Introduction
Systemic lupus erythematosus (SLE) is an autoimmune
inflammatory disease which involves multiple organs. The disease is
progressively aggravated without intervention. Therapy with
immunosuppressants controls the progression of the disease in the
early stages (1–3). However, successful treatment of severe
lupus nephritis (LN), particularly when complicated with acute
kidney injury (AKI), cardiovascular or neuropsychiatric
involvement, remains a problem. Peritoneal dialysis (PD) has the
advantages of hemodynamic stability, residual renal function (RRF)
preservation, home dialysis and lower costs. Both of PD and
hemodialysis dialysis are used to treat kidney failure. PD uses the
lining of the abdominal cavity (peritoneal membrane) and a solution
(dialyusate) to remove waste and extra fluid from the body.
Hemodialysis uses a man-made membrane (dialyzer) to filter waste
and remove extra fluid from the blood. Previously, studies have
investigated the effects of PD in the treatment of AKI (4–7).
However, few observations with regard to PD as a treatment method
for severe LN patients with multiple-organ dysfunction have been
reported. Therefore, the aim of the present study was to
investigate the effect of PD as therapy for patients with severe
LN.
Patients and methods
Patients
In total, 13 patients, including 10 females and 3
males with a mean age of 36.3±13.3 years (age range, 18–54 years)
that had been admitted to the Department of Nephrology at Jinling
Hospital (Nanjing, China) between November 2003 and September 2010,
were included in the study. All the patients were diagnosed with
severe LN with rapid progressive glomerulonephritis (RPGN). Among
the patients, four individuals had heart diseases, including
enlargement of the cardiothoracic ratio, slight or moderate
pericardial effusion and pulmonary artery hypertension, and one
patient had lupus encephalopathy. The present study was approved by
the ethics committee of Nanjing University (Nanjing, China). All of
the patients approved the present study and gave their informed
consents.
Renal biopsy
A renal biopsy was performed in all the patients.
According to the 2003 International Society of Nephrology/Renal
Pathology Society classification criteria (2,6), seven
patients were class IV, five patients were class IV + V and one
patient was class III. Two patients had thrombotic microangiopathy.
The crescent formations from 10 to 83% of glomeruli were observed
in 9 patients.
Surgery and dialysis prescription
Open surgery was performed for PD catheter
insertion. Patients were initially advised to have three to four
daytime exchanges of one liter dextrose solution (1.5%). After one
week, the patients were discharged and the PD prescription was
changed to two liters dextrose solution [1.5 or 2.5% according to
the urine (UV) and ultrafiltration volume] with three or four
daytime exchanges.
Data collection
All the patients underwent a peritoneal
equilibration test at the outpatient department in the first month
following discharge. Dialysate, urinary protein, urinary sediment,
blood biochemistry, blood routine, autoantibody titer and
complement levels were examined every two months. PD efficiency
(Kt/V, Ccl) was also evaluated. The levels of albumin, hemoglobin,
serum creatinine were detected using a Blood biochemistry detection
kit (Tiangen, Beijing, China). Other observational indexes included
RRF, nutrition, systemic lupus erythematosus disease activity index
(SLE-DAI) and immunosuppressant and infectious complications.
Statistical analysis
Measurement data are expressed as the mean ±
standard deviation. The Student's t-test was used to analyze the
differences between the parameters prior to and following
treatment. P<0.05 was considered to indicate a statistically
significant difference. Analyses were performed using SPSS software
(SPSS, Inc., Chicago, IL, USA).
Results
Clinical and pathological features at
the baseline
Clinical and pathological observations at the
baseline are shown in Table I. All
the patients had severe renal dysfunction and the levels of serum
creatinine (Scr) ranged between 4.24 and 9.48 mg/dl. Oliguria and
anuria were present in five and two patients, respectively. Anemia
was identified in 10 patients, with hemoglobin (Hb) levels ranging
between 4.9 and 10.4 g/dl. All the patients exhibited
hypoalbuminemia with an average albumin (Alb) level of 29.7±5.7
g/l, and eight patients had severe hypoalbuminemia with a level of
<30 g/l. In total, four cases were complicated with cardiac
insufficiency, including chest distress, enlargement of the heart
shadow and congestion of the lungs, as observed by X-ray.
 | Table I.Baseline clinical and renal
pathological characteristics. |
Table I.
Baseline clinical and renal
pathological characteristics.
| Case | Gender | Age (years) | BUN (mg/dl) | Scr (mg/dl) | Pathology | Crescents (%) |
|---|
| 1 | F | 41 | 50 | 6.6 | Class IV, TMA | 0 |
| 2 | F | 51 | 94 | 4.2 | Class IV + V | 10,
fibrocellular |
| 3 | M | 24 | 137 | 9.5 | Class IV + V | 83,
fibrocellular |
| 4 | F | 24 | 79 | 7.5 | Class IV, 53%
glomerular sclerosis | 40,
fibrocellular |
| 5 | F | 54 | 103 | 6.1 | Class IV, TMA | 0 |
| 6 | F | 21 | 106 | 5.5 | Class IV + V | 27,
fibrocellular |
| 7 | M | 44 | 93 | 6.7 | Class IV, 59%
glomerular sclerosis | 28, cellular |
| 8 | M | 18 | 77 | 4.4 | Class V + IV | 77, cellular |
| 9 | F | 43 | 120 | 8.2 | Class IV | 84, cellular |
| 10 | F | 41 | 112 | 5.4 | Class IV | 0 |
| 11 | F | 41 | 69 | 4.3 | Class IV | 16, cellular |
| 12 | F | 35 | 44 | 9.3 | Class IV + V,
TMA | 6.5, fibrocellular;
Class IV 16, fibro |
| 13 | F | 46 | 46 | 8.5 | Class III | 26.7
fibrocellular |
Oral prednisone at a dose of 20–30 mg/day was
administered to all the patients. Six patients received intravenous
methylprednisolone pulse therapy (0.5 g/day for three days per
patient) prior to PD.
Treatment and follow-up
The dialysis dose was six liters per day in 10 cases
and 4 liters per day in the other three cases. Two cases were
administered continuous ambulatory peritoneal dialysis (CAPD),
while 11 cases received daytime ambulatory peritoneal dialysis
(Table II). During the follow-up
examinations, Kt/V calculations were performed in all the patients
and the results were all >1.7.
 | Table II.Clinical and dialysis indexes of the
13 patients prior to discharge. |
Table II.
Clinical and dialysis indexes of the
13 patients prior to discharge.
|
|
|
|
|
| Alb (g/l) | Scr (mg/dl) | UV (ml/day) |
|---|
|
|
|
|
|
|
|
|
|
|---|
| Case | Dialysis prescription
(ml/day) | Time (days) | Edema | UFV (ml/day) | Pre PD | Discharge | Pre PD | Discharge | Pre PD | Discharge |
|---|
| 1 | CAPD, 6000 | 14 | No | 1000 | 38.3 | 39.3 | 6.58 | 5.68 |
0 | 100 |
| 2 | DAPD, 6000 | 27 | No | 200 | 27.2 | 21.6 | 4.24 | 8.04 |
0 | 400 |
| 3 | DAPD, 4000 | 11 | No | 100 | 26.6 | 26.6 | 9.48 | 3.80 | 1400 | 400 |
| 4 | DAPD, 6000 | 8 | No | 100 | 21.0 | 21.2 | 7.49 | 7.30 |
300 |
800 |
| 5 | DAPD, 6000 | 7 | No |
0 | 27.9 | 30.3 | 6.14 | 6.83 |
200 |
200 |
| 6 | DAPD, 6000 | 12 | No | 700 | 31.1 | 31.6 | 5.52 | 4.22 |
700 | 1000 |
| 7 | DAPD, 6000 | 9 | No |
0 | 29.1 | 29.1 | 6.69 | 6.29 |
800 |
800 |
| 8 | DAPD, 6000 | 11 | No | 600 | 28.3 | 27.6 | 5.37 | 5.39 |
150 |
200 |
| 9 | CAPD, 6000 | 23 | No | 1500 | 23.8 | 27.4 | 8.23 | 4.26 |
370 |
500 |
| 10 | DAPD, 4000 | 9 | No | 100 | 33.0 | 34.3 | 5.42 | 3.89 |
810 |
900 |
| 11 | DAPD, 4000 | 16 | No | 100 | 40.4 | 38.6 | 4.26 | 3.73 |
265 | 1100 |
| 12 | DAPD, 6000 | 8 | No |
0 | 29.5 | 31.1 | 9.33 | 7.72 |
300 | 1500 |
| 13 | DAPD, 6000 | 7 | No | 100 | 30.4 | 39.3 | 8.46 | 6.58 |
600 |
100 |
Short-term effects
Following PD, there were no patients with edema and
the blood pressure was stable. No case developed heart failure and
the indicators of renal function and serum Alb levels were all
improved (Table II).
In total, 10 patients received oral mycophenolate
(MMF) one month following PD. The dosage of MMF was initiated at
0.75–1.5 g twice daily and the concentration was measured after one
or two months with three plasma samples, according to the strategy
developed by Shaw et al (8);
the dosage was titrated to maintain an area under the time
concentration curve between 0 and 12 h of MMF at 20–45 mg/h/l. The
additional three patients were treated with prednisone alone and
the daily dosage of prednisone started at ~0.6 mg/kg/day.
Long-term effects
The mean follow-up time was 8.1±6.3 months (range,
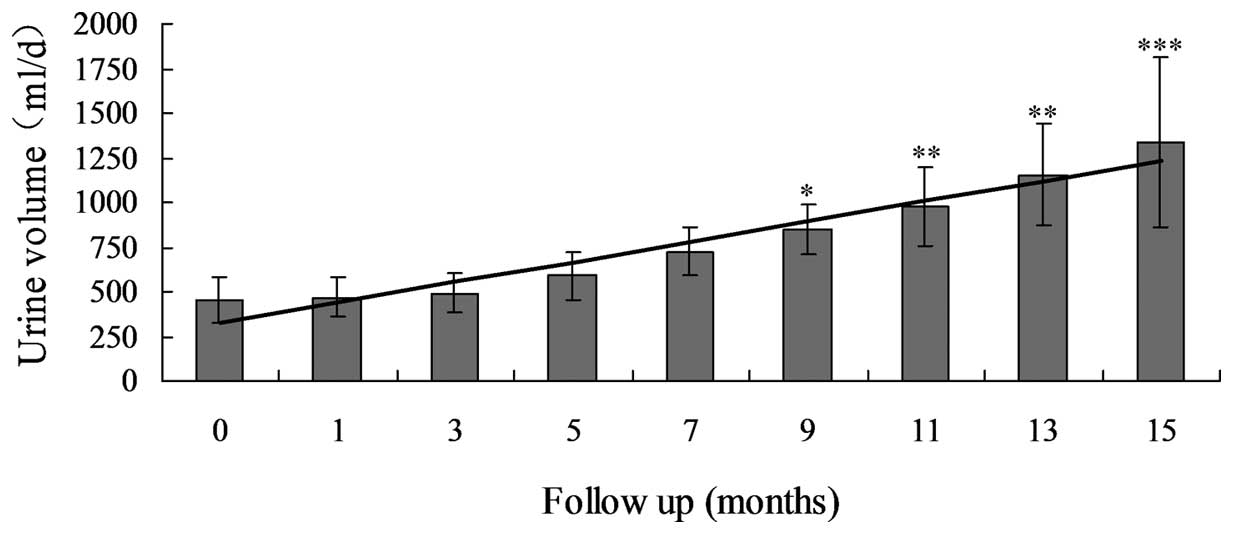
4–26 months). At the last follow-up examination, the UV had
significantly increased between 454.1±428.6 and 1333.6±475.8 ml
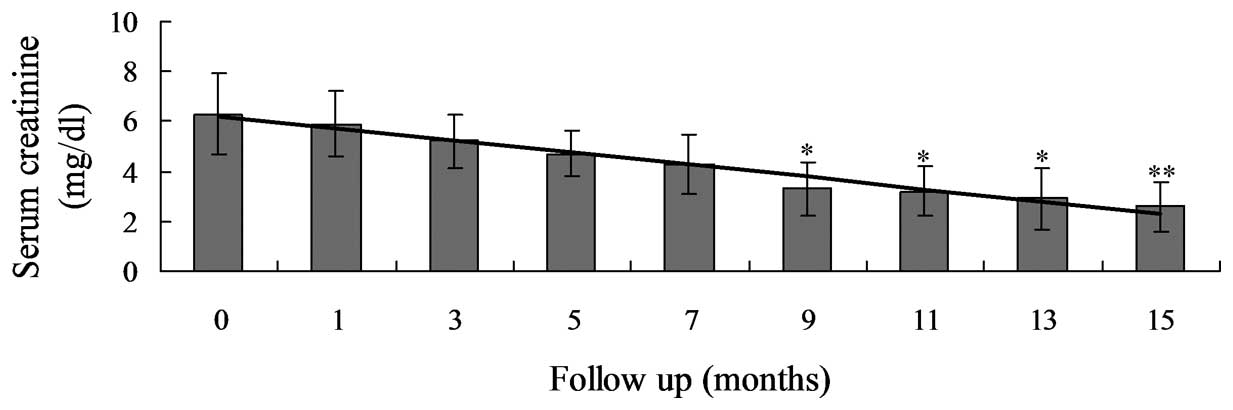
(P<0.0001; Table III; Fig. 1) and the Scr levels had significantly
decreased between 6.3±1.6 and 2.6±1.0 mg/dl (P<0.0001; Table III; Fig.
2). The residual glomerular filtration rate also significantly
increased from 4.6±3.2 to 17.4±6.7 ml/min (P<0.01). In addition,
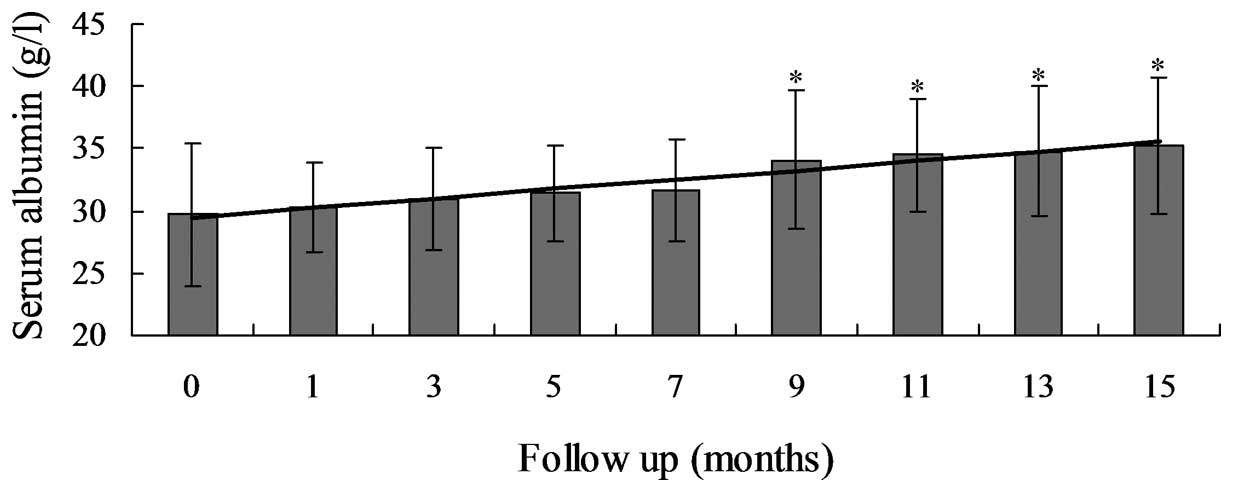
serum Alb and Hb levels markedly increased between 29.7±5.7 and
35.2±5.5 g/l (P=0.031) and between 8.7±1.8 and 9.8±1.8 g/dl
(P=0.016), respectively (Table
III; Fig. 3). Proteinuria and
hematuria were markedly decreased in six cases. Serum antinuclear
antibody levels were negative in five patients and had decreased
markedly in the additional eight patients. Anti-double-stranded DNA
(anti-ds-DNA) antibody tests were positive in five patients at the
baseline, but became negative at last follow-up examination in four
patients. The anti-ds-DNA antibody titer decreased in the remaining
patient. The SLE-DAI decreased significantly between 15±4 and 6±2
(P<0.001). In total, 10 patients discontinued PD treatment due
to the recovery of renal function, while three patients continued
with a decreased dosage of PD for the improvement of renal
function.
 | Table III.Clinical and dialysis indexes of the
13 patients during the follow-up. |
Table III.
Clinical and dialysis indexes of the
13 patients during the follow-up.
|
|
|
|
| rGFR (ml/min) | Alb (g/l) | Scr (mg/dl) | Hb (g/dl) | UV (ml/day) |
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Case | Dialysis
prescription (ml/day) | Follow-up
(months) | Outcome | Pre PD | Post PD | Pre PD | Post PD | Pre PD | Post PD | Pre PD | Post PD | Pre PD | Post PD |
|---|
| 1 | CAPD, 6000 | 26 | Discontinued |
0.0 | 11.6 | 38.3 | 43.8 | 6.58 | 2.70 |
4.9 | 12.3 |
0 | 2000 |
| 2 | DAPD, 6000 | 4 | Discontinued |
0.0 | 21.6 | 27.2 | 25.1 | 4.24 | 1.29 |
7.9 |
7.8 |
0 | 1500 |
| 3 | DAPD, 4000 | 6 | Continued |
7.3 | 18.1 | 26.6 | 38.7 | 9.48 | 3.18 |
9.0 | 10.7 | 1400 | 1300 |
| 4 | DAPD, 6000 | 4 | Discontinued |
7.1 | 19.9 | 21.0 | 34.6 | 7.49 | 3.05 |
9.3 |
6.7 |
300 |
600 |
| 5 | DAPD, 6000 | 4 | Discontinued |
9.2 | 19.1 | 27.9 | 35.7 | 6.14 | 1.61 |
6.9 |
9.2 |
200 | 1500 |
| 6 | DAPD, 6000 | 16 | Continued |
4.0 | 10.1 | 31.1 | 38.2 | 5.52 | 3.60 |
8.9 | 10.3 |
700 | 1400 |
| 7 | DAPD, 6000 | 5 | Discontinued |
5.5 |
7.9 | 29.1 | 29.1 | 6.69 | 4.33 |
8.4 |
8.7 |
800 | 1000 |
| 8 | DAPD, 6000 | 9 | Continued |
1.5 |
9.4 | 28.3 | 35.5 | 5.37 | 2.70 | 10.4 |
8.8 |
150 |
670 |
| 9 | CAPD, 6000 | 9 | Discontinued |
1.8 | 16.3 | 23.8 | 29.4 | 8.23 | 2.56 |
8.3 | 10.3 |
370 | 1500 |
| 10 | DAPD, 4000 | 5 | Discontinued |
9.2 | 23.0 | 33.0 | 38.6 | 5.42 | 1.42 | 11.5 | 12.7 |
810 | 2100 |
| 11 | DAPD, 4000 | 5 | Discontinued |
5.8 | 13.2 | 40.4 | 38.4 | 4.26 | 1.62 | 10.3 | 10.4 |
265 | 1100 |
| 12 | DAPD, 6000 | 7 | Discontinued |
3.6 | 27.9 | 29.5 | 38.7 | 9.33 | 1.55 |
5.4 |
9.5 |
300 | 1800 |
| 13 | DAPD, 6000 | 5 | Discontinued |
5.4 | 28.0 | 30.4 | 34.3 | 8.46 | 1.76 |
4.8 | 11.6 |
600 | 1600 |
Complications
During the follow-up period, only one case developed
peritonitis following diarrhea, and recovered via the
administration of antibiotics. Two cases who received MMF treatment
developed pneumonia, while one case developed peritoneal
leakage.
Discussion
In total, 13 severe LN patients with AKI that had
undergone PD therapy were reported in the study. PD was
demonstrated to be a simple, safe, gentle and efficient renal
replacement therapy method, with the ability to correct AKI-induced
metabolic, electrolytic and acid-base disorder and volume overload.
Compared with hemodialysis (HD), PD has been associated with faster
recovery of renal function in AKI and better maintenance of
residual function in patients with CKD (9,10). This
may be due to the improved cardiovascular tolerance associated with
this renal replacement therapy method. This method presents
episodes of hypotension (and consequently renal ischemia), and to
be a lower level of activation of the inflammatory pathway, since
the blood is not in contact with artificial membranes (11–13). PD
is particularly suitable for patients with refractory heart failure
or hemodynamically instable conditions where systemic
anticoagulation should be avoided.
Data on PD treatment in severe LN cases are limited.
Immunosuppressive therapy is the basic process to control lupus
activity, however, this is limited in patients with complications
such as RPGN or AKI. All the patients in the present study
developed renal dysfunction to varying degrees, with Scr levels
ranging between 4.24 and 9.48 mg/dl. Conditions worsened due to
oliguria and congestive heart failure, particularly following
methylprednisolone pulse therapy. In this situation, PD therapy was
selected for support. The results demonstrated that PD had marked
effects for these patients. The majority of patients had time for
the recovery of renal function and, more importantly, these
patients reached a stable homeostasis following the administration
of PD for one month, thus, it was possible to reapply
immunosuppressants. PD is suitable therapy for patients with a high
catabolism, oliguria, anuria, severe innutrition, water-sodium
retention, prerenal failure and cardiovascular problems. All of the
patients were followed up until the present study was
completed.
It has been reported that patients behave
differently within a short time period due to rapidly progressive
LN. In 10–20% of these patients, renal function may recover or
partly recover within a four-month period (14), allowing the cessation of HD. It has
been reported that 10–28% of LN patients require renal replacement
therapy to achieve partial remission from renal failure (15). In the present study, the 13 patients
were found to have azotemia caused by active LN, prerenal factors
or steroids. There were a number of indicators, including
enlargement of kidney size. Patients with active pathological
changes, including crescents or loop necrosis, should be treated
actively. Compared with HD, PD has little influence on the
hemodynamics and preserves the RRF well (16,17). PD
has been shown to be more conducive to renal function recovery. In
the 10 patients receiving immunosuppressive therapy assisted with
PD, the RRF improved, the UV increased and Scr levels decreased
gradually. In total, 76.9% (10/13) of the patients discontinued the
PD treatment and had the PD catheters removed. For these patients,
PD maintained the capability balance and removed nitrogen
production. Thus, PD offered great support and safeguard for
further treatment with immunosuppressants. In addition, nutrition
improved since there was no limit in protein or other nutrient
intake during PD. Therefore, we hypothesize that PD should be
applied early for these LN patients. In order to preserve the RRF,
body weight, UV and ultrafiltration should be monitored during PD.
These parameters are not only used to decide the hypertonic
dialysate in patients with heavy edema, but may also be used to
estimate the RRF.
Immunosuppressants may be applied timely and
reasonably during the PD process in order to further treat the
primary disease. Previous studies have largely focused on the
impact of immunological insults on SLE patients following dialysis
(18). Nevertheless, the
nonimmunological effects are also important. Therefore, renal
survival may be more representative of predialysis lupus severity
compared with the SLE-DAI or serological lupus activity. The use of
immunosuppressive therapy in LN patients with severe renal
insufficiency remains controversial. Due to long-term exposure to
steroids or cytotoxic drugs, LN patients have been shown to have
accelerated atherosclerosis and increased risks of infectious
complications (19).
PD patients with LN have significantly lower
predialysis levels of serum Alb and Hb compared with non-LN
patients. In addition, LN patients are more likely to suffer from
various infections due to hypoimmunity (19). Previous studies have revealed that
when compared with non-LN patients undergoing CAPD, LN patients had
a lower Alb level, more complications associated with infection and
a poorer life quality (20–23). In the study by Andrews et al
(24), risk factors for infectious
complications included a significantly lower level of Alb at the
start of PD as well as the patients being on immunosuppressives. In
previous studies, a significantly higher incidence of peritonitis
and other infections was observed in LN patients undergoing PD. The
incidence of exit site infections was higher in the study by Liang
et al (23). In addition, the
overall mortality rate was higher in the SLE group (32%) as
compared with the control group (9%). The effect of
immunosuppression on the high incidence rate of peritonitis and
other infections in PD patients is further documented by a study
from Guy's Hospital (19). As well
as short-term effects, peritonitis may have additional serious side
effects. Peritonitis can induce exacerbations of the disease and
may contribute to the development of encapsulating peritoneal
sclerosis, particularly in lupus patients (25). Therefore, these results indicate that
PD may not be the first choice for renal replacement therapy in
lupus patients undergoing immunosuppressive therapy. However, we
hypothesize that the purpose of PD adjuvant therapy in LN patients
is not to treat the protopathy, but to protect the RRF and improve
the azotemia and nutrition of the patients in order to earn time
and improve the condition for further immunosuppressant treatment.
Thus, all the patients in the present study continued to receive
immunosuppressive therapy during PD and a number of them required
immunosuppressants such as MMF. LN in these patients was shown to
be controlled following treatment. Autoantibody levels in a number
of patients turned negative, complement levels were elevated to a
normal level, urinalysis was improved, RRF was recovered and
dialysis treatment was ceased.
The incidence of infectious complications was high.
In order to prevent these complications, prednisone treatment was
decreased to 10 mg/day prior to surgery. Interventions to reduce
catheter-associated infections included sterile placement
techniques, appropriate local dressing and catheter care. Full-time
doctors and nurses performed these procedures. Follow-up via
telephone was regularly conducted by nurses. In the group of 13
patients, only one individual developed peritonitis and two
patients developed pneumonia. In addition, the incision was
difficult to heal and leakage of the dialysate occurred easily.
Thus, two pockets were ligated during surgery and a small dosage of
dialysate was applied following surgery; the initial dose was 1,000
ml per time and this was performed three to four times daily,
gradually increasing the dose. All the patients healed without
infection, with the exception of one case that had dialysate
leakage. Following pausing PD and undergoing hemofiltration, the
leakage was stopped and PD was continued.
The cost of PD is lower than hemofiltration. In the
present study, the expenses of PD were $120 per week at a dose of
6,000 ml per day, while the expenses of hemofiltration were at
least $714 per week. PD patients were able to manage by themselves
conveniently without any interruption of daily life. The time of
renal function recovery was difficult to estimate. A total of 10
patients in the group recovered in 1–4 months following PD. One
case achieved gradual remission with PD over 15 months. Long-term
hemofiltration is likely to result in a heavy economic burden.
PD not only improves the fluid and electrolyte
imbalance, but also significantly reduces the effect of systemic
cytokines. PD can clear cytokines, including interleukin (IL)-6,
IL-10 and tumor necrosis factor-α (26). Future research should focus on the
associations among inflammatory factors, severity and duration in
the acute phase of SLE.
In conclusion, PD is not only a replacement method,
but also a therapy. PD is an adjuvant method that may be used for
treating LN patients complicated with severe organ dysfunction. PD
can preserve the RRF, improve the nutritional status of the
patients and provide conditions and guarantees for further
immunosuppressive therapy of SLE. In contrast to others studies,
the results of the present study, with low infection and mortality
rates coupled with a high rate of recovery of renal function,
indicate that PD can be considered as a treatment option for
patients with severe LN and AKI who require ongoing
immunosuppressive therapy.
References
|
1
|
Zhu X, Li F, Yang B, Liang J, Qin H and Xu
J: Effects of ultraviolet B exposure on NDA methylation in patients
with systemic lupus erythematosus. Exp Ther Med. 5:1219–1225.
2013.PubMed/NCBI
|
|
2
|
Fayaz A, Pirson Y, Cosyns JP, Yango L and
Lambert M: Pauci-immune necrotizing and crescentic
glomerulonephritis in a patient with systemic lupus erythematosus.
Clin Nephrol. 69:290–293. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao L, Liu G, Li C, Li Y, Wang Z, Zhou Z,
Tong S and Wu X: Specificity of anti-SSB as a diagnostic marker for
the classification of systemic lupus erythematosus. Exp Ther Med.
5:1710–1714. 2013.PubMed/NCBI
|
|
4
|
Soni S and Saboo S: High-volume peritoneal
dialysis in acute kidney injury. Kidney Int. 75:11192009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ren W, Chen W, Pan HX, Lan L, Wang P,
Huang YH, Kong M and Wang K: Clinical application of right
low-position modified peritoneal dialysis catheterization. Exp Ther
Med. 5:457–460. 2013.PubMed/NCBI
|
|
6
|
Rietveld A and Berden JH: Renal
replacement therapy in lupus nephritis. Nephrol Dial Transpl.
23:3056–3060. 2008. View Article : Google Scholar
|
|
7
|
Zhang ZY, Zhou CH, Li MX and Yu YW:
Long-term efficacy of intermittent peritoneal dialysis using
various doses. Exp Ther Med. 3:519–524. 2012.PubMed/NCBI
|
|
8
|
Shaw LM, Nicholls A, Hale M, Armstrong VW,
Oellerich M, Yatscoff R, Morris RE, Holt DW, Venkataramanan R,
Haley J, Halloran P, Ettenger R, Keown P and Morris RG: Therapeutic
monitoring of mycophenolic acid. A consensus panel report. Clin
Biochem. 31:317–322. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang A, Wang Y, Wang G, Zhou Z and Yang X:
Infective endocarditis associated with acute renal railure: Repeat
renal biopsy and successful recovery. Exp Ther Med. 1:433–436.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chionh CY, Ronco C, Finkelstein FO, Soni
SS and Cruz DN: Acute peritoneal dialysis: what is the ‘adequate’
dose for acute kidney injury? Nephrol Dial Transplant.
25:3155–3160. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stenvinkel P, Chung SH, Heimbürger O and
Lindholm B: Malnutrition, inflammation, and atherosclerosis in
peritoneal dialysis patients. Perit Dial Int. 21(Suppl 3):
S157–S162. 2001.PubMed/NCBI
|
|
12
|
Li L, Wang R, Shi HH, Xie L, Li JD, Kong
WC, Tang JT, Ke DN and Zhao LY: In vitro study on the feasibility
of magnetic stent hyperthermia for the treatment of cardiovascular
restenosis. Exp Ther Med. 6:347–354. 2013.PubMed/NCBI
|
|
13
|
Takahashi T, Kubota M, Nakamura T, Ebihara
I and Koide H: Interleukin-6 gene expression in peripheral blood
mononuclear cells from patients undergoing hemodialysis or
continuous ambulatory peritoneal dialysis. Ren Fail. 22:345–354.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coplon NS, Diskin CJ, Petersen J and
Swenson RS: The long-term clinical course of systemic lupus
erythematosus in end-stage renal disease. N Engl J Med.
308:186–190. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheigh JS and Stenzel KH: End-stage renal
disease in systemic lupus erythematosus. Am J Kidney Dis. 21:2–8.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou F, Ji J, Song Q, Peng Z, Zhang G and
Wang Y: Pulmonary fat embolism and related effects during femoral
intramedullary surgery: An experimental study in dogs. Exp Ther
Med. 6:469–474. 2013.PubMed/NCBI
|
|
17
|
Gabriel DP, Caramori JT, Martin LC,
Barretti P and Balbi AL: Continuous peritoneal dialysis compared
with daily hemodialysis in patients with acute kidney injury. Perit
Dial Int. 29(Suppl 2): S62–S71. 2009.PubMed/NCBI
|
|
18
|
Rodby RA, Korbet SM and Lewis EJ:
Persistence of clinical and serologic activity in patients with
systemic lupus erythematosus undergoing peritoneal dialysis. Am J
Med. 83:613–618. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altieri P, Sau G, Cao R, Barracca A,
Menneas A, Micchittu B, Cabiddu G, Esposito P and Pani A:
Immunosuppressive treatment in dialysis patients. Nephrol Dial
Transplant. 17(Suppl 8): 2–9. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Siu YP, Leung KT, Tong MK, Kwan TH and Mok
CC: Clinical outcomes of systemic lupus erythematosus patients
undergoing continuous ambulatory peritoneal dialysis. Nephrol Dial
Transplant. 20:2797–2802. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tachaudomdach C, Kantachuvesiri S,
Changsirikulchai S, Wimolluck S, Pinpradap K and Kitiyakara C:
Connective tissue growth factor gene expression and decline in
renal function in lupus nephritis. Exp Ther Med. 3:713–718.
2012.PubMed/NCBI
|
|
22
|
Weng CH, Hsu CW, Yu CC, Yen TH, Yang CW
and Hung CC: Peritoneal dialysis and hemodialysis in systemic lupus
erythematosus patients: comparison of clinical outcomes. Kidney
Blood Press Res. 32:451–456. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang CC, Huang CC, Wang IK, Chang CT,
Chen KH, Weng CH, Lin JL, Hung CC, Yang CW and Yen TH: Impact of
renal survival on the course and outcome of systemic lupus
erythematosus patients treated with chronic peritoneal dialysis.
Ther Apher Dial. 14:35–42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Andrews PA, Warr KJ, Hicks JA and Cameron
JS: Impaired outcome of continuous ambulatory peritoneal dialysis
in immunosuppressed patients. Nephrol Dial Transplant.
11:1104–1108. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Odama UO, Shih DJ and Korbet SM:
Sclerosing peritonitis and systemic lupus erythematosus: a report
of two cases. Perit Dial Int. 19:160–164. 1999.PubMed/NCBI
|
|
26
|
Szakszon K, Csízy I and Szabó T: Early
introduction of peritoneal dialysis may improve survival in severe
sepsis. Pediatr Emerg Care. 25:599–602. 2009. View Article : Google Scholar : PubMed/NCBI
|