Introduction
Coronary bifurcation lesions represent a complex
lesion subtype, and treatment via a percutaneous coronary
intervention (PCI) has been associated with reduced procedural
success rates, an increased rate of restenosis and major adverse
cardiovascular events (MACE) (1–4). In
previous decades, considerable progress has occurred in the field
of PCI, including the use of plaque debulking [rotational
atherectomy (ROTA) or directional atherectomy and ablative lasers],
cutting balloon, bare-metal stents (BMS), and particularly the
development of drug-eluting stents (DES) (5–8).
Previous studies have indicated that DES are able to significantly
improve the incidence of myocardial infarction (MI), target vessel
revascularization (TVR) and MACE following PCI of bifurcation
coronary lesions, compared with BMS (9–12).
Despite advances in device technology, the treatment of coronary
bifurcation lesions remains a technically challenging field. An
alternative to PCI is the coronary artery bypass graft; however,
PCI is preferred due to minimal invasion (13).
It has been estimated that bifurcation lesions
comprise 15–20% of all PCI procedures (14,15).
Stenting of a bifurcation lesion may result in a significant
reduction in the angiographic diameter of the ostium of the side
branch (SB), primarily as a result of plaque shifting, ostial
recoil and propagation of dissection (16,17).
ROTA has been advocated for the treatment of bifurcation lesions as
it appears to effectively remove plaque while minimizing injury to
adjacent normal arterial segments to a greater extent compared with
standard balloon angioplasty (BA), while additionally avoiding
‘snow’ plaque shifting (18,19). However, the role and long-term
outcomes of ROTA for the treatment of bifurcation coronary lesions
in the DES era remain unknown and require further investigation.
Diabetes mellitus (DM) is a major contributor to the development of
coronary artery disease (CAD) as well as to the outcomes following
various manifestations of the disease. DM patients have a higher
prevalence of complex coronary lesions such as triple-vessel,
bifurcation and ostial lesions. Therefore, the aim of the present
study was to determine the long-term outcomes of bifurcation
lesions following a ROTA.
Materials and methods
Study subjects
This retrospective study enrolled 863 consecutive
patients that had undergone elective PCI with bifurcation coronary
lesions at Juntendo University Hospital (Tokyo, Japan) between
January 2007 and December 2009. Finally, 337 patients met the
inclusion criteria of the present study, which comprised the use of
ROTA for the treatment of bifurcation coronary lesions. The
baseline characteristics and follow-up clinical information of the
patients were obtained from medical record reviews. The study was
approved by the Ethics Committee of Juntendo University
Hospital.
All laboratory measurements were performed
immediately following patient admission. Renal function was
evaluated via the estimated glomerular filtration rate (eGFR),
which was calculated according to the simplified ‘Modification of
Diet in Renal Disease’ equation, as follows: eGFR (ml/min/1.73
m2) = 186 × (Scr)−1.154 ×
(Age)−0.203 × (0.742, if female), where Scr is the serum
creatine level (20). All coronary
angiograms obtained from the study patients were reviewed by
board-certified interventional cardiologists.
Diabetes mellitus (DM) was defined as the patient
receiving active treatment with insulin or an oral antidiabetic
agent, or if the patient exhibited an abnormal blood glucose level
following overnight fasting or abnormal glucose tolerance test
results according to the World Health Organization criteria
(21). The patients were divided
into DM and non-DM groups.
Procedures
All baseline, procedural, and follow-up angiograms
were performed immediately following the administration of 200 µg
intracoronary nitroglycerin, and the treated lesion was evaluated
using two or more angiographic projections. The Cardiovascular
Measurement System (Medis Medical Imaging Systems, Leiden,
Netherlands) was used by two experienced angiographers to perform
quantitative coronary angiography. The reference diameter, lesion
length, % of diameter stenosis (%DS) and minimal lumen diameter
(MLD) were measured using the view showing the smallest luminal
diameter in the diastolic frames.
Lesions were classified according to the modified
American College of Cardiology/American Heart Association grading
system as type A, B1, B2 or C (22).
Type A and B1 lesions were categorized as simple, while type B2 and
C lesions were designated complex. The degree of coronary
calcification was judged as follows: Grade 1, calcification
difficult to recognize; grade 2, easily recognized; grade 3,
recognized in >50% of a single coronary artery; and grade 4,
recognized in nearly the entire length of a single coronary
artery.
Louvard et al (23) defined a bifurcation lesion as ‘a
coronary artery narrowing occurring adjacent to, and/or involving,
the origin of a significant SB’. A significant SB was defined as a
branch (typically with a diameter of >1.5 mm) that would result
in detrimental effects if lost, due to the associated symptoms,
location of ischemia and loss of viability, collateral vessels and
ventricular function (23). The
diameter of SB was classified as small (≤2.0 mm), medium (2.0–2.5
mm) or large (≥2.5 mm). Lesion angulation was measured at baseline
in a working view, which provided the optimal SB ostium
visualization, by measuring the distal angle between the SB and the
main vessel (MV) distal to the bifurcation. When angulation was
<70°, the bifurcation lesion was defined as Y-shape, while
bifurcation lesions with angulation of >70° were defined as
T-shape. The bifurcation lesion type was classified according to
the classification of Medina et al (24).
A true bifurcation coronary lesion was defined as a
coronary lesion with ≥50% luminal diameter stenosis in the parent
vessel and the ostium of an SB arising from the lesion, which were
observed to be ≥2.0 mm in diameter by visual estimation, in
addition to specific plaque geography meeting the Medina
classification (24), as follows:
(1,1,1), (1,0,1), (0,1,1).
A simple stenting strategy was defined as stenting
of the MV only, and provisional stenting of the SB only if bailout
of the SB was necessary, while a complex stenting strategy was
defined as routine stenting in the MV and SB using a variety of
techniques, including crush, mini-crush, modified crush, culotte,
simultaneous kissing stent and T-stent (25).
Events
Acute gain and late loss were defined as the
difference between pre- and post-procedural MLD, and between
post-procedural and follow-up MLD. Procedural success was defined
as the achievement of <30% angiographic residual stenosis in the
MV, <50% in the SB by quantitative coronary angiography and
thrombolysis in MI flow grade 3 in the MV and SB, with no
periprocedural or in-hospital complications such as mortality, MI
or emergent bypass surgery. MI was defined as the MB isoform of
creatine kinase level was >3 times the normal value, with or
without the occurrence of new abnormal Q-waves in ≥2 contiguous
leads.
Acute thrombosis was defined as the occurrence of
acute closure of the target vessel <24 h after the index
procedure. Subacute thrombosis was defined as the occurrence of
acute closure of the target vessel after 24 h but within 1 month,
and late thrombosis was defined as the occurrence of acute closure
≥1 month after the index procedure (26).
Angiographic evaluations were performed at regular
intervals. In addition, a repeat coronary angiography was scheduled
within 5–9 months after the index procedure, unless earlier
intervention was required due to symptoms or a history of
myocardial ischemia. Follow-up was discontinued after December
2010.
Target lesion (TL) restenosis was defined as a
stenosis diameter of ≥50% within the stented segment and the 5-mm
proximal and distal persistent area at follow-up angiography. In
addition, TL revascularization (TLR) was defined as any
revascularization or bypass surgery of the original TL, which was
performed in the presence of angiographic restenosis of ≥50% by
quantitative angiography in the presence of ischemic symptoms or
objective evidence of ischemia, or in the presence of angiographic
restenosis ≥70% with no evident ischemic symptoms or indications of
ischemia. The TL was considered to be the area covered by the stent
plus a 5-mm margin proximal and distal to the edges of the stent
(27). Furthermore, TVR was defined
as clinically driven PCI or coronary artery bypass grafting of the
treated vessel. MACE was defined as a composite of clinical events
including TLR, TL restenosis, non-fatal MI (Q- or non-Q-wave MI),
acute thrombosis, subacute thrombosis and cardiac fatality
(27).
Statistical analysis
Discrete variables are presented as frequency counts
and percentages. Continuous variables were expressed as the mean ±
standard deviation when normally distributed, or as the median with
interquartile range if not.
The χ2 test, two-tailed independent
Students t-test and Wilcoxon/Kruskal-Wallis test were used to
compare proportions and mean/median values. Independent predictors
of long-term outcomes were identified using Cox's proportional
hazards analysis and logistic regression analysis. Kaplan-Meier
accumulated survival curves were drawn and log-rank values were
calculated to assess their statistical significance.
Data analysis was performed using JMP software,
version 8.0 (SAS Institute Inc., Cary. NC. USA). P≤0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 337 patients met the study criteria and
were enrolled into the present study. The patients had a mean age
of 68.1±9.1 years (age range, 52–86 years old), and 283 subjects
(84.0%) were male. In total, 178 patients (52.8%) had a history of
DM, 28 patients had a history of PCI and 39 patients were receiving
hemodialysis treatment due to end-stage renal disease. Detailed
baseline demographics and clinical risk factors in DM and non-DM
patients are presented in Table I.
Baseline patient characteristics were comparable between the two
groups (P>0.05), with the exception of reduced eGFR and an
increased number of hemodialysis patients in the DM group compared
with the non-DM group.
 | Table I.Baseline demographics and risk
factors in DM and non-DM patients. |
Table I.
Baseline demographics and risk
factors in DM and non-DM patients.
| Parameter | DM group
(n=178) | No DM group
(n=159) | Total cohort
(n=337) | P-value |
|---|
| Baseline
demographics |
|
|
|
|
| Age
(years)a | 68.7±8.8 | 67.4±9.3 | 68.1±9.1 | 0.19 |
| Gender
(male)b | 148 (83.1) | 135 (84.9) | 283 (84.0) | 0.66 |
| Risk factors |
|
|
|
|
| BMI
(kg/m2)c | 22.9 (21.1–25) | 23.8
(21.6–25.2) | 23.3
(21.4–25.1) | 0.13 |
| Waist
(cm)c | 85 (82–90) | 86 (82–90) | 86 (82–90) | 0.36 |
| Current
smokingb | 78 (43.8) | 64 (40.2) | 142 (42.3) | 0.21 |
|
Hypertensionb | 132 (74.2) | 119 (74.8) | 251 (74.5) | 0.86 |
|
Hyperlipidemiab | 131 (73.6) | 120 (75.5) | 251 (74.5) | 0.69 |
|
MSb | 80 (44.9) | 55 (34.8) | 134 (40.0) | 0.06 |
| Family
historyb | 51 (28.7) | 50 (31.5) | 101 (30.0) | 0.58 |
|
Previous PCIb | 17 (9.6) | 11 (6.9) | 28 (8.3) | 0.38 |
| Serum
creatininec | 0.88
(0.74–1.12) | 0.85
(0.74–1.00) | 0.86
(0.74–1.04) | 0.08 |
|
eGFRc | 85.9
(67.7–102.5) | 92.3
(77.0–107.9) | 88.4
(72.1–105.1) | 0.03 |
| ESRD on
hemodialysisb | 27 (15.2) | 12 (7.6) | 39 (11.6) | 0.03 |
Angiographic and procedure
characteristics
A total of 211 cases (62.6%) exhibited severe
calcification (grade 3 or 4) and 334 cases (99.1%) exhibited
complex lesions (type B2 or C). Furthermore, 211 lesions (62.6%)
had an angulation of >70°, while 126 lesions (37.4%) exhibited
an angulation of <70°. The bifurcation of the left anterior
descending artery/diagonal (193 lesions, 57.3%) and left main/left
anterior descending/left circumflex artery (69 lesions, 20.5%) were
among the most frequently involved locations. There were 202 cases
(59.9%) with involvement of the MV and SB, with 155 cases (50.0%)
classified as type (1,1,1), 20 cases (5.9%) as type (1,0,1) and 27
cases (8.0%) as type (0,1,1). In addition, there were 146 cases
(43.3%) with a medium SB diameter, 100 cases (29.7%) with a large
SB diameter and 152 cases (45.1%) with a true bifurcation lesion.
Each case was treated with an average of 1.2±0.4 ROTA burrs, with a
mean size of 2.9±0.3 mm. The mean values of MLD pre-PCI and
post-PCI were 0.5±0.3 and 2.6±0.4 mm, respectively. There were 23
cases (6.8%) treated with BA (including cutting balloon), 46 cases
(13.7%) with BMS and 268 cases (79.5%) with DES. A total of 287
cases (85.2%) were treated with a simple stenting technology and 29
cases (8.6%) with a complex stenting technology, with a mean total
stent length was 33.0±16.9 mm. Furthermore, 43 cases (12.8%)
received SB stents and 95 cases (28.3%) received final kissing-BA.
In total, 319 cases (94.7%) exhibited immediate procedural success.
Detailed angiographic and procedural characteristics in the DM and
non-DM patients were comparable (P>0.05) and are presented in
Table II.
 | Table II.Angiographic and procedural
characteristics in DM and non-DM patients. |
Table II.
Angiographic and procedural
characteristics in DM and non-DM patients.
| A, Angiographic
characteristicsa |
|
|
|
|
|---|
|
|---|
| Parameter | DM group
(n=178) | No DM group
(n=159) | Total cohort
(n=337) | P-value |
|---|
| Severe
calcification (Grade 3/4) | 113 (63.5) | 98 (61.6) | 211 (62.6) | 0.73 |
| Complex lesion
(Type B2/C) | 178 (100.0) | 156 (98.1) | 334 (99.1) | 0.10 |
| Calcification
severity (Grade 3/4) | 113 (63.5) | 98 (61.6) | 211 (62.6) | 0.73 |
| Bifurcation
angulation |
|
|
| 0.42 |
| Y
(<70) | 115 (64.6) | 96 (60.4) | 211 (62.6) |
|
| T
(>70) | 63 (35.4) | 63 (39.6) | 126 (37.4) |
|
| Bifurcation
location |
|
|
| 0.23 |
| LM,
LAD, LCX | 30 (16.9) | 39 (24.5) | 69 (20.5) |
|
| LM,
intermediate branch, LAD | 1 (0.6) | 4 (2.5) | 5 (1.5) |
|
| LAD,
diagonal branch | 106 (59.6) | 87 (54.7) | 193 (57.3) |
|
| LAD,
septal branch | 7 (3.9) | 7 (4.4) | 14 (4.2) |
|
| LCX,
obtuse marginal branch | 14 (7.9) | 11 (6.9) | 25 (7.4) |
|
| RCA,
right ventricular branch | 20 (11.2) | 11 (6.9) | 31 (9.2) |
|
| Bifurcation
type | 109 (61.2) | 93 (58.5) | 202 (59.9) | 0.42 |
|
(1,1,1) | 86 (48.3) | 69 (43.3) | 155 (50.0) |
|
|
(1,0,1) | 9 (5.1) | 11 (6.9) | 20 (5.9) |
|
|
(0,1,1) | 14 (7.9) | 13 (8.2) | 27 (8.0) |
|
|
(1,1,0) | 32 (18.0) | 36 (22.6) | 68 (20.2) |
|
|
(1,0,0) | 6 (3.4) | 2 (1.3) | 8 (2.4) |
|
|
(0,1,0) | 28 (15.7) | 21 (13.2) | 49 (14.5) |
|
|
(0,0,1) | 3 (1.7) | 7 (4.4) | 10 (3.0) |
|
| Branch vessel
size |
|
|
| 0.24 |
| Medium
(2.0–2.5 mm) | 83 (46.6) | 63 (39.6) | 146 (43.3) |
|
| Large
(≥2.5 mm) | 46 (25.8) | 54 (34.0) | 100 (29.7) |
|
| True
bifurcation lesions | 84 (47.2) | 68 (42.8) | 152 (45.1) | 0.42 |
|
| B, Procedural
characteristics |
|
|
|
|
|
| Parameter | DM group
(n=178) | No DM group
(n=159) | Total cohort
(n=337) | P-value |
|
| ROTA
numberb | 1.2±0.4 | 1.3±0.4 | 1.2±0.4 | 0.26 |
| ROTA
sizeb | 2.9±0.3 | 2.9±0.3 | 2.9±0.3 | 0.14 |
| MLD
pre-PCIb | 0.5±0.3 | 0.5±0.3 | 0.5±0.3 | 0.25 |
| MLD
post-PCIb | 2.7±0.4 | 2.6±0.4 | 2.6±0.4 | 0.71 |
| Total stent length
(mm)b | 33.1±18.4 | 32.8±15.0 | 33.0±16.9 | 0.88 |
| PCI
strategya |
|
|
| 0.30 |
| Balloon
angioplasty | 11 (6.2) | 12 (7.6) | 23 (6.8) |
|
|
Bare-metal stents | 29 (16.3) | 17 (10.7) | 46 (13.7) |
|
|
Drug-eluting stents | 138 (77.5) | 130 (81.8) | 268 (79.5) |
|
| Stenting
strategya |
|
|
| 0.63 |
| Simple
stenting technology | 153 (86.0) | 134 (84.3) | 287 (85.2) |
|
| Complex
stenting technology | 13 (7.3) | 16 (10.1) | 29 (8.6) |
|
| Use of
side-branch stents | 17 (9.6) | 26 (16.4) | 43 (12.8) | 0.06 |
| Final
kissing-balloon angioplasty | 45 (25.3) | 50 (31.4) | 95 (28.3) | 0.21 |
|
Procedural success | 171 (96.1) | 148 (93.1) | 319 (94.7) | 0.23 |
Quantitative coronary angiographic data in the two
groups are presented in Table III.
Baseline lesion length, reference diameter, MLD and %DS prior to
the procedure were comparable between the DM and non-DM patients
(P>0.05). MLD, %DS and acute gain following the procedure were
also comparable between two groups. DM patients exhibited a
significantly reduced MLD value (1.97±0.92 vs. 2.26±0.73 mm;
P=0.0038), increased %DS value (27.9±21.3 vs. 20.2±13.3%; P=0.022)
and late loss (0.70±0.45 vs. 0.42±0.36 mm; P=0.0047) compared with
the non-DM patients.
 | Table III.Quantitative coronary angiographic
data of DM and non-DM patients. |
Table III.
Quantitative coronary angiographic
data of DM and non-DM patients.
| Parameter | DM group
(n=178) | No DM group
(n=159) | Total cohort
(n=337) | P-value |
|---|
| Lesion length
(mm) | 21.8±6.5 | 23.1±6.4 | 22.4±6.4 |
0.07 |
| Reference diameter
(mm) | 2.73±0.36 | 2.75±0.33 | 2.74±0.35 |
0.55 |
| Minimal lumen
diameter (mm) |
|
|
|
|
|
Pre-PCI | 0.48±0.28 | 0.45±0.31 | 0.47±0.29 |
0.44 |
|
Post-PCI | 2.66±0.42 | 2.64±0.39 | 2.65±0.41 |
0.71 |
|
Follow-up | 1.97±0.92 | 2.26±0.73 | 2.11±0.85 | <0.01 |
| Diameter stenosis
(%) |
|
|
|
|
|
Pre-PCI | 82.4±10.1 | 83.4±11.4 | 82.9±10.7 |
0.41 |
|
Post-PCI | 5.2±4.0 | 5.7±4.6 | 5.5±4.3 |
0.60 |
|
Restudy | 27.9±21.3 | 20.2±13.3 | 24.3±18.1 |
0.02 |
| Acute
gain (mm) | 2.18±0.47 | 2.19±0.48 | 2.18±0.47 |
0.87 |
| Late
loss (mm) | 0.70±0.45 | 0.42±0.36 | 0.57±0.41 | <0.01 |
Clinical events for long-term
outcomes
Mean clinical follow-up periods were 53.7±19.1 and
50.5±19.8 months in the DM and non-DM groups, respectively
(P=0.13). Cumulative clinical events for long-term outcomes are
shown in Table IV. During the
follow-up period, there were 8 cases of cardiac fatality in each
group, no cases of acute thrombosis in either group, 1 case of
non-fatal MI and 2 cases of subacute thrombosis in the non-DM
group. The rates of TLR [28 (15.7%) vs. 8 cases (5.0%), P=0.0011],
TL restenosis [46 (25.8%) vs. 20 cases (12.6%), P=0.0019] and MACE
[36 (20.2%) vs. 19 cases (12.0%), P=0.039] were significantly
higher in the DM group compared with the non-DM group.
 | Table IV.Long-term outcomes in DM and non-DM
patients. |
Table IV.
Long-term outcomes in DM and non-DM
patients.
| Long-term
outcome | DM group
(n=178) | No DM group
(n=159) | Total cohort
(n=337) | P-value |
|---|
| Mean follow-up
(months)a | 53.7±19.1 | 50.5±19.8 | 52.2±19.4 |
0.13 |
| Cardiac
mortality | 8 (4.5) | 8 (5.0) | 16 (4.7) |
0.82 |
| TLR | 28 (15.7) | 8 (5.0) | 36 (10.7) | <0.01 |
| TVR | 7 (3.9) | 12 (7.6) | 22 (6.5) |
0.15 |
| Target lesion
restenosis | 46 (25.8) | 20 (12.6) | 66 (19.6) | <0.01 |
| Non-fatal MI | 0 (0.0) | 1 (0.6) | 1 (0.3) |
0.47 |
| SAT | 0 (0.0) | 2 (1.3) | 2 (0.6) |
0.22 |
| MACE | 36 (20.2) | 19 (12.0) | 55 (16.3) |
0.04 |
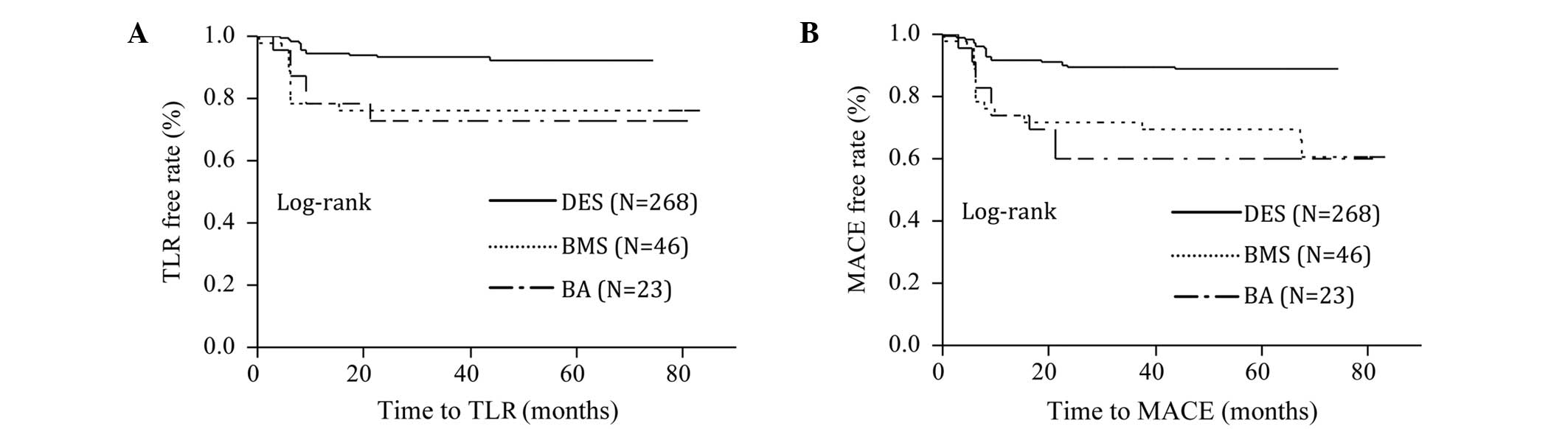
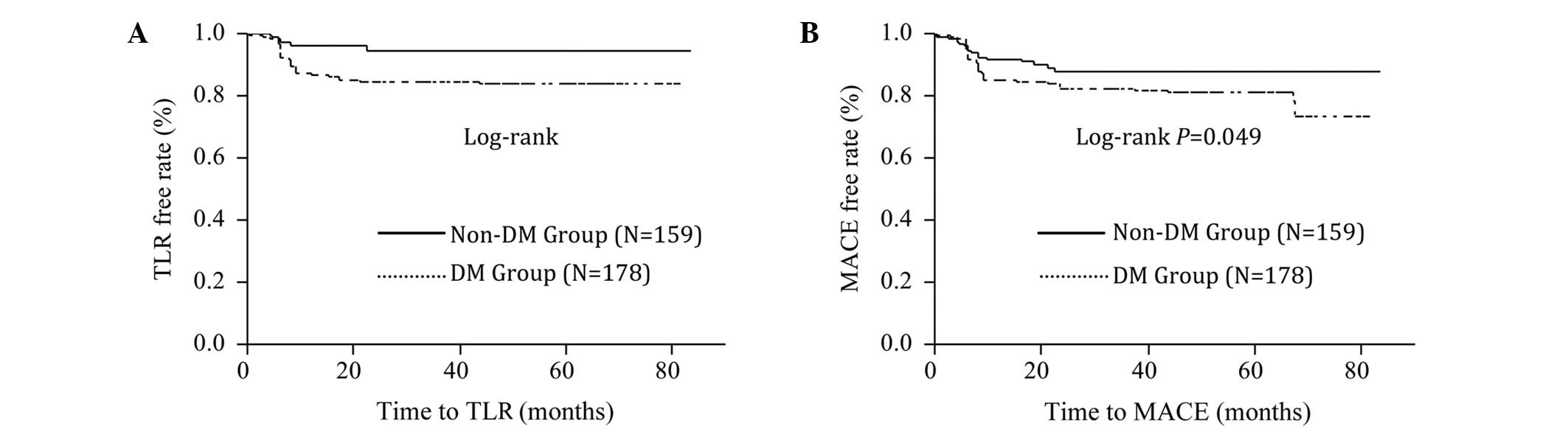
Kaplan-Meier survival curves indicated that the
rates of TLR and MACE were significantly higher in the non-DES
group (Fig. 1A and B; log-rank
P<0.0001) and DM group (Fig. 2A and
B; log-rank P<0.05).
Multivariate Cox proportional hazard analysis
(Table V) showed that, following
adjustment, DM (HR, 3.06; 95%CI, 1.41–7.36; P=0.0039), current
smoker status (HR, 2.25; 95%CI, 1.03–4.73; P=0.043) and DES (HR,
0.40; 95%CI, 0.23–0.69; P=0.0013) were independent predictors of
TLR, while DM and DES were independent predictors of MACE. In
addition, logistic regression analysis demonstrated that DM
(P=0.0053), current smoker status (P=0.0059) and DES stenting
(P<0.0001) were independent predictors of TL restenosis at
follow-up (Table VI).
 | Table V.Multivariate Cox proportional hazard
models for TLR and MACE. |
Table V.
Multivariate Cox proportional hazard
models for TLR and MACE.
|
| TLR (multivariate,
adjusted) | MACE (multivariate,
adjusted) |
|---|
|
|
|
|
|---|
| Parameter | HR (95%CI) | P-value | HR (95%CI) | P-value |
|---|
| Age (10 year
increase) | 1.13 (0.75,
1.75) | 0.56 | 1.38 (0.78,
2.52) |
0.27 |
| Gender
(female) | 0.65 (0.19,
1.87) | 0.44 | 0.74 (0.29,
1.76) |
0.51 |
| Metabolism
syndrome | 1.98 (0.93,
4.26) | 0.077 | 1.59 (0.87,
2.88) |
0.13 |
| Diabetes
mellitus | 3.06 (1.41,
7.36) | 0.0039 | 1.55 (1.10,
2.22) |
0.01 |
| Hypertension | 1.02 (0.48,
2.19) | 0.97 | 1.51 (0.82,
2.71) |
0.18 |
| Hyperlipidemia | 1.98 (0.94,
4.10) | 0.073 | 1.85 (0.97,
3.40) |
0.06 |
| Current smoker | 2.25 (1.03,
4.73) | 0.043 | 1.61 (0.88,
2.99) |
0.12 |
| ESRD on
hemodialysis | 1.10 (0.37,
2.78) | 0.85 | 2.16 (0.94,
4.42) |
0.07 |
| True
bifurcation | 1.07 (0.51,
2.26) | 0.87 | 1.20 (0.66,
2.21) |
0.54 |
| Bifurcation
angulation (>70°) | 0.74 (0.33,
1.57) | 0.44 | 0.85 (0.46,
1.56) |
0.61 |
| Calcified
severity | 1.20 (0.58,
2.60) | 0.64 | 1.26 (0.68,
2.30) |
0.46 |
| Simple stent
strategy | 1.16 (0.44,
2.88) | 0.76 | 1.16 (0.54,
2.42) |
0.70 |
| Final
kissing-balloon angioplasty | 0.98 (0.41,
2.58) | 0.97 | 0.87 (0.43,
1.86) |
0.70 |
| PCI strategy
(DES) | 0.40 (0.23,
0.69) | 0.0013 | 0.41 (0.26,
0.63) | <0.01 |
 | Table VI.Logistic regression analysis for
target lesion restenosis at restudy. |
Table VI.
Logistic regression analysis for
target lesion restenosis at restudy.
| Parameter | Estimated
coefficient | P-value |
|---|
| Age |
0.02 |
0.35 |
| Gender
(female) | −0.94 |
0.07 |
| Metabolism
syndrome |
0.08 |
0.82 |
| Diabetes
mellitus |
0.47 |
0.01 |
| Hypertension |
0.09 |
0.80 |
| Hyperlipidemia |
0.55 |
0.14 |
| Current smoker |
0.98 |
0.01 |
| ESRD on
hemodialysis |
0.01 |
0.98 |
| True
bifurcation |
0.20 |
0.55 |
| Bifurcation
angulation (>70°) | −0.17 |
0.62 |
| Severe
calcification |
0.04 |
0.91 |
| Complex stenting
strategy | −0.79 |
0.09 |
| Final
kissing-balloon angioplasty |
0.62 |
0.17 |
| PCI strategy
(DES) |
1.51 | <0.01 |
Discussion
The results of the present study indicated that in
bifurcation lesions treated with ROTA, patients that have undergone
non-DES and DM patients exhibited higher rates of TLR and MACE.
Bifurcation coronary lesion represents a complex lesion subtype and
remains a major interventional challenge, despite the advances
associated with DES. The complexity of treating bifurcations arises
from a number of technical and clinical challenges, including
variations in bifurcation anatomy (different bifurcation location,
type and angulation) and dynamic differences in anatomy during
treatment (plaque shifting and dissection causing flow problems),
as well as time-consuming and technically challenging managements,
including wire trapping and subsequent requirement of wire
replacement, stent deformation, incomplete lesion coverage, stent
overlap and large metal burden in the arteries (2,14,15,28).
Previous studies have indicate that DES is superior
to BMS in the treatment of bifurcation lesions with lower in-stent
restenosis or TLR (9,11,12,29). In
the present study, significant reductions in TLR rates (15.7 vs.
5.0%; P=0.0011) and MACE rates (20.2 vs. 12.0; P=0.039) were
observed in the DES group compared with the BA and BMS groups, and
the use of DES was an independent protector for TLR and MACE. DES
has emerged as the preferred stent platform for the treatment of
coronary bifurcations. However, due to the aforementioned causes,
PCI for bifurcation remains technically challenging, with reduced
procedural success rates and worse clinical outcomes compared with
non-bifurcation lesions, despite recent advances in interventional
cardiology and the introduction of DES (30).
ROTA may provide a safe and effective means of
treating this difficult lesion subtype. ROTA is performed using a
high-speed rotating burr containing diamond chips, which
selectively ablates calcified plaque within the coronary artery
while deflecting normal elastic tissue away from the burr,
resulting in a near circular lumen with a focally smooth and
polished surface (31,32). The small particles are able to pass
harmlessly through the distal myocardial capillary bed (33). This technique has been particularly
useful for heavily calcified lesions that cannot be easily
approached using BA or directional atherectomy (34). In the present study, 62.6% of the
subjects had severe calcified lesions (grade 3 or 4). A number of
previous studies have reported the use of ROTA for the treatment of
bifurcation lesions. Nageh et al (35) evaluated the role of ROTA in a
non-randomized study and observed that the ROTA group exhibited a
higher success rate and lower in-hospital event rate. Furthermore,
during a mean follow-up period of 15 months, ROTA was associated
with reduced cardiac events and target lesion revascularization
compared with BA. Furthermore, Dauerman et al (36) compared the clinical outcomes between
mechanical debulking (directional or rotational coronary
atherectomy) and BA for true bifurcation lesions. At the 1-year
follow-up, the incidence of TVR was markedly reduced in the
debulking group compared with the BA group. In addition, Ito et
al (28) have demonstrated the
safety and feasibility of ROTA and provisional SB stenting to treat
SB ostial lesions of true severe bifurcation coronary artery
disease. The authors suggested that ROTA of an SB ostium prior to
MV stenting may be performed in patients undergoing complex
bifurcation lesion angiography (28).
Based on the aforementioned findings of previous
studies, the combination of ROTA and DES placement appears to be a
promising approach for the treatment of bifurcation lesions. In the
present study, which included a mean follow-up period of 52.2±19.4
months, the rates of TLR and MACE were 10.7 and 16.3%,
respectively, in all cohorts, and 7.1 and 10.8% in DES group,
respectively. Considering the extended period of follow-up, it
appears to be a low incidence of TLR and MACE.
The results of previous studies have indicated that
DM is a consistent clinical predictor of worse outcomes following
BA, BMS and DES implantation (37–39).
Patients with DM exhibit a higher risk of mortality and elevated
restenosis rates following stenting compared with patients without
DM, despite the application of DES. In the present study, increased
rates of TLR (15.7 vs. 5.0%; P=0.0011), target lesion restenosis
(25.8 vs. 12.6%; P=0.0019) and MACE (20.2 vs. 12.0%; P=0.039) were
observed in the DM group compared with the non-DM group. After
adjusting for other factors, DM remained an independent risk factor
of TLR, TL restenosis and MACE.
Diabetes is associated with hormonal and vascular
abnormalities that promote the proliferation of smooth muscle cells
after vascular injury, including injury from catheter-based
interventions, including BA, stenting implantation and ROTA
(40). Increased smooth muscle
proliferation in diabetic patients may by induced by mitogens, such
as platelet-derived growth factor and insulin-like growth factor,
that stimulate cell growth and deleterious vascular effects,
including endothelial dysfunction and excessive extracellular
matrix production (41). In
addition, DM is markedly associated with the loss of endothelial
cells, increased platelet activation, hypercoagulability and the
release of vasoconstrictive substances (42). This may explain why DM remained the
‘Achilles’ heel’ of bifurcation lesions following the introduction
of DES and ROTA (43). Therefore,
patients with DM that receive ROTA and DES for bifurcation lesions
may require adjunctive systemic pharmacotherapy to modify the
underlying pathophysiological mechanisms responsible for neointimal
formation and atherosclerosis progression.
There were a number of limitations in the present
study. It was a retrospective and single-institution study with no
randomization. Furthermore, the ROTA procedure and stenting
strategy were performed at the operator's discretion. A large,
randomized, multicenter clinical study is required for more
accurate evaluation of this interventional approach.
In conclusion, the results of the present study
demonstrate that, although ROTA and DES evidently improved
long-term outcomes in patients with bifurcation lesions, DM
remained an independent risk factor for TLR, TL restenosis and
MACE. In the future, more emphasis should be placed on the
management of DM in bifurcation lesions treated with ROTA.
Intensive and systemic pharmacotherapy to control neointimal
formation and atherosclerosis progression may be required for
treating this particular patient population.
Glossary
Abbreviations
Abbreviations:
|
BA
|
balloon angioplasty
|
|
BMS
|
bare-metal stents
|
|
DES
|
drug-eluting stents
|
|
DM
|
diabetes mellitus
|
|
eGFR
|
estimated glomerular filtration
rate
|
|
MACE
|
major adverse cardiovascular
events
|
|
MI
|
myocardial infarction
|
|
MLD
|
minimum luminal diameter
|
|
PCI
|
percutaneous coronary intervention
|
|
ROTA
|
rotational atherectomy
|
|
TLR
|
target lesion revascularization
|
|
TVR
|
target vessel revascularization
|
|
%DS
|
percentage of diameter stenosis
|
References
|
1
|
Garot P, Lefèvre T, Savage M, et al:
Nine-month outcome of patients treated by percutaneous coronary
interventions for bifurcation lesions in the recent era: A report
from the Prevention of Restenosis with Tranilast and its Outcomes
(PRESTO) trial. J Am Coll Cardiol. 46:606–612. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Melikian N and Di Mario C: Treatment of
bifurcation coronary lesions: a review of current techniques and
outcome. J Interv Cardiol. 16:507–513. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Al Suwaidi J, Yeh W, Cohen HA, et al:
Immediate and one-year outcome in patients with coronary
bifurcation lesions in the modern era (NHLBI dynamic registry). Am
J Cardiol. 87:1139–1144. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al Suwaidi J, Berger PB, Rihal CS, et al:
Immediate and long-term outcome of intracoronary stent implantation
for true bifurcation lesions. J Am Coll Cardiol. 35:929–936. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Delhaye C, Wakabayashi K, Maluenda G,
Ben-Dor I, Torguson R, Xue Z, Suddath WO, Satler LF, Pichard AD,
Kent KM, et al: Safety and efficacy of bivalirudin for percutaneous
coronary intervention with rotational atherectomy. J Interv
Cardiol. 23:223–229. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang Z, Bai J, Su SP, Wang Y, Liu MH, Bai
QC, Tian JW, Xue Q, Gao L, An CX, et al: Cutting-balloon
angioplasty before drug-eluting stent implantation for the
treatment of severely calcified coronary lesions. J Geriatr
Cardiol. 11:44–49. 2014.PubMed/NCBI
|
|
7
|
Liu Y, Zhou X, Jiang H, Gao M, Wang L, Shi
Y and Gao J: Percutaneous coronary intervention strategies and
prognosis for graft lesions following coronary artery bypass
grafting. Exp Ther Med. 9:1656–1664. 2015.PubMed/NCBI
|
|
8
|
Toutouzas K, Anousakis-Vlachochristou N
and Tousoulis D: Everolimus-Eluting Stents or Bypass Surgery for
Coronary Disease. N Engl J Med. 373:5802015.PubMed/NCBI
|
|
9
|
Pendyala L, Jabara R, Hou D, et al: Review
of percutaneous therapy for bifurcation lesions in the era of
drug-eluting stents. Minerva Cardioangiol. 56:89–105.
2008.PubMed/NCBI
|
|
10
|
Chen JL, Gao RL, Yang YJ, et al: Short and
long-term outcomes of two drug eluting stents in bifurcation
lesions. Chin Med J (Engl). 120:183–186. 2007.PubMed/NCBI
|
|
11
|
Galassi AR, Colombo A, Buchbinder M, et
al: Long-term outcomes of bifurcation lesions after implantation of
drug-eluting stents with the mini-crush technique. Catheter
Cardiovasc Interv. 69:976–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan M, de Suárez Lezo J, et al:
Drug-eluting stents for the treatment of bifurcation lesions: A
randomized comparison between paclitaxel and sirolimus stents. Am
Heart J. 153:15.e1–e7. 2007. View Article : Google Scholar
|
|
13
|
Lassen JF, Holm NR, Stankovic G, Lefèvre
T, Chieffo A, Hildick-Smith D, Pan M, Darremont O, Albiero R,
Ferenc M and Louvard Y: Percutaneous coronary intervention for
coronary bifurcation disease: consensus from the first 10 years of
the European Bifurcation Club meetings. EuroIntervention.
10:545–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Colombo A: Bifurcation lesions. Ital Heart
J. 6:475–488. 2005.PubMed/NCBI
|
|
15
|
Sharma SK, Sweeny J and Kini AS: Coronary
bifurcation lesions: a current update. Cardiol Clin. 28:55–70.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dauerman HL, Higgins PJ, Sparano AM, et
al: Mechanical debulking versus balloon angioplasty for the
treatment of true bifurcation lesions. J Am Coll Cardiol.
32:1845–1852. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dzavik V: Progress in percutaneous
management of coronary bifurcation lesions. Minerva Cardioangiol.
53:379–401. 2005.PubMed/NCBI
|
|
18
|
Villanueva EV, Wasiak J and Petherick ES:
Percutaneous transluminal rotational atherectomy for coronary
artery disease. Cochrane Database Syst Rev.
4:CD0033342003.PubMed/NCBI
|
|
19
|
Nageh T, Kulkarni NM and Thomas MR:
High-speed rotational atherectomy in the treatment of
bifurcation-type coronary lesions. Cardiology. 95:198–205. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Levey AS, Bosch JP, Lewis JB, et al: A
more accurate method to estimate glomerular filtration rate from
serum creatinine: A new prediction equation. Modification of Diet
in Renal Disease Study Group. Ann Intern Med. 130:461–470. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
World Health Organization: Definition,
diagnosis and classification of diabetes mellitus and its
complications: Report of a WHO consultation. Part 1. Diagnosis and
classification of diabetes mellitus (Geneva). World Health
Organization. 1999.
|
|
22
|
Ryan TJ, Faxon DP, Gunnar RM, Kennedy JW,
King SB III, Loop FD, Peterson KL, Reeves TJ, Williams DO and
Winters WL Jr: Guidelines for percutaneous transluminal coronary
angioplasty. A report of the American College of
Cardiology/American Heart Association Task Force on Assessment of
Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee
on Percutaneous Transluminal Coronary Angioplasty). Circulation.
78:486–502. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Louvard Y, Thomas M, Dzavik V,
Hildick-Smith D, Galassi AR, Pan M, Burzotta F, Zelizko M, Dudek D,
Ludman P, et al: Classification of coronary artery bifurcation
lesions and treatments: Time for a consensus! Catheter Cardiovasc
Interv. 71:175–183. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Medina A, de Suárez Lezo J and Pan M: A
new classification of coronary bifurcation lesions. Rev Esp
Cardiol. 59:1832006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang F, Dong L and Ge J: Simple versus
complex stenting strategy for coronary artery bifurcation lesions
in the drug-eluting stent era: A meta-analysis of randomised
trials. Heart. 95:1676–1681. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mauri L, Hsieh WH, Massaro JM, Ho KK,
D'Agostino R and Cutlip DE: Stent thrombosis in randomized clinical
trials of drug-eluting stents. N Engl J Med. 356:1020–1029. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mehran R, Dangas G, Abizaid AS, Mintz GS,
Lansky AJ, Satler LF, Pichard AD, Kent KM, Stone GW and Leon MB:
Angiographic patterns of in-stent restenosis: Classification and
implications for long-term outcome. Circulation. 100:1872–1878.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ito H, Piel S, Das P, et al: Long-term
outcomes of plaque debulking with rotational atherectomy in
side-branch ostial lesions to treat bifurcation coronary disease. J
Invasive Cardiol. 21:598–601. 2009.PubMed/NCBI
|
|
29
|
Stinis CT, Hu SP, Price MJ, et al:
Three-year outcome of drug-eluting stent implantation for coronary
artery bifurcation lesions. Catheter Cardiovasc Interv. 75:309–314.
2010.PubMed/NCBI
|
|
30
|
Levine GN, Bates ER, Blankenship JC,
Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA,
Hollenberg SM, et al: 2011 ACCF/AHA/SCAI Guideline for Percutaneous
Coronary Intervention: executive summary: a report of the American
College of Cardiology Foundation/American Heart Association Task
Force on Practice Guidelines and the Society for Cardiovascular
Angiography and Interventions. Circulation. 124:2574–2609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bersin RM and Simonton CA: Rotational and
directional coronary atherectomy. Catheter Cardiovasc Interv.
58:485–499. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Saland KE, Cigarroa JE, Lange RA, et al:
Rotational atherectomy. Cardiol Rev. 8:174–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Reisman M: Technique and strategy of
rotational atherectomy. Cathet Cardiovasc Diagn. (Suppl 3): 2–14.
1996.PubMed/NCBI
|
|
34
|
Kaufmann UP and Meyer BJ: Atherectomy
(directional, rotational, extractional) and its role in
percutaneous revascularization. Curr Opin Cardiol. 10:412–419.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nageh T, Kulkarni NM and Thomas MR:
High-speed rotational atherectomy in the treatment of
bifurcation-type coronary lesions. Cardiology. 95:198–205. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dauerman HL, Higgins PJ, Sparano AM, et
al: Mechanical debulking versus balloon angioplasty for the
treatment of true bifurcation lesions. J Am Coll Cardiol.
32:1845–1852. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smith SC Jr, Faxon D, Cascio W, et al:
Prevention Conference VI: Diabetes and Cardiovascular Disease.
Writing Group VI: Revascularization in diabetic patients.
Circulation. 105:e165–e169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Elezi S, Kastrati A, Pache J, et al:
Diabetes mellitus and the clinical and angiographic outcome after
coronary stent placement. J Am Coll Cardiol. 32:1866–1873. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ortolani P, Balducelli M, Marzaroli P, et
al: Two-year clinical outcomes with drug-eluting stents for
diabetic patients with de novo coronary lesions: results from a
real-world multicenter registry. Circulation. 117:923–930. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kornowski R, Mintz GS, Kent KM, et al:
Increased restenosis in diabetes mellitus after coronary
interventions is due to exaggerated intimal hyperplasia. A serial
intravascular ultrasound study. Circulation. 95:1366–1369. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sobel BE: Acceleration of restenosis by
diabetes: pathogenetic implications. Circulation. 103:1185–1187.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Iijima R, Ndrepepa G, Mehilli J, et al:
Impact of diabetes mellitus on long-term outcomes in the
drug-eluting stent era. Am Heart J. 154:688–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Diletti R, Garcia-Garcia HM, Bourantas CV,
van Geuns RJ, Van Mieghem NM, Vranckx P, Zhang YJ, Farooq V, Iqbal
J, Wykrzykowska JJ, et al: RESOLUTE All Comers Investigators:
Clinical outcomes after zotarolimus and everolimus drug eluting
stent implantation in coronary artery bifurcation lesions: Insights
from the RESOLUTE All Comers Trial. Heart. 99:1267–1274. 2013.
View Article : Google Scholar : PubMed/NCBI
|