Introduction
Gestational diabetes mellitus (GDM) refers to
abnormal glucose tolerance, a common complication that occurs in
some women for the first time during the gestation period (1). According to the latest diagnostic
criteria of the International Association of Diabetes and Pregnancy
Study Groups (IADPSG), the incidence of GDM has increased to 17.8%
(2). GDM may lead to many
complications in mothers and infants and pose a serious threat to
their health. The short- and long-term effects of GDM on pregnant
and lying-in women and perinatal infants have aroused considerable
attention (2–4).
Early diagnosis and the timely treatment of GDM have
always been a focus of clinicians. In recent years, clinical
studies have shown that the onset of GDM was closely associated
with a number of factors, including gestational hypertension,
polyhydramnios, infection, advanced age and family history of
diabetes and obesity in pregnancy (3–5). Further
clinical investigation is required to verify the precise
relationship between the onset of GDM and some of the above
influencing factors in order to make a clear diagnosis and identify
treatment for this disease.
At present, the treatment of GDM is mainly focused
on the monitoring, evaluation and control of blood sugar. In
clinic, two types of indicators are monitored, the instant blood
glucose (such as fasting blood glucose) and the long-term blood
glucose monitoring indicator, such as glycosylated hemoglobin
(HbAlc). However, the two indicators have their shortcomings.
Fasting blood glucose is greatly influenced by previous diet,
mental state and other factors such as stress, and exhibits great
fluctuations, making it difficult to control. HbA1c only reflects
the blood glucose level during the previous 2–3 months and has a
relatively shorter observation period for GDM, thus, it is not
sensitive. Glycated albumin (GA) reflects the average blood glucose
within 2–3 weeks and also provides a short-term monitoring
indicator. Additionally, the level of GA was not affected by the
red blood cell lifetime. The level of GA has been utilized to
monitor the status of blood glucose control in diabetics. However,
few reports besides Asian studies are currently available on the
relationship between GA and the occurrence of GDM and blood glucose
control.
Materials and methods
Selection of cases
Pregnant and lying-in women with GDM who had
undergone childbirth in the Womens Hospital, School of Medicine,
Zhejiang University (Zhejiang, China), between August 2013 and May
2014 were selected for this study. Additionally, pregnant women
without GDM were selected as normal controls. The study was
approved by the Institutional Ethics Committee of Women's Hospital,
School of Medicine, Zhejiang University (Zhejian, China). Written
informed consent was obtained from each participant. The diagnostic
criteria as stipulated by IADPSG, included a 75 g glucose tolerance
test and fasting blood glucose (FGP; after a 12-h fast).
Specific diagnostic criteria were as follows: i)
Oral glucose tolerance test (OGTT; 75 g), for which the subjects
were required to fast for 8–14 h prior to the test, and received
300 ml liquid composed of 75 g glucose orally in 5 min. Elbow
venous blood (3 ml) was withdrawn during the fasting state and at 1
and 2 h after glucose load to measure the blood glucose, using the
glucose oxidase method (Glucose Kit; BioSino Bio-Technology and
Science Inc., Beijing, China). ii) IADPSG criteria: If one or more
of the following criteria were exceeded during the OGTT, a
diagnosis of GDM was considered. Blood glucose levels were measured
at fasting, 5.1 mmol/l; at 1 h, 10.0 mmol/l; and at 2 h, 8.5
mmol/l. If fasting blood glucose was ≥7.0 mmol/l or HbAlc ≥6.5% or
random blood glucose was ≥11.1 mmol/l, the diagnosis was overt
diabetes mellitus.
Determination of influencing
factors
An influencing factor record (questionnaire) for
pregnant and lying-in women with gestational diabetes mellitus was
created. Information of the aforementioned and normal control
cases, including mother's age, mother's degree of education, urban
resident or not, cesarean delivery, BMI, weight, height,
hypertensive disorders complicating pregnancy (HDP), in
vitro fertilization (IVF), preterm birth, GA-L, FGP, family
hypertension history, and family diabetes history was recorded.
Detection method of GA-L and FGP
Detection of GA was carried out using the Lucica
GA-L glycated albumin assay kit (Lucica® GA-L; Asahi Kasei Corp.,
Tokyo, Japan), which utilizes bromcresol purple, and has higher
specificity for albumin, as compared to the previously used
bromocresol green, which was not as specific. The levels of GA and
serum albumin (GA-L) were expressed as a percentage to exclude the
influence of serum albumin on the detection result. Blood glucose
was measured using the glucose oxidase method.
Definition and assignment of major
variables
For dependent variables GD cases were assigned as 1
and control subjects were assigned 0 (Table I). Definitions of some of the
independent variables were as follows: Elderly parturient women,
age>35 years; BMI, weight in kilograms divided by the square of
height in meters; IVF, in vitro fertilization; hypertensive
disorders complicating pregnancy (HDP) were considered as a disease
characterized by the coexistence of pregnancy and hypertension
(systolic pressure ≥140 mmHg and/or diastolic pressure ≥90 mmHg) or
with urinary protein ≥0.3 g/24 h, or positive random urinary
protein after pregnancy for 20 weeks, and in severe cases, with
convulsion; preterm birth was considered as the birth of a baby at
>28 weeks and <37 weeks gestational age; family hypertension
history included one or several relatives with hypertension; and
family diabetic history included one or several relatives with
diabetes (Fig. 1).
 | Table I.Assignment table of some influencing
factors of GDM pregnant women. |
Table I.
Assignment table of some influencing
factors of GDM pregnant women.
| Indicator |
| Assignment |
|---|
| Mothers age >35
years | Yes | 1 |
|
| No | 0 |
| Permanent job | Yes | 1 |
|
| No | 0 |
| Pregnancy
hypertension | Yes | 1 |
|
| No | 0 |
| HDP | Yes | 1 |
|
| No | 0 |
| Family diabetic
history | Yes | 1 |
|
| No | 0 |
| College
education | Yes | 1 |
|
| No | 0 |
| External
fertilization | Yes | 1 |
|
| No | 0 |
| Preterm birth | Yes | 1 |
|
| No | 0 |
| Cesarean
delivery | Yes | 1 |
|
| No | 0 |
| Weight | Constant
argument |
|
| Height | Constant
argument |
|
| BMI | Constant
argument |
|
| GA-L | Constant
argument |
|
| FGP | Constant
argument |
|
Statistical analysis
EpiData 3.1 (The EpiData Association, Odense,
Denmark) was used to construct the database with the original data,
and then the data were transformed into a recognizable format. SPSS
16.0 software (IBM Corporation, Chicago, IL, USA) was used for
statistical analysis for conducting single and multiple factor
conditional logistic regression analysis and to calculate the odds
ratio (OR) and 95% confidence interval (CI). If OR, 95% CI did not
include 1, the result was considered statistically significant.
Results
General condition
The total number of subjects included in the study
was 1,554, of which 893 pregnant women were confirmed with GDM and
661 healthy pregnant women served as the normal controls. The
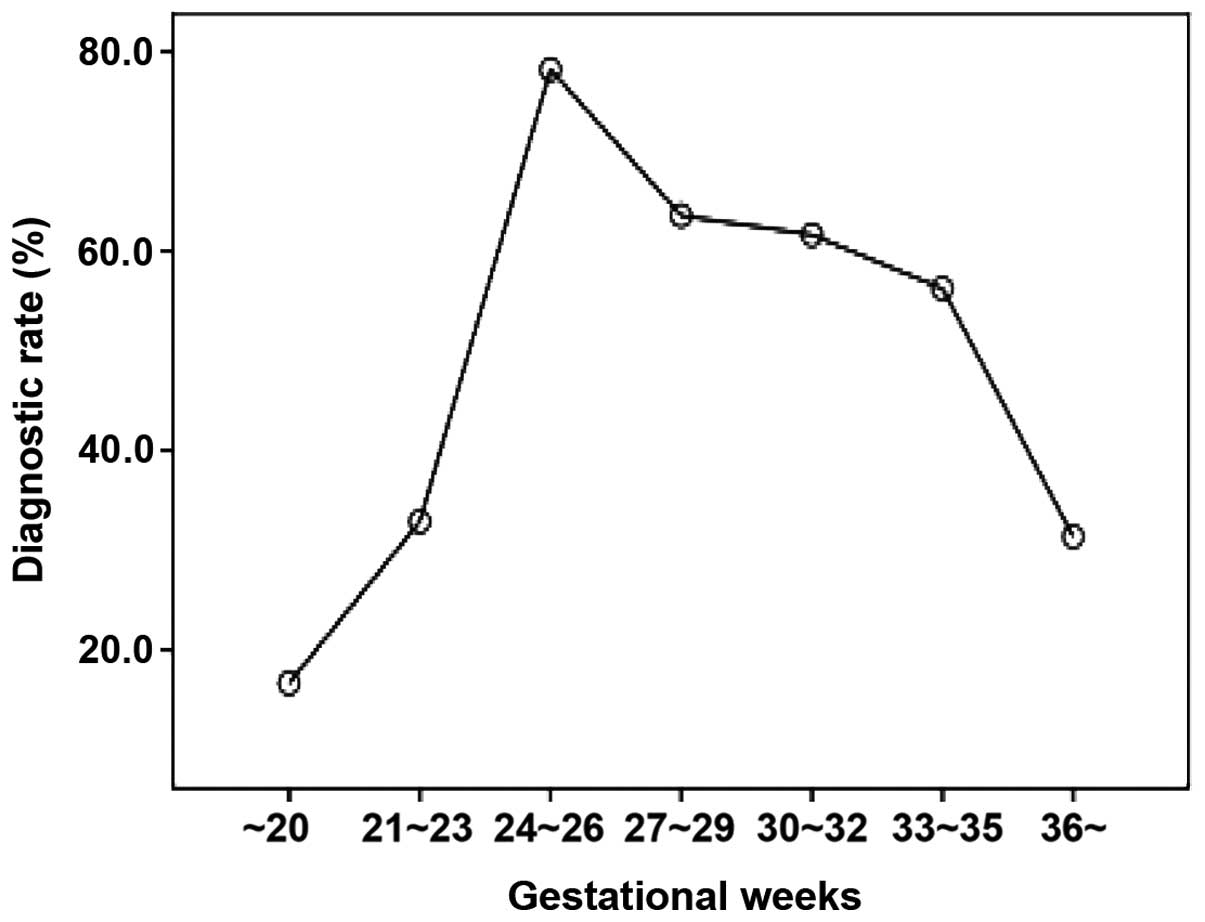
median pregnancy weeks of the GDM cases was 26 weeks and
gestational weeks with a relatively higher diagnostic rate were
24–26 weeks (diagnostic rate, 78.22%), 27–29 weeks (diagnostic
rate, 63.57%), and 30–32 weeks (diagnostic rate, 61.64%) (Fig. 1).
Changes of FGP concentration and GA-L
of pregnant women in different gestational weeks
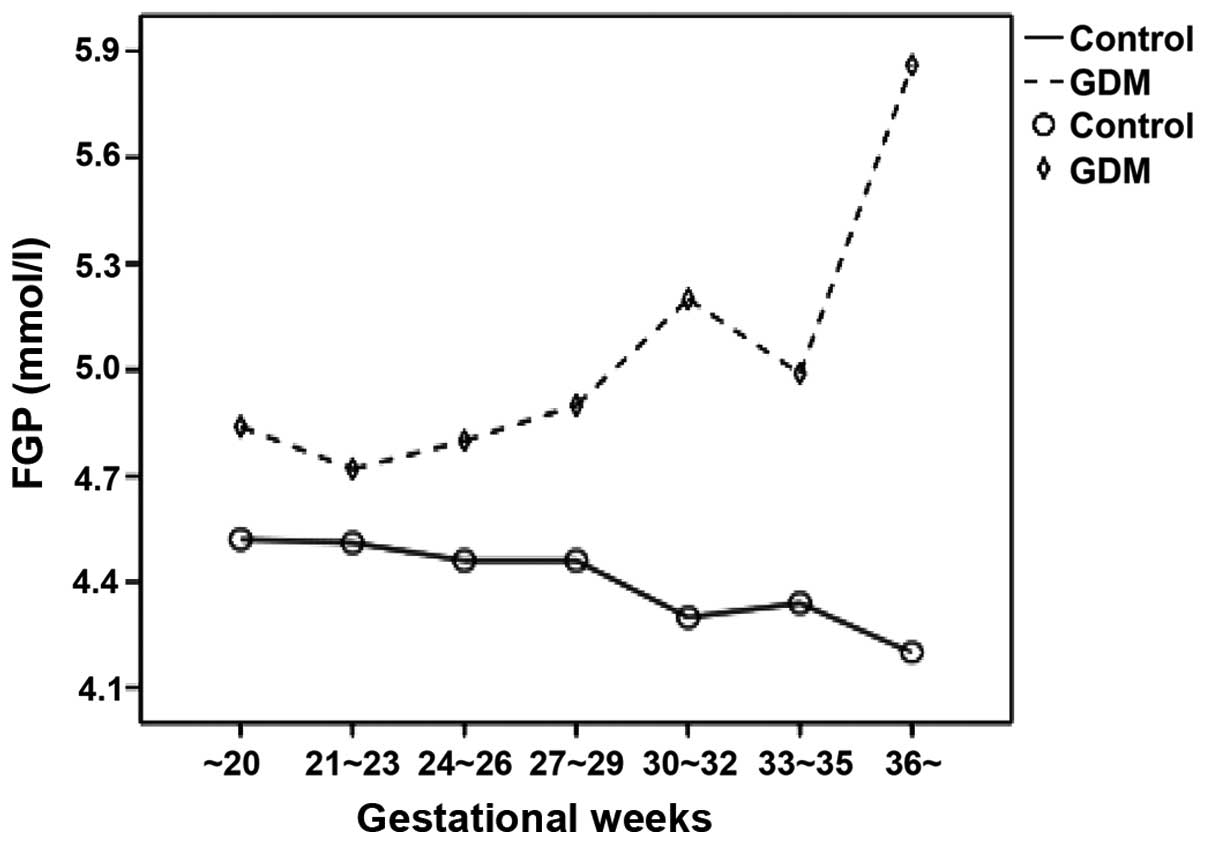
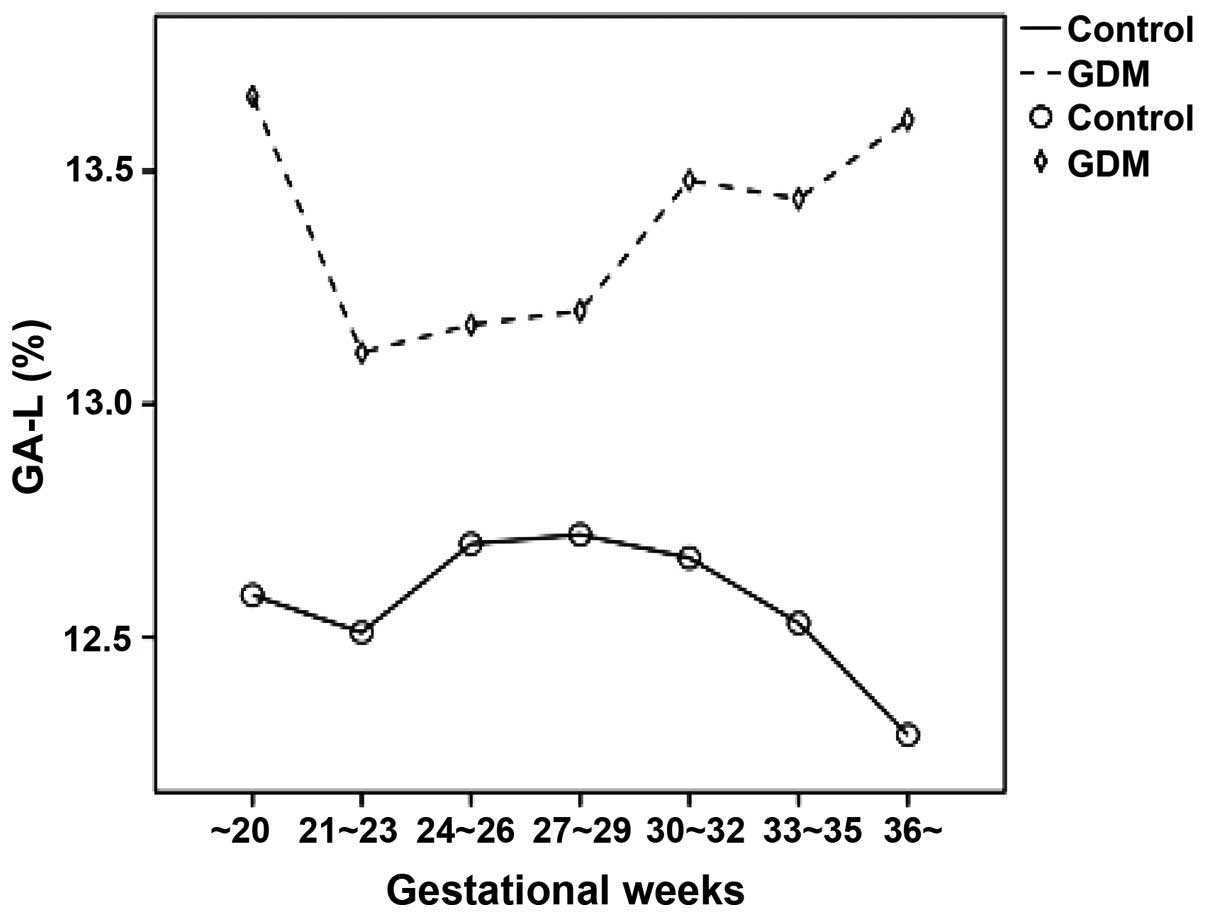
The FGP concentration and GA-L level in the pregnant
women with GDM at different gestational weeks were relatively
higher compared to the pregnant women in the normal control group.
As the gestational weeks increased, the FGP concentration and GA-L
value of the pregnant women in the normal control group gradually
decreased while those of the pregnant women with GDM markedly
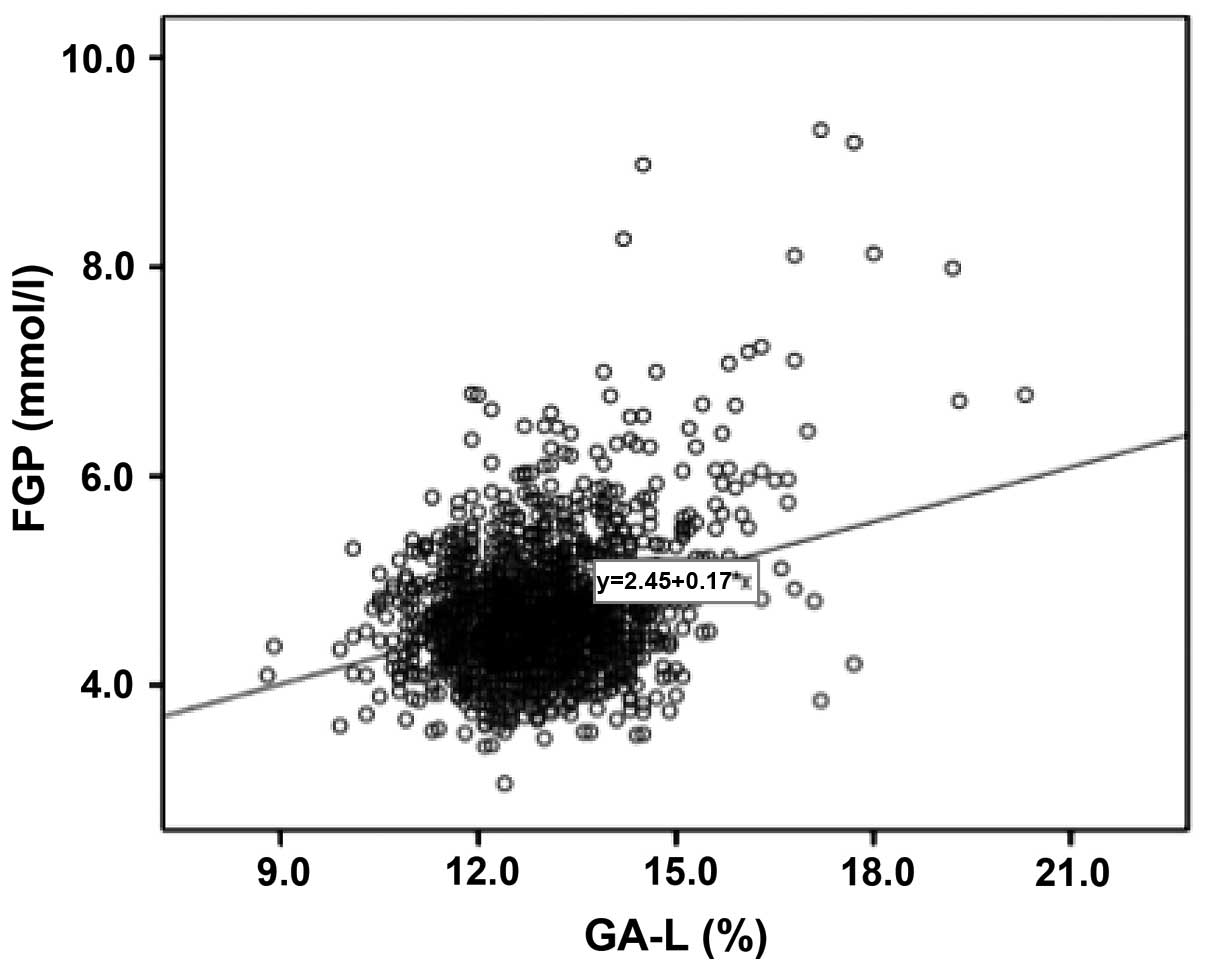
increased (Figs. 2 and 3). The results showed that FGP
concentration and GA-L value in all pregnant women were
significantly correlated (P=0.000; R2=0.103) (Fig. 4).
Single factor analysis of the general
condition of puerperas
The result of the single factor analysis (Table II) revealed that factors that were
statistically different with the occurrence of GDM included:
Mother's age >35 years (OR=1.802; 95% CI, 1.306–2.487),
complication of pregnancy hypertension (OR=3.381; 95% CI,
1.910–5.983), family hypertension history (OR=1.678; 95% CI,
1.132–2.486), family diabetic history (OR=3.848; 95% CI,
2.101–7.047), cesarean delivery (OR=1.544; 95% CI, 1.261–1.891),
height (OR=0.033; 95% CI, 0.004–0.307), BMI (OR=1.039; 95% CI,
1.006–1.073), GAL (OR=1.637; 95% CI, 1.479–1.813), and FGP
(OR=5.460; 95% CI, 4.246–7.020).
 | Table II.Single factor analysis of GDM. |
Table II.
Single factor analysis of GDM.
| Factor | P-value | OR (95% CI) |
|---|
| Mothers age |
|
|
| >35 years | 0.000 | 1.802
(1.306–2.487) |
| HDP | 0.000 | 3.381
(1.910–5.983) |
| FHH | 0.010 | 1.678
(1.132–2.486) |
| FDH | 0.000 | 3.848
(2.101–7.047) |
| College
education | 0.795 | 0.965
(0.736–1.265) |
| External
fertilization | 0.262 | 1.494
(0.741–3.009) |
| Preterm birth | 0.977 | 1.006
(0.657–1.541) |
| Cesarean
delivery | 0.000 | 1.544
(1.261–1.891) |
| Weight | 0.372 | 1.005
(0.994–1.017) |
| Height | 0.003 | 0.033
(0.004–0.307) |
| BMI | 0.019 | 1.039
(1.006–1.073) |
| GA-L | 0.000 | 1.637
(1.479–1.813) |
| FGP | 0.000 | 5.460
(4.246–7.020) |
Factors that had no direct correlation with GDM
included: College education (OR=0.965; 95% CI, 0.736–1.265),
external fertilization (OR=1.494; 95% CI, 0.741–3.009), preterm
birth (OR=1.006; 95% CI, 0.657–1.541), and weight (OR=1.005; 95%
CI, 0.994–1.017).
Multiple conditional logistic
regression analysis
The variables with statistical significance from the
single factor analysis were considered as independent variables,
and included, mother's age >35 years, complication of pregnancy
hypertension, family hypertension history, family diabetic history,
cesarean delivery, height, BMI, GA-L, and FGP, respectively.
Following the results of the multiple conditional logistic
regression analysis occurrence and non-occurrence of GDM were
considered as dependent variables. The multiple conditional
logistic regression model was employed to analyze the results from
the complication of pregnancy hypertension (OR=3.302; 95% CI,
1.705–6.394), family hypertension history (OR=2.970; 95% CI,
1.520–5.801), GA-L (OR=1.556; 95% CI, 0.940–2.012) and FGP
(OR=5.431; 95% CI, 4.097–7.198) (Table
III and Fig. 3).
 | Table III.Multiple conditional logistic
regression analysis of risk factors of GDM. |
Table III.
Multiple conditional logistic
regression analysis of risk factors of GDM.
| Factor | Regression
coefficient | Standard error | Wald | P-value | OR (95% CI) |
|---|
| HDP | 1.194 | 0.337 |
12.546 | 0.000 | 3.302
(1.705–6.394) |
| Family diabetic
history | 1.088 | 0.342 |
1.103 | 0.001 | 2.970
(1.520–5.801) |
| FGP | 1.692 | 0.144 | 138.519 | 0.000 | 5.431
(4.097–7.198) |
| GA-L | 0.442 | 0.061 |
52.664 | 0.000 | 1.556
(0.940–2.012) |
Discussion
GDM poses a serious threat to mothers and infants
(6). GDM may be controlled using a
series of treatments, including kinesitherapy, diet control, and
insulin. Other methods, such as strengthening the monitoring and
caring of pregnant mothers and infants, and selecting appropriate
delivery methods and delivery days based on the condition of
pregnant mothers, may decrease the complications of mothers and
infants and improve the prognosis of perinatal infants. Therefore,
a timely diagnosis of diabetes is crucial. Diagnostic criteria of
GDM have been unified, and include NDDG, ADA and IADPSG criteria
(7). In China, such criteria remain
to be established. Since 2011, our hospital has begun to utilize
IADPSG criteria as diagnostic criteria of GDM. IADPSG criteria do
not include the data of patients from the Chinese mainland, which
accounts for a large proportion of the overall Asian population.
The time period for GDM screening and diagnosis in Chinese pregnant
women was investigated in the present study. Our results indicated
that 24–29 weeks was the most appropriate time period to screen the
pregnant women with GDM.
The cause of GDM is different to that of general
diabetes. WHO defined GDM as an independent diabetes type that is
different from type 1 and type 2 diabetes. GDM is regarded as an
endocrine and metabolic disease characterized by hereditary
inclination and induced by various factors (5,8–10). Similar to general type 2 diabetes,
gestational diabetes has numerous common risk factors, and specific
causes of diseases associated with pregnancy. In a previous study
(10), a multiple factor analysis
was conducted and the results revealed that GA-L, FGP, HDP and
family diabetic history were closely associated with the onset of
GDM.
GDM may result in the elevation of the blood glucose
levels of patients, causing damage to mothers and infants. After
the insulin resistance and insulin secretion function of GDM
patients was impaired, their insulin secretion peak exhibited a
delay, eventually leading to hyperglycemia. Hyperglycemia
subsequently resulted in numerous short-term effects, including the
complication of early preeclampsia in pregnant women with GDM, the
increasing incidence of abortion, preterm birth and cesarean
delivery, the significant increase of fetal malformation, fetal
macrosomia, birth trauma, neonatal hypoglycemia and other diseases.
Long-term effects included a higher risk of type 2 diabetes on
puerperas themselves and a higher risk of obesity in adolescence,
as well as hypertension and diabetes in adulthood of their
offspring (2–4,10,11).
Adverse pregnancy outcomes appeared to be closely associated with
the blood glucose. The results of the present study have shown that
the concentration of FGP and value of GA-L in pregnant women with
GDM at different gestational weeks increased. The FGP concentration
and GA-L value of the pregnant women with GDM increased, indicating
that the glucose metabolic pathways of pregnant women with GDM
underwent a form of decompensation. In pancreatic β-cells,
increased β-cell volume and increased insulin secretion reaction
occurs to compensate for their insulin resistance during pregnancy.
It is considered that abnormal glucose tolerance develops in
pregnant women when this compensatory effect is insufficient. The
presence of pancreatic β-cell dysfunction in GDM has been
demonstrated (12).
GA reflects the recent blood glucose control state
of pregnant women with GDM. In the studies conducted by Yoshiuchi
et al (13), it was confirmed
that GA and the blood glucose in patients with type 1 and type 2
diabetes were closely correlated with HbA1c, with GA being a better
indicator for monitoring the blood glucose control of patients with
diabetes. According to a report by the Japan Glycated Albumin Study
Group of the Japanese Society of Diabetes and Pregnancy, the
frequencies of neonatal complications (neonatal hypoglycemia,
polycythemia, and respiratory disorder) and babies that are large
for gestational age were significantly higher in the group whose GA
value at the end of pregnancy was ≥15.8% compared with those of the
group whose GA value was <15.8% (14). Based on these results, the Japanese
Society of Diabetes and Pregnancy strongly recommended that GA
measurement was useful for the prevention of perinatal
complications in mothers and fetus/infants (15).
Our results demonstrated that GA and the
concentration of FGP on pregnant women with and without GDM were
closely correlated. The multi-factor regression analysis revealed
that the level of GA in pregnant women was closely associated with
the condition irrespective of GDM. Since GDM usually occurred
during pregnancy and the course was relatively short, FGP and
dynamic blood glucose monitoring may have some drawbacks.
Additionally, HbA1c may not reflect the short-term change of blood
glucose resulting from dietary changes or insulin treatment.
Compared with HbA1c, the half-life period of GA was 17–19 days and
it was able to reflect the average blood glucose within 2–3 weeks,
making it more sensitive to short-term blood glucose change.
Therefore, it was suggested that GA be applied to reflect the
recent blood glucose control in pregnant women with GDM.
Pregnant women with GDM suffered a higher risk (2-
to 4-fold) of experiencing complications by HDP than pregnant women
without diabetes. Previous findings (16–18) have
identified that GDM and HDP may have a common origin. Severe
insulin resistance and hyperinsulinemia were closely associated
with the complication of GDM by HDP. During the second and third
trimester, placentae secreted various types of insulin antagonists,
such as estriol, which reached a peak during the 32–34 weeks,
resulting in a gradual increase of insulin resistance. The
resulting hyperinsulinemia, in turn, caused the hyperfunction of
the sympathetic nervous system, retention of sodium and water, with
HDP aggravating the insulin resistance. Inflammatory factor
adiponectin was able to adjust the glycerolipid metabolism of the
body, protect blood vessel endothelium, and reduce blood pressure.
Adiponectin is known to play an important role in the
patho-mechanism of GDM and HDP (19).
Family history of diabetes has been associated with
the occurrence of GDM. Duman et al (17) studied 650 cases of outpatient
pregnant women in Turkey and showed that family diabetic history
was a predominant risk that predisposes pregnant women to
developing GDM. Additionally, previous studies (20,21) have
suggested that the history of diabetes immediate family members and
previous GDM history of pregnant women potentially increased the
incidence of GDM during gestation. The results of the present study
are consistent with findings of the aforementioned studies.
Acknowledgements
The present study was supported by the Department of
Education of Zhejiang Province (N20120047).
References
|
1
|
Yuen L and Wong VW: Gestational diabetes
mellitus: Challenges for different ethnic groups. World J Diabetes.
6:1024–1032. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sacks DA, Hadden DR, Maresh M,
Deerochanawong C, Dyer AR, Metzger BE, Lowe LP, Coustan DR, Hod M,
Oats JJ, et al: HAPO Study Cooperative Research Group: Frequency of
gestational diabetes mellitus at collaborating centers based on
IADPSG consensus panel-recommended criteria: The Hyperglycemia and
Adverse Pregnancy Outcome (HAPO) Study. Diabetes Care. 35:526–528.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Köck K, Köck F, Klein K, Bancher-Todesca D
and Helmer H: Diabetes mellitus and the risk of preterm birth with
regard to the risk of spontaneous preterm birth. J Matern Fetal
Neonatal Med. 23:1004–1008. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alfadhli EM: Gestational diabetes
mellitus. Saudi Med J. 36:399–406. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
World Health Orgnization: Definition
diagnosis and classification of diabetes mellitus and its
complications. Geneva: World Health Orgnization. 1999.
|
|
6
|
Hartling L, Dryden DM, Guthrie A, Muise M,
Vandermeer B, Aktary WM, Pasichnyk D, Seida JC and Donovan L:
Screening and diagnosing gestational diabetes mellitus. Evid Rep
Technol Assess (Full Rep). 210:1–3272012.
|
|
7
|
Agarwal MM: Gestational diabetes mellitus:
An update on the current international diagnostic criteria. World J
Diabetes. 6:782–791. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poomalar GK: Changing trends in management
of gestational diabetes mellitus. World J Diabetes. 6:284–295.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harder T and Plagemann A: A role for
gestational diabetes in the excess maternal transmission of type 2
diabetes? Diabetes Care. 23:431–432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Buchanan TA, Xiang AH and Page KA:
Gestational diabetes mellitus: Risks and management during and
after pregnancy. Nat Rev Endocrinol. 8:639–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ovesen PG, Jensen DM, Damm P, Rasmussen S
and Kesmodel US: Maternal and neonatal outcomes in pregnancies
complicated by gestational diabetes. a nation-wide study. J Matern
Fetal Neonatal Med. 28:1720–1724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hashimoto K and Koga M: Indicators of
glycemic control in patients with gestational diabetes mellitus and
pregnant women with diabetes mellitus. World J Diabetes.
6:1045–1056. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yoshiuchi K, Matsuhisa M, Katakami N,
Nakatani Y, Sakamoto K, Matsuoka T, Umayahara Y, Kosugi K, Kaneto
H, Yamasaki Y, et al: Glycated albumin is a better indicator for
glucose excursion than glycated hemoglobin in type 1 and type 2
diabetes. Endocr J. 55:503–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu I, Hiramatsu Y, Omori Y and
Nakabayashi M: Glycated albumin reflects maternal and perinatal
outcome in a multicenter study in Japan. Diabetes and Pregnancy.
10:27–31. 2010.
|
|
15
|
Koga M: Glycated albumin; clinical
usefulness. Clin Chim Acta. 433:96–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gordin D, Forsblom C, Groop PH, Teramo K
and Kaaja R: Risk factors of hypertensive pregnancies in women with
diabetes and the influence on their future life. Ann Med.
46:498–502. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Duman NB: Frequency of gestational
diabetes mellitus and the associated risk factors. Pak J Med Sci.
31:194–197. 2015.PubMed/NCBI
|
|
18
|
Pan J, Zhang F, Zhang L, Bao Y, Tao M and
Jia W: Influence of insulin sensitivity and secretion on glycated
albumin and hemoglobin A1c in pregnant women with gestational
diabetes mellitus. Int J Gynaecol Obstet. 121:252–256. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miehle K, Stepan H and Fasshauer M:
Leptin, adiponectin and other adipokines in gestational diabetes
mellitus and pre-eclampsia. Clin Endocrinol (Oxf). 76:2–11. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan LY, Wong SF and Ho LC: Diabetic
family history is an isolated risk factor for gestational diabetes
after 30 years of age. Acta Obstet Gynecol Scand. 81:115–117. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neelakandan R and Sethu PS: Early
universal screening for gestational diabetes mellitus. J Clin Diagn
Res. 8:OC12–OC14. 2014.PubMed/NCBI
|