Introduction
Medulloblastoma (MB) is a malignant epithelial tumor
of the central nervous system, which often occurs in children
(1). The underlying factors
contributing to MB are currently unknown. MB primary tumors are
able to develop in any part of the brain; however, they
predominantly occur in the cerebellar vermis, above the top of the
fourth ventricle. The initial clinical symptoms of MB include
headaches, vomiting and unstable walking, which may progress to
diplopia, ataxia and vision loss due to increased intracranial
pressure and cerebellar damage (2).
Tumorigenesis, development, invasion, metastasis,
and malignancy are closely associated with angiogenesis (3). The vascular endothelial cell growth
factor (VEGF) is an effective pro-angiogenic growth factor and is
an important regulator of angiogenesis, in which new blood vessels
are formed from existing ones in order to increase blood supply
(4). Angiogenesis has a key role in
the malignant transformation of normal tissues (5), and alterations in the expression levels
of numerous vascular growth factors, including VEGF, have been
detected during the development of various tumor types (6). Furthermore, VEGF has been associated
with the development of numerous diseases, including coronary heart
disease (7), cardiac X syndrome
(8), hypertension (9), cerebrovascular disease (10), and diabetic nephropathy (11).
Previous studies have detected an association
between upregulation of micro (mi)RNA-210 expression levels and
elevated expression levels of VEGF in kidney tissue samples, during
bone necrosis, and in glioblastoma (12–14).
However, to the best of our knowledge, the relationship between
miRNA-210 and VEGF in patients with MB has yet to be investigated.
In the present study, the expression levels of miRNA-210 and VEGF
in MB primary and secondary tumor tissues, tumor adjacent tissues
and in the cerebrospinal fluid (CSF) were detected, in order to
investigate the association between miRNA-210 and VEGF, and their
roles in MB metastasis.
Materials and methods
Subjects
A total of 86 adult patients with cerebellar MB, who
were admitted to the People's Hospital of Laiwu (Laiwu, China)
between January 2011 and June 2014, were enrolled in the present
study. Of the 86 patients, 50 were male and 36 were female. The age
range was 18–46 years, with a mean age of 35.6±8.6 years. All
patients underwent surgery to remove the tumor. Following the
initial surgery, MB metastasis to the subarachnoid space occurred
in 11 patients, including 5 male and 6 female patients. The age
range of these 11 patients was 21–39 years, with a mean age of
29.3±8.1 years. The metastatic MB tissue was similarly removed by
surgery. The tumor tissues of primary MB and metastatic MB, tumor
adjacent tissues and CSF were collected. Written informed consent
was obtained from all patients prior to the study. The study was
approved by the Ethics Review Board of the People's Hospital of
Laiwu.
Reagents and instruments
The miRcute miRNA Isolation kit, miRcute miRNA cDNA
First Strand Synthesis kit, miRcute miRNA Quantitative Fluorescence
Detection kit, SuperReal PreMix (SYBR Green), and TIANScript II
cDNA First Strand Synthesis kit, were all obtained from Tiangen
Biotech Co., Ltd. (Beijing, China). Rabbit anti-human polyclonal
VEGF antibody (cat. no. ab46154), rabbit anti-human polyclonal
β-actin antibody (cat. no. ab129348), horseradish peroxidase
(HRP)-conjugated sheep anti-rabbit immunoglobulin (Ig)G (cat. no.
ab6721), and goat anti-rabbit biotinylated secondary monoclonal
antibody (cat. no. ab128978) were purchased from Abcam (Cambridge,
MA, USA). TRIzol® reagent was obtained from Liaoning Yisheng
Biological Pharmaceutical Co., Ltd. (Shenyang, China). The
bicinchoninic acid (BCA) protein assay kit was purchased from Zhong
Ke Rui Tai Biotech Co. (Beijing, China). The Image Lab 3.0 software
and high-performance iQ5 Real-Time PCR Detection system were
obtained from Bio-Rad Laboratories, Inc. (Hercules, CA, USA).
Primers were designed using Primer Premier 5.0 software (Premier
Biosoft International, Palo Alto, CA, USA) and were synthesized by
Sangon Biotech Co., Ltd. (Shanghai, China).
Immunohistochemistry
MB tumor tissues and tumor adjacent tissues were
fixed with 10% formalin and embedded in paraffin. Tissues were cut
into 4 µm sections, which were then dewaxed in graded xylene and
dehydrated in graded alcohols. In order to inactivate endogenous
peroxidase, tissue sections were incubated with 3% hydrogen
peroxide for 10 min at room temperature. Antigen retrieval was
performed in a microwave (92°C for 15 min). After blocking in 5%
goat serum (Beijing Zhongshan Golden Bridge Biological Technology
Co., Ltd., Beijing, China), rabbit anti-human VEGF polyclonal
antibody (dilution 1:200) was added and incubated at room
temperature for 1 h, prior to incubation with biotinylated goat
anti-rabbit antibody (dilution 1:1,000) at 37°C for 30 min. The
tissue sections were then incubated with 3,3-diaminobenzidine
chromogenic substrate reagent (Abcam) and counterstained with
haematoxylin (Abcam). Following differentiation with hydrochloric
acid and the dimethylbenzene transparency procedure, tissue
sections were mounted with neutral gum.
Evaluation of immunohistochemical
staining results
Tissue sections were observed under a microscope
(magnification, 400×; Olympus BX50; Olympus Corporation, Tokyo,
Japan). Cells with brown or tan granules in the cytoplasm or on the
membrane were defined as positive. Images of five fields at
high-magnification were randomly captured and positive cells were
counted. At least 100 cells were counted. The positive rate
corresponded to the ratio of the number of positive cells to the
total number of cells counted.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression levels of VEGF mRNA and miRNA-210
were detected in tissue and CSF samples using RT-qPCR. Prior to RNA
extraction, tissue samples were homogenized using a homogenizer
(PRO 200 homogenizer; Pro Scientific, Inc., Oxford, CT, USA). For
analysis of VEGF mRNA expression levels, total RNA was extracted
from tumor tissues and tumor adjacent tissues using TRIzol®
reagent, and the total RNA was reverse transcribed into cDNA. The
primers used were as follows: Forward, 5′-TTG CCT TGC TGC TCT ACC
TC-3′ and reverse, 5′-AAA TGC TTT CTC CGC TCT GA-3′ for VEGF; and
forward, 5′-TGA CGT GGA CAT CCG CAA AG-3′ and reverse, 5′-CTG GAA
GGT GGA CAG CGA GG-3′ for β-actin. β-actin was used as an internal
control. PCR-iQ5 thermal cycler (Bio-Rad Laboratories, Inc.) was
used to perform PCR. The PCR cycling procedures were as follows:
Pre-denaturation at 94°C for 2 min, 35 cycles of denaturation at
94°C for 30 sec, annealing at 55°C for 30 sec and extension at 71°C
for 1 min, and a final extension at 71°C for 2 min. The
2﹣ΔΔCt method (15) was
used to calculate the relative expression levels of VEGF and
β-actin.
The following primers were used to analyze the
expression levels of miRNA-210 in the CSF: Forward,
5′-CTGTGCGTGTGACAGCGGCTGA-3′ and reverse,
5′-GCGAGCACAGAATTAATACGAC-3′ for miRNA-210; and forward,
5′-CGCTTCGGCAGCACATATACTA-3′ and reverse,
5′-CGCTTCACGAATTTGCGTGTCA-3′ for U6. U6 was used as an internal
control. The PCR cycling procedures were as follows:
Pre-denaturation at 95°C for 10 min, 40 cycles of denaturation at
95°C for 15 sec, annealing at 60°C for 1 min and extension at 72°C
for 2 min, and final extension at 72°C for 4 min. The
2﹣ΔΔCt method was used to calculate the relative
expression levels of miRNA-210 and U6.
Western blotting
Prior to protein extraction, tissue samples were
homogenized using a homogenizer (PRO 200 homogenizer; Pro
Scientific, Inc.). Total protein was extracted from the tissue
sections and CSF using radioimmunoprecipitation assay buffer
(Beyotime Institute of Biotechnology, Shanghai, China) and the
protein concentration was determined using the BCA protein assay
kit. Protein samples (30 µg) were separated by 10% SDS-PAGE, after
which they were transferred onto a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA). After blocking with 5% non-fat
milk for 1 h at room temperature, the membrane was incubated with
primary rabbit anti-human VEGF polyclonal antibody (1:1,000
dilution) and rabbit anti-human β-actin polyclonal antibody
(1:5,000 dilution) at 4°C overnight. After washing with 150 mmol/l
Tris-buffered saline containing 5% Tween (Amresco, LLC, Solon, OH,
USA), the membrane was incubated with sheep anti-rabbit
HRP-conjugated IgG (1:3,000) at room temperature for 1 h.
Subsequently, the membrane was developed via incubation with
Enhanced Chemiluminescence Reagent Plus (Abcam). The western blot
images were analyzed using Image Lab 3.0 software. β-actin was used
as an internal control. The relative value of VEGF was defined as
the grey value ratio of VEGF:β-actin.
Statistical analysis
SPSS 18.0 software (SPSS Inc., Chicago, IL, USA) was
used for statistical analysis. Data are presented as the mean ±
standard deviation. One-way analysis of variance was performed in
order to compare the differences between the various groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
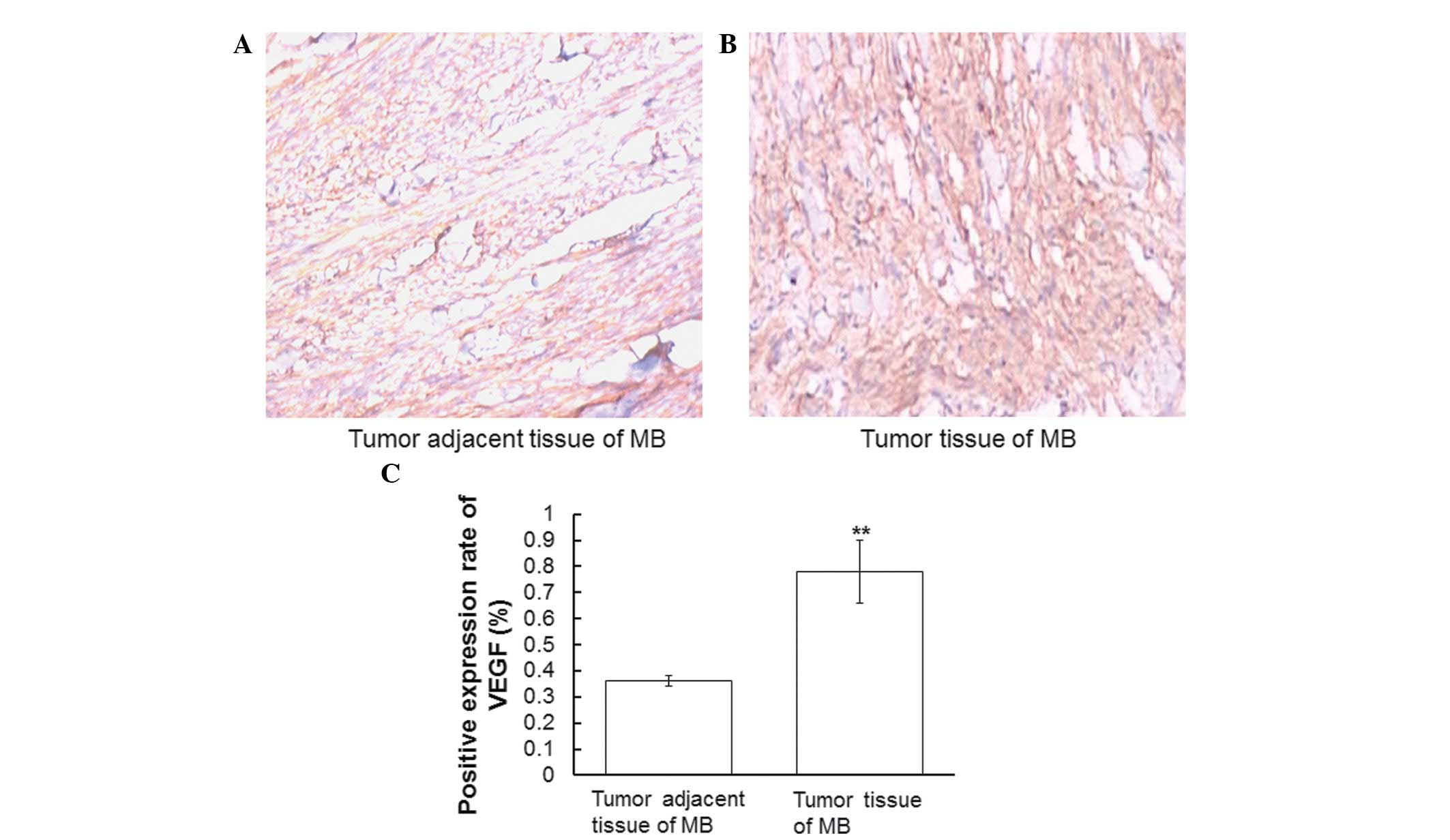
VEGF is highly expressed in tumor
tissues of MB
In order to determine the expression levels of VEGF
in MB tumor tissues and tumor adjacent tissues, immunohistochemical
analyses were performed. Representative immunohistochemical
staining results are presented in Fig.
1A and B. Cells with brown or tan granules in the cytoplasm or
on the membrane were defined as positive. Positive cells were
counted and the positive expression rate was defined as the ratio
of the number of positive cells to the total number of cells. The
positive expression rate of VEGF was significantly higher in the MB
tumor tissues, as compared with in the tumor adjacent tissues
(P<0.01; Fig 1C). These results
indicate that VEGF expression levels in MB tumor tissues are
upregulated, as compared with tissues adjacent to the tumor.
Protein and mRNA expression levels of
VEGF are upregulated in the tumor tissues of patients with MB
In order to analyze the mRNA and protein expression
levels of VEGF, RT-qPCR and western blotting of tumor and tumor
adjacent tissues from patients with MB were conducted. As compared
with in the tumor adjacent tissues, VEGF mRNA expression levels in
the tumor tissues from patients with MB were significantly
upregulated (P<0.01; Fig. 2A).
Concordantly, VEGF protein expression levels were significantly
increased in the MB tumor tissues, as compared with in the tumor
adjacent tissues (P<0.01; Fig. 2B and
C). These results suggest that VEGF mRNA and protein expression
levels are upregulated in the tumor tissues of patients with
MB.
Protein and mRNA expression levels of
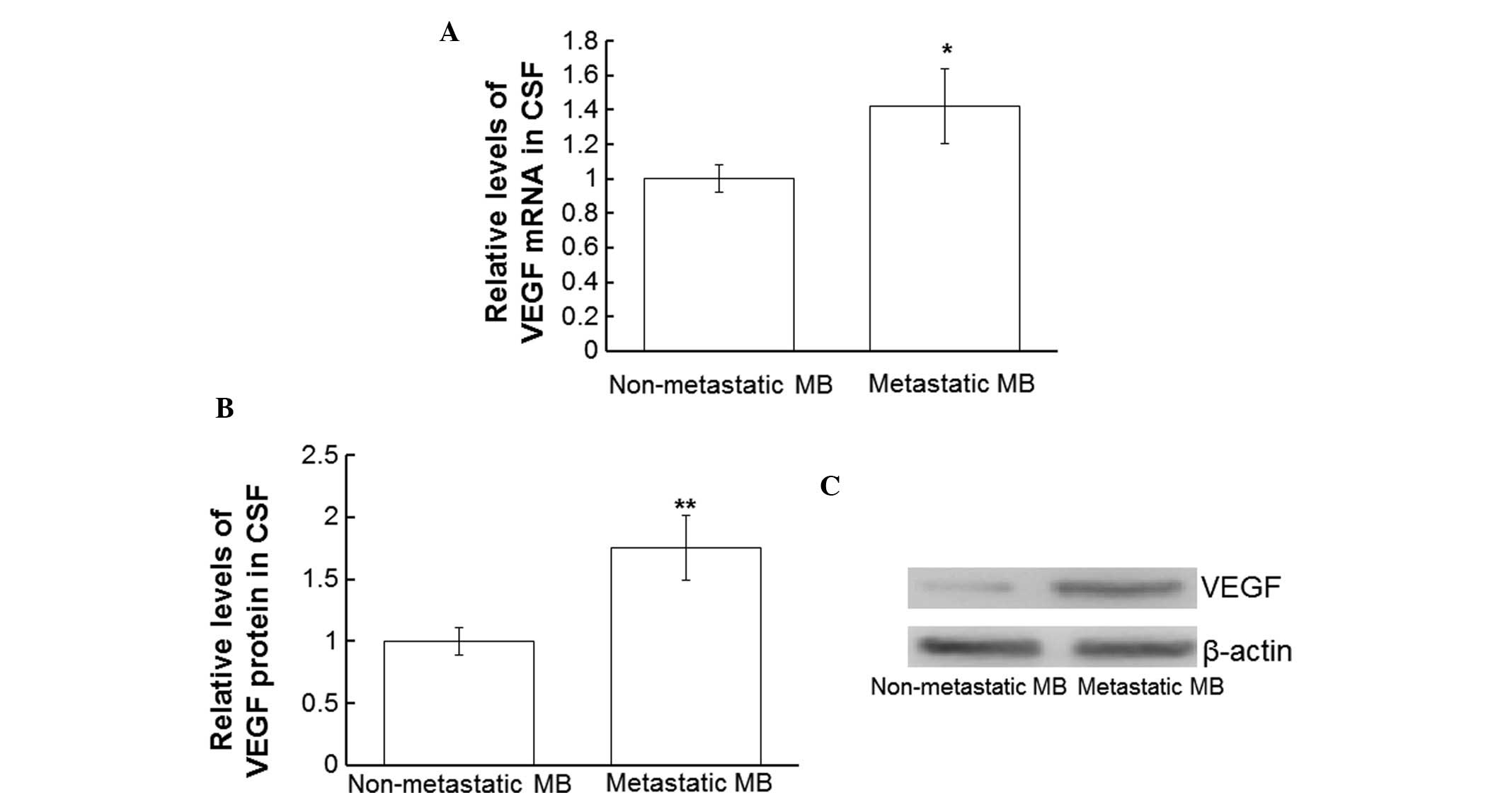
VEGF are upregulated in the CSF of patients with metastatic MB
In order to compare the expression levels of VEGF
mRNA and protein in the CSF of patients with and without secondary
MB tumors, RT-qPCR and western blotting were performed using CSF
collected from all patients. The VEGF mRNA expression levels in the
CSF from patients with metastatic MB were significantly
upregulated, as compared with in the CSF from patients without
secondary tumors (P<0.05; Fig.
3A). In addition, VEGF protein expression levels in the CSF
from patients with metastatic MB were significantly upregulated, as
compared with in the CSF from patients without metastases
(P<0.01; Fig. 3B and C). These
results suggest that VEGF is upregulated in the CSF of patients
with metastatic MB.
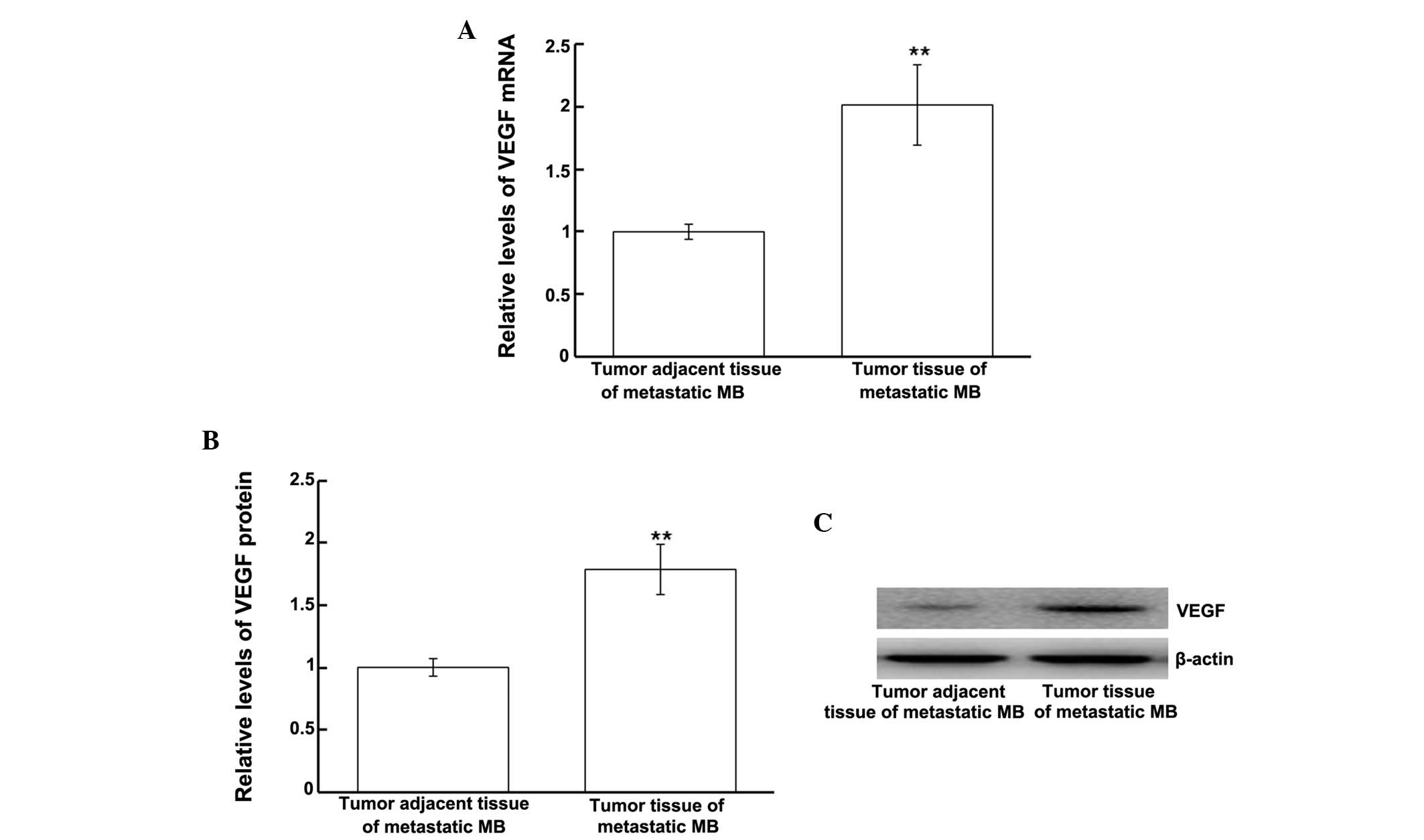
Protein and mRNA expression levels of
VEGF are upregulated in tumor tissues from patients with metastatic
MB
In order to investigate the mRNA and protein
expression levels of VEGF in tumor tissues from patients with
metastatic MB, tumor and tumor adjacent tissues from only the
patients with secondary tumors were analyzed. VEGF mRNA and protein
expression levels were measured using RT-qPCR and western blotting,
respectively. The VEGF mRNA expression levels in the tumor tissues
from patients with metastatic MB were significantly upregulated, as
compared with in the tumor adjacent tissues (P<0.01; Fig. 4A). Similarly, western blotting
results demonstrated that VEGF protein expression levels were
significantly upregulated in the tumor tissues from patients with
metastatic MB, as compared with in the tumor adjacent tissues
(P<0.01; Fig. 4B). These results
indicate that VEGF mRNA and protein expression levels are
upregulated in the tumors of patients with metastatic MB.
Expression levels of miRNA-210 are
increased in patients with MB
In order to analyze the expression levels of
miRNA-210 in patients with MB, RT-qPCR of MB tumor tissue, tumor
adjacent tissue, CSF and metastatic tumor tissue samples was
conducted. miRNA-210 expression levels in the tumor tissues of
patients with MB were significantly upregulated, as compared with
in the tumor adjacent tissues (P<0.01; Fig. 5A). Similarly, miRNA-210 expression
levels in the CSF from patients with metastatic MB were
significantly increased, as compared with in the CSF from patients
with primary MB (P<0.01; Fig.
5B). In addition, miRNA-210 expression levels in the tumor
tissues of patients with metastatic MB were significantly
upregulated, as compared with in the tumor adjacent tissues
(P<0.01; Fig. 5C). These results
indicate that miRNA-210 levels are upregulated in patients with
MB.
Discussion
Hypoxia is able to induce expression of VEGF and
hypoxia inducible factor-1α, which in turn may activate downstream
factors involved in tumor invasion and metastasis (16); therefore, the expression levels of
VEGF may be used as an indicator for assessing tumor invasion and
metastasis. In the present study, VEGF mRNA and protein expression
levels were significantly upregulated in the tumor tissues of
patients with MB and metastatic MB; thus indicating the occurrence
of hypoxia in MB. Furthermore, upregulated VEGF may have a role in
promoting tumor angiogenesis.
The detection of VEGF expression in brain tissue
can, to some extent, reveal the pathological process of brain tumor
invasion (17). In the present
study, immunohistochemical staining demonstrated that the positive
expression rate of VEGF was elevated in MB tumor tissues, as
compared with in non-tumor tissues adjacent to MB. This indicates
that the expression levels of VEGF may be associated with the
degree of MB infiltration. The metastasis of MB primary tumors
occurs predominantly via the CSF (18); therefore, the levels of VEGF in the
CSF of patients with MB and metastatic MB were also analyzed in the
present study. The results demonstrated that VEGF levels were
significantly upregulated in the CSF of patients with metastatic
MB, as compared with in patients with primary MB. The reason for
this may be that VEGF expression increases the transcriptional
activation of numerous downstream molecules, and in doing so
initiates the invasion and metastasis of MB (19), ultimately leading to the metastasis
of MB via the CSF.
Previous studies have detected miRNA-210-mediated
regulation of VEGF expression and angiogenesis in various
processes, including tumor formation and development (20), angiogenesis and nerve repair in the
brain (21), capillary formation
(22), and ligament repair (23). In addition, Szabó et al
(24) demonstrated that VEGF mRNA is
a target of miRNA-210; therefore, miRNA-210 has the potential to be
used as a specific biomarker in the early diagnosis and treatment
of diseases associated with VEGF (25). In the present study, consistent with
the alterations in the expression levels of VEGF, miRNA-210
expression levels were elevated in the tumor tissues of patients
with MB and metastatic MB, and in the CSF of patients with
metastatic MB. This suggests that miRNA-210 may regulate the
expression of VEGF in patients with MB.
In conclusion, VEGF and miRNA-210 expression levels
were upregulated in the tumor tissues of patients with MB, and most
significantly in patients with metastatic MB; thus suggesting that
miRNA-210 is able to regulate the metastasis of MB via regulation
of VEGF expression.
Acknowledgements
The authors of the present study would like to thank
Dr Quanxiang Wang (Department of Neurosurgery, People's Hospital of
Laiwu) for his valuable help in the study design, analysis, and
writing of the present study.
References
|
1
|
Sardiñas N, Marcos R, Pestaña EM, Vargas
J, Chi-Ramírez D, Rojas E, Esteban EM and Zarrabeitía L: Tumors of
the posterior fossa in children. Rev Neurol. 28:1153–1158. 1999.(In
Spanish). PubMed/NCBI
|
|
2
|
Packer RJ, Cogen P, Vezina G and Rorke LB:
Medulloblastoma: Clinical and biologic aspects. Neuro Oncol.
1:232–250. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yan XY: Angiogenesis: A promising strategy
for tumor therapy. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao.
26:180–193. 2010.(In Chinese).
|
|
4
|
Lohela M, Bry M, Tammela T and Alitalo K:
VEGFs and receptors involved in angiogenesis versus
lymphangiogenesis. Curr Opin Cell Biol. 21:154–165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roberts E, Cossigny DA and Quan GM: The
role of vascular endothelial growth factor in metastatic prostate
cancer to the skeleton. Prostate Cancer. 2013:4183402013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mao X, Wang T, Liu Y, Irwin MG, Ou JS,
Liao XL, Gao X, Xu Y, Ng KF, Vanhoutte PM and Xia Z:
N-acetylcysteine and allopurinol confer synergy in attenuating
myocardial ischemia injury via restoring HIF-1α/HO-1 signaling in
diabetic rats. PLoS One. 8:e689492013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vimala N, Mittal S, Kumar S, Dadhwal V and
Sharma Y: A randomized comparison of sublingual and vaginal
misoprostol for cervical priming before suction termination of
first-trimester pregnancy. Contraception. 70:117–120. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mende A, Takano H, Kodama Y, Nakamura T,
Umetani K, Fujioka D, Saito Y, Kobayashi T, Kawabata K, Obata JE,
et al: Relation between transcardiac gradient of VEGF and coronary
flow response in humans. Int J Cardiol. 119:156–162. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stumpf C, Jukic J, Yilmaz A, Raaz D,
Schmieder RE, Daniel WG and Garlichs CD: Elevated VEGF-plasma
levels in young patients with mild essential hypertension. Eur J
Clin Invest. 39:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xiong N, Zhang Z, Huang J, Chen C, Zhang
Z, Jia M, Xiong J, Liu X, Wang F, Cao X, et al: VEGF-expressing
human umbilical cord mesenchymal stem cells, an improved therapy
strategy for Parkinson's disease. Gene Ther. 18:394–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim NH, Jung HH, Cha DR and Choi DS:
Expression of vascular endothelial growth factor in response to
high glucose in rat mesangial cells. J Endocrinol. 165:617–624.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Wang Y, Liu F, Lou YL, Deng J and
Cui SP: Dynamic changes of the expression of hypoxia inducible
factor-1α and the target genes miR-210, vascular endothelial growth
factor in the kidney after ischemic-reperfusion injury. Zhong Hua
Shi Yan Wai Ke Za Zhi. 28:2074–2076. 2011.(In Chinese).
|
|
13
|
Cha HS, Bae EK, Koh JH, Chai JY, Jeon CH,
Ahn KS, Kim J and Koh EM: Tumor necrosis factor-alpha induces
vascular endothelial growth factor-C expression in rheumatoid
synoviocytes. J Rheumatol. 34:16–19. 2007.PubMed/NCBI
|
|
14
|
Agrawal R, Pandey P, Jha P, Dwivedi V,
Sarkar C and Kulshreshtha R: Hypoxic signature of microRNAs in
glioblastoma: Insights from small RNA deep sequencing. BMC
Genomics. 15:6862014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu SQ, Yu HC, Gong YZ and Lai NS:
Quantitiative measurement of HLA-B27 mRNA in patients with
ankylosing spondylitis - correlation with clinical activity. J
Rheumatol. 33:1128–1132. 2006.PubMed/NCBI
|
|
16
|
Brahimi-Horn MC, Chiche J and Pouysségur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen AL and Colman H: Glioma biology and
molecular markers. Cancer Treat Res. 163:15–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Barai S, Bandopadhayaya GP, Julka PK,
Dhanapathi H, Haloi AK and Seith A: Cerebellar medulloblastoma
presenting with skeletal metastasis. J Postgrad Med. 50:110–112.
2004.PubMed/NCBI
|
|
19
|
Slongo ML, Molena B, Brunati AM, Frasson
M, Gardiman M, Carli M, Perilongo G, Rosolen A and Onisto M:
Functional VEGF and VEGF receptors are expressed in human
medulloblastomas. Neuro Oncol. 9:384–392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quero L, Dubois L, Lieuwes NG, Hennequin C
and Lambin P: miR-210 as a marker of chronic hypoxia, but not a
therapeutic target in prostate cancer. Radiother Oncol.
101:203–208. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai
H, Liu J, Wang Y, Fu Y and Yang GY: MicroRNA-210 overexpression
induces angiogenesis and neurogenesis in the normal adult mouse
brain. Gene Ther. 21:37–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lou Y, Gao F, Xie A, Guo F, Deng Z and
Wang Y: MicroRNA-210 modified human umbilical vein endothelial
cells induce capillary formation. Zhongguo Xiu Fu Chong Jian Wai Ke
Za Zhi. 26:587–591. 2012.(In Chinese). PubMed/NCBI
|
|
23
|
Shoji T, Nakasa T, Yamasaki K, Kodama A,
Miyaki S, Niimoto T, Okuhara A, Kamei N, Adachi N and Ochi M: The
effect of intra-articular injection of microRNA-210 on ligament
healing in a rat model. Am J Sports Med. 40:2470–2478. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szabó DR, Luconi M, Szabó PM, Tóth M,
Szücs N, Horányi J, Nagy Z, Mannelli M, Patócs A, Rácz K and Igaz
P: Analysis of circulating microRNAs in adrenocortical tumors. Lab
Invest. 94:331–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alaiti MA, Ishikawa M, Masuda H, Simon DI,
Jain MK, Asahara T and Costa MA: Up-regulation of miR-210 by
vascular endothelial growth factor in ex vivo expanded CD34+ cells
enhances cell-mediated angiogenesis. J Cell Mol Med. 16:2413–2421.
2012. View Article : Google Scholar : PubMed/NCBI
|