0.14 and 0.70±0.14 vs. 1.58±0.28, P<0.05).
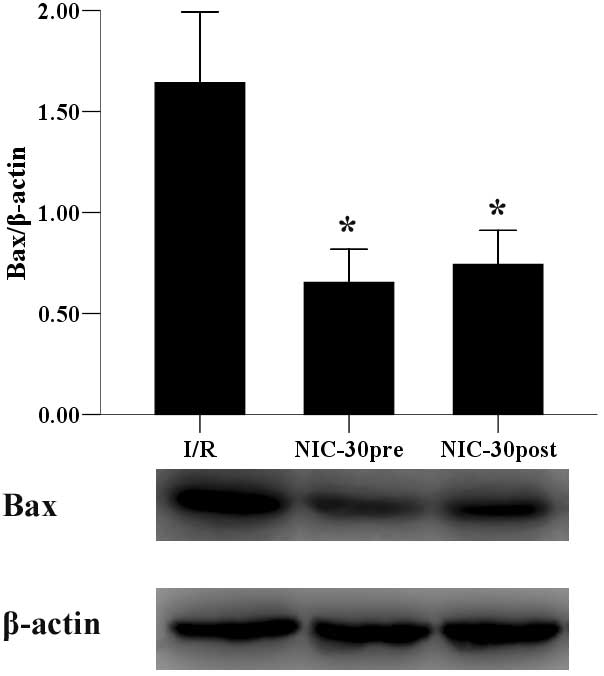
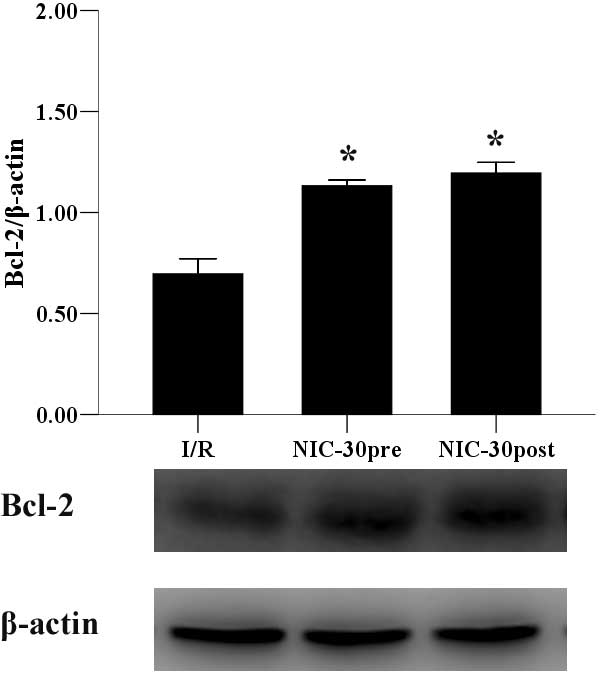
Preconditioning and postconditioning
with nicorandil regulates the expression of Bax and Bcl-2
proteins
Finally, the expression levels of Bax and Bcl-2
proteins were determined. Preconditioning and postconditioning with
nicorandil (30 µmol/l) significantly suppressed the expression of
Bax (0.65±0.16 and 0.74±0.17 vs. 1.64±0.35, P<0.05) (Fig. 7) and upregulated Bcl-2 expression
(1.13±0.03 and 1.20±0.05 vs. 0.70±0.07, P<0.05) (Fig. 8), as compared with the I/R group.
Discussion
Hypercholesterolemia is a major risk factor for the
induction and progression of IHD, and the incidence of myocardial
infarction is higher in patients with chronic hypercholesterolemia,
as hypercholesterolemia modifies the responses of the myocardium to
I/R and cardioprotective interventions (7–9). Various
studies have demonstrated that the myocardia of patients with
hypercholesterolemia are more vulnerable to I/R-induced myocardial
injury (10–15). In the present study, rats fed a high
cholesterol diet for 8 weeks demonstrated significantly increased
levels of TC and LDL-C. Hypercholesterolemia significantly
exacerbated myocardial I/R injury by increasing the myocardial
infarct size (44.04±2.70 vs. 39.04±1.90%, P<0.05) and the
percentage of apoptotic cardiomyocytes (54.64±1.88 vs. 46.06±2.74%,
P<0.05), as compared with the normocholesterolemic control rats
subjected to I/R (data not shown). The results of the present study
are consistent with previous studies conducted on Yucatan pigs
(10) and New Zealand rabbits
(11).
Nicorandil is a hybrid agent with two distinct
mechanisms of pharmacological action; it opens KATP channels,
thereby dilating peripheral and coronary resistance arterioles, in
addition to activating sGC through its nitrate-like effect, which
increases cyclic guanosine monophosphate (cGMP) levels and
subsequently dilates the systemic veins and epicardial coronary
arteries. Therefore, nicorandil increases coronary blood flow,
reduces preload and afterload, and exerts an anti-anginal effect
(19). Although perfusion with
nicorandil had previously been demonstrated to produce
pharmacological preconditioning-induced cardioprotection in normal
hearts (21,23), few studies have been conducted on
whether it still exerts cardioprotective effects on
hypercholesterolemic hearts, particularly when administrated at the
onset of reperfusion or reoxygenation. In the present study, five
different concentrations of nicorandil were administrated either
before ischemia, or at the onset of reperfusion, in order to induce
pharmacological preconditioning and postconditioning, respectively.
To the best of our knowledge, the present study has demonstrated
for the first time that pharmacological preconditioning and
postconditioning with nicorandil (1–100 µmol/l) reduced myocardial
necrosis and apoptosis induced by I/R, in a concentration-dependent
manner in hypercholesterolemic rats. The present study also
demonstrated that, for anti-infarct and anti-apoptosis, the optimal
concentration of respective nicorandil preconditioning and
postconditioning was 30 µmol/l, as no further improvements were
determined at 100 µmol/l.
The KATP channels, including the mitoKATP and
sarcolemmal KATP (sarcKATP) channels, are important in
cardioprotection; and the mitoKATP channel in particular has been
demonstrated to be the final effector of cardioprotection (21). The opening of the mitoKATP channels
ensures the preservation of mitochondrial integrity and protects
cellular function, which subsequently mediates the cardioprotective
effects of ischemic and pharmacological preconditioning and
postconditioning (5,6). Accordingly, pharmacological mitoKATP
opening may be a putative therapeutic strategy to reduce I/R
injury, particularly in hypercholesterolemic hearts with impaired
KATP channels (15). Nicorandil is a
KATP channel opener, and a previous study by Sato et al
(27) demonstrated that nicorandil
concentrations as low as 10 µmol/l may open mitoKATP channels,
whereas sarcKATP activation requires exposure to concentrations as
high as 1 mmol/l. In previous studies, nicorandil has limited the
infarct size (21), blunted the rate
of cardiomyocyte death (27), and
reduced oxidative stress-induced cellular apoptosis (22) by opening mitoKATP channels. In the
present study, the cardioprotective effects of nicorandil
preconditioning and postconditioning were partially but
significantly ameliorated by the mitoKATP channel blocker 5-HD,
indicating that the cardioprotective effects of nicorandil in
hypercholesterolemic hearts were partially mediated by the
selective activation of mitoKATP channels.
In cardiomyocytes, NO is the major activator of sGC,
which results in the generation of cGMP-induced cardioprotection
against I/R injury (28). However,
previous studies have demonstrated that the myocardial NO/sGC
pathway may be impaired in hypercholesterolemia. For example,
Prasan et al (14)
demonstrated that NO production is decreased in the hearts of
hypercholesterolemic rabbits during I/R, and Schwemmer et al
(29) detected decreased cGMP levels
in the hearts of hypercholesterolemic guinea pigs. Furthermore,
previous studies have also demonstrated that cardioprotective
mechanisms, such as preconditioning and postconditioning, in which
the NO/sGC pathway has an important role, are abolished in
hyperlipidemia (16–18). Therefore, we hypothesize that NO
donors may have induced cardioprotection against I/R injury in
hypercholesterolemic hearts. Giricz et al (30) and Tang et al (31) have previously reported that
administration of an NO donor failed to induce cardioprotective
effects in hypercholesterolemic animals. However, in the present
study, as an NO donor, nicorandil preconditioning and
postconditioning protected the hypercholesterolemic hearts against
I/R-induced necrosis and apoptosis. Furthermore, the
cardioprotective effects associated with nicorandil were
significantly suppressed by cotreatment with OQD, an sGC inhibitor,
suggesting that the cardioprotective effects of nicorandil in
hypercholesterolemic hearts may also be associated with its NO/sGC
dependent mechanism. The discrepancy between the present and
previous studies may be due to the dual effects of nicorandil,
particularly considering that the NO signaling pathway has been
associated with the opening of mitoKATP channels (32,33) and
that the NO released from nicorandil activates mitoKATP channels
(34), thus making it difficult to
separate the dual mechanisms of nicorandil action.
Myocardial injury during I/R implicates two
morphologically and biologically distinct pathways, necrosis and
apoptosis. Apoptosis is a fundamental process of cell death, which
is mediated by a family of aspartate-specific cysteine proteases,
known as caspases. Of the 14 caspases characterized to date,
caspase-3 plays a critical role in the apoptosis of cardiomyocytes
and thus represents the final common pathway of the caspase cascade
(35). Early studies demonstrated
that nicorandil inhibited hypoxia-induced apoptosis in
cardiomyocytes and human pulmonary arterial endothelial cells, by
reducing caspase-3 activation (25,36).
Similarly, the present study demonstrated that nicorandil
preconditioning and postconditioning also suppressed cardiomyocyte
apoptosis, by downregulating the expression of caspase-3 in
hypercholesterolemic hearts.
The apoptosis pathway is also regulated by members
of the Bcl-2 family, which are associated with cell survival by
regulating the permeability of mitochondria (37). The Bcl-2 family is composed of
anti-apoptotic members, such as Bcl-2 and Bcl-extra large, and
pro-apoptotic members, such as Bax, Bcl-2-associated death promoter
and Bcl-2 homologous antagonist/killer. It has been suggested that
protein interactions between Bcl-2 family members may play an
important pathophysiological role in the control of apoptotic
processes in cardiomyocytes. Nishikawa et al (25) demonstrated that nicorandil inhibited
hypoxia-induced Bcl-2 downregulation and Bax upregulation in
mitochondria, and thus protected cardiomyocytes. Furthermore, Wang
et al (26) also found that,
when administered before/during ischemia or at the onset of
reperfusion, nicorandil increased Bcl-2 expression and reduced Bax
expression in normal Sprague-Dawley rats. In agreement with these
observations, the present study demonstrated that respective
nicorandil preconditioning and postconditioning significantly
upregulated the expression of Bcl-2 and downregulated the
expression of Bax in hypercholesterolemic hearts, suggesting that
the cardioprotective effects of nicorandil may be mediated by
regulation of the Bcl-2 family.
In conclusion, the present study demonstrated that
pharmacological preconditioning and postconditioning with
nicorandil can protect hypercholesterolemic hearts against
I/R-induced necrosis and apoptosis, in a concentration-dependent
manner. Furthermore, the cardioprotective effects of nicorandil may
be due to the dual pharmacological mechanisms of mitoKATP channel
opening and a NO/sGC dependent mechanism, in addition to the
regulation of the following apoptosis-related proteins: Caspase-3,
Bax and Bcl-2. Therefore, nicorandil may be of potential clinical
benefit to patients suffering from both IHD and
hypercholesterolemia. The nicorandil conditioning strategy may be
capable of reducing myocardial injury in patients presenting with
acute myocardial infarction, cardiac arrest, undergoing
percutaneous coronary intervention or cardiac surgery such as
coronary artery bypass grafting.
Acknowledgements
The present study was funded by Liaoning Provincial
Science and Technology Projects, Shenyang, China (grant no.
2013021011).
References
|
1
|
Frank A, Bonney M, Bonney S, Weitzel L,
Koeppen M and Eckle T: Myocardial ischemia reperfusion injury: From
basic science to clinical bedside. Semin Cardiothorac Vasc Anesth.
16:123–132. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hausenloy DJ and Yellon DM:
Preconditioning and postconditioning: Underlying mechanisms and
clinical application. Atherosclerosis. 204:334–341. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murry CE, Jennings RB and Reimer KA:
Preconditioning with ischemia: A delay of lethal cell injury in
ischemic myocardium. Circulation. 74:1124–1136. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao ZQ, Corvera JS, Haulkos ME, Kerendi
F, Wang NP, Guyton RA and Vinten-Joansen J: Inhibition of
myocardial injury by ischemic postconditioning during reperfusion
comparision with ischemic preconditioning. Am J Physiol Heart Circ
Physiol. 285:579–588. 2003. View Article : Google Scholar
|
|
5
|
Gerczuk PZ and Kloner RA: Protecting the
heart from ischemia: An update on ischemic and pharmacologic
conditioning. Hosp Pract. 39:35–43. 2011. View Article : Google Scholar
|
|
6
|
Andreadou I, Iliodromitis EK, Koufaki M
and Kremastinos DT: Pharmacological pre- and post- conditioning
agents: Reperfusion-injury of the heart revisited. Mini Rev Med
Chem. 8:952–959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sack MN and Murphy E: The role of
comorbidities in cardioprotection. J Cardiovasc Pharmacol Ther.
16:267–272. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Balakumar P, Singh H, Singh M and
Anand-Srivastava MB: The impairment of preconditioning-mediated
cardioprotection in pathological conditions. Pharmacol Res.
60:18–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferdinandy P, Schulz R and Baxter GF:
Interaction of cardiovascular risk factors with myocardial
ischemia/reperfusion injury, preconditioning, and postconditioning.
Pharmacol Rev. 59:418–458. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Osipov RM, Bianchi C, Feng J, Clements RT,
Liu Y, Robich MP, Glazer HP, Sodha NR and Sellke FW: Effect of
hypercholesterolemia on myocardial necrosis and apoptosis in the
setting of ischemia-reperfusion. Circulation. 120(Suppl): S22–S30.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang TD, Chen WJ, Su SS, Lo SC, Lin WW and
Lee YT: Increased cardiomyocyte apoptosis following ischemia and
reperfusion in diet-induced hypercholesterolemia: Relation to Bcl-2
and Bax proteins and caspase-3 activity. Lipids. 37:385–394. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu HR, Tao L, Gao E, Qu Y, Lau WB, Lopez
BL, Christopher TA, Koch W, Yue TL and Ma XL: Rosiglitazone
inhibits hypercholesterolaemia-induced myeloperoxidase
upregulation-a novel mechanism for the cardioprotective effects of
PPAR agonists. Cardiovasc Res. 81:344–352. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Onody A, Csonka C, Giricz Z and Ferdinandy
P: Hyperlipidemia induced by a cholesterol-rich diet leads to
enhanced peroxynitrite formation in rat hearts. Cardiovasc Res.
58:663–670. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasan AM, McCarron HC, Zhang Y and Jeremy
RW: Myocardial release of nitric oxide during ischaemia and
reperfusion: Effects of L-arginine and hypercholesterolaemia. Heart
Lung Circ. 16:274–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Genda S, Miura T, Miki T, Ichikawa Y and
Shimamoto K: K(ATP) channel opening is an endogenous mechanism of
protection against the no-reflow phenomenon but its function is
compromised by hypercholesterolemia. J Am Coll Cardiol.
40:1339–1346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Görbe A, Varga ZV, Kupai K, Bencsik P,
Kocsis GF, Csont T, Boengler K, Schulz R and Ferdinandy P:
Cholesterol diet leads to attenuation of ischemic
preconditioning-induced cardiac protection: the role of connexin
43. Am J Physiol Heart Circ Physiol. 300:H1907–H1913. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu N, Zhang X, Guan Y, Shu W, Jia P and
Jia D: Hypercholesterolemia abrogates the cardioprotection of
ischemic postconditioning in isolated rat heart: Roles of glycogen
synthase kinase-3β and the mitochondrial permeability transition
pore. Cell Biochem Biophys. 69:123–130. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma LL, Zhang FJ, Qian LB, Kong FJ, Sun JF,
Zhou C, Peng YN, Xu HJ, Wang WN, Wen CY, et al:
Hypercholesterolemia blocked sevoflurane-induced cardioprotection
against ischemia-reperfusion injury by alteration of the
MG53/RISK/GSK3β signaling. Int J Cardiol. 168:3671–3678. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horinaka S: Use of nicorandil in
cardiovascular disease and its optimization. Drugs. 71:1105–1119.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
IONA Study Group: Effect of nicorandil on
coronary events in patients with stable angina: The Impact Of
Nicorandil in Angina (IONA) randomised trial. Lancet.
359:1269–1275. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuchida A, Miura T, Tanno M, Sakamoto J,
Miki T, Kuno A, Matsumoto T, Ohnuma Y, Ichikawa Y and Shimamoto K:
Infarct size limitation by nicorandil: Roles of mitochondrial
K(ATP) channels, sarcolemmal K(ATP) channels, and protein kinase C.
J Am Coll Cardiol. 40:1523–1530. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Akao M, Teshima Y and Marbán E:
Antiapoptotic effect of nicorandil mediated by mitochondrial
atp-sensitive potassium channels in cultured cardiac myocytes. J Am
Coll Cardiol. 40:803–810. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu C, Minatoguchi S, Arai M, Wang N, Chen
XH, Bao N, Kawamura I, Yasuda S, Kobayashi H, Wu DJ, Takemura G and
Fujiwara H: Nicorandil improves post-ischemic myocardial
dysfunction in association with opening the mitochondrial K(ATP)
channels and decreasing hydroxyl radicals in isolated rat hearts.
Circ J. 70:1650–1654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagata K, Obata K, Odashima M, Yamada A,
Somura F, Nishizawa T, Ichihara S, Izawa H, Iwase M, Hayakawa A,
Murohara T and Yokota M: Nicorandil inhibits oxidative
stress-induced apoptosis in cardiac myocytes through activation of
mitochondrial ATP-sensitive potassium channels and a nitrate-like
effect. J Mol Cell Cardiol. 35:1505–1512. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa S, Tatsumi T, Shiraishi J,
Matsunaga S, Takeda M, Mano A, Kobara M, Keira N, Okigaki M,
Takahashi T and Matsubara H: Nicorandil regulates Bcl-2 family
proteins and protects cardiac myocytes against hypoxia-induced
apoptosis. J Mol Cell Cardiol. 40:510–519. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang A, Chen F, Xie Y, Guo Z and Yu Y:
Protective mechanism of nicorandil on rat myocardial
ischemia-reperfusion. J Cardiovasc Med (Hagerstown). 13:511–515.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato T, Sasaki N, O'Rourke B and Marbán E:
Nicorandil, a potent cardioprotective agent, acts by opening
mitochondrial ATP-dependent potassium channels. J Am Coll Cardiol.
35:514–518. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costa AD, Pierre SV, Cohen MV, Downey JM
and Garlid KD: cGMP signalling in pre- and post-conditioning: The
role of mitochondria. Cardiovasc Res. 77:344–352. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwemmer M, Sommer O, Koeckerbauer R and
Bassenge E: Cardiovascular dysfunction in hypercholesterolemia
associated with enhanced formation of AT1-receptor and of
eicosanoids. JCardiovasc Pharmacol Ther. 5:59–68. 2000. View Article : Google Scholar
|
|
30
|
Giricz Z, Görbe A, Pipis J, Burley DS,
Ferdinandy P and Baxter GF: Hyperlipidaemia induced by a
high-cholesterol diet leads to the deterioration of
guanosine-3′,5′-cyclic monophosphate/protein kinase G-dependent
cardioprotection in rats. Br J Pharmacol. 158:1495–1502. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang XL, Stein AB, Shirk G and Bolli R:
Hypercholesterolemia blunts NO donor-induced late preconditioning
against myocardial infarction in conscious rabbits. Basic Res
Cardiol. 99:395–403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasaki N, Sato T, Ohler A, O'Rourke B and
Marbán E: Activation of mitochondrial ATP-dependent potassium
channels by nitric oxide. Circulation. 101:439–445. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Harada N, Miura T, Dairaku Y, Kametani R,
Shibuya M, Wang R, Kawamura S and Matsuzaki M: NO donor-activated
PKC-δ plays a pivotal role in ischemic myocardial protection
through accelerated opening of mitochondrial K-ATP channels. J
Cardiovasc Pharmacol. 44:35–41. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuno A, Critz SD, Cohen MV and Downey JM:
Nicorandil opens mitochondrial K(ATP) channels not only directly
but also through a NO-PKG-dependent pathway. Basic Res Cardiol.
102:73–79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Iliodromitis EK, Lazou A and Kremastinos
DT: Ischemic preconditioning: Protection against myocardial
necrosis and apoptosis. Vasc Health Risk Manag. 3:629–637.
2007.PubMed/NCBI
|
|
36
|
Wang H, Zuo X, Wang Q, Yu Y, Xie L, Wang
H, Wu H and Xie W: Nicorandil inhibits hypoxia-induced apoptosis in
human pulmonary artery endothelial cells through activation of
mitoKATP and regulation of eNOS and the NF-κB pathway. Int J Mol
Med. 32:187–194. 2013.PubMed/NCBI
|
|
37
|
Gustafsson AB and Gottlieb RA: Bcl-2
family members and apoptosis, taken to heart. Am J Physiol Cell
Physiol. 292:C45–C51. 2007. View Article : Google Scholar : PubMed/NCBI
|