Introduction
Angiocardiopathy is one of the most dangerous
diseases to human health, accounting for 50% of mortality in
developed countries (1). Coronary
heart disease (CHD) may be the most lethal form of cardiovascular
disease. Statistical data from the World Health Organization
indicates that CHD will become the most significant cause of
mortality worldwide by 2020 (2–4).
Thrombolytic therapy, coronary artery bypass grafting (CABG),
percutaneous coronary intervention (PCI) and angiogenesis therapy
are therapeutic options. These therapies enable revascularization
of the ischemic myocardium and markedly improve the symptoms of
CHD; however, they are associated with certain shortcomings. For
example, certain patients are not able to undergo the risk of CABG
and PCI, while others may not be able to afford the treatment.
Alternative therapies targeting angiogenesis, including stem cell
therapy, remain in development and are not yet routinely used in
clinical practice.
Cardiac shockwave therapy (CSWT) is a novel,
noninvasive approach that has been shown to ameliorate myocardial
ischemia and improve cardiac function (5–9). In
early clinical trials CSWT was found to alleviate angina and
improve cardiopulmonary fitness in patients with myocardial
ischemia (6,9). Furthermore, findings from previous
studies suggest that CSWT can reduce the ischemic burden and
relieve angina by enhancing angiogenesis and revascularization in
the ischemic myocardium (5–7). Animal studies in vivo and human
clinical studies have shown that the low-energy pulse waves
generated by CSWT induce a ‘cavitation effect’ (violent collapse of
micron-sized bubbles within and outside cells), exerting a
mechanical shear force on myocardial and vascular endothelial
cells. It has been reported that CSWT promotes revascularization
and improves ventricular remodeling in an acute myocardial
infarction pig model (5,9). Furthermore, results of our previous
study showed that CSWT improved the clinical symptoms of CHD in a
randomized, double-blind experiment (10); however, although CSWT has shown
promising efficacy against the symptoms of CHD, the mechanism is
not clear. Two different studies previously revealed that CSWT
upregulated the vascular endothelial growth factor (VEGF) level in
a chronic myocardial ischemia porcine model (11,12).
Based on previous studies, we hypothesized that CSWT would induce
VEGF, interleukin-8 (IL-8), stromal cell-derived factor 1 (SDF-1)
and matrix metalloproteinase 9 (MMP-9) secretion in CHD patients,
and that the increase in those cytokines would accelerate the
proliferation, differentiation and recruitment of endothelial
progenitor cells (EPCs), promote revascularization in the ischemic
myocardium and improve cardiac function. The aim of this study was
to test this hypothesis.
Patients and methods
Patients
A total of 23 male and 3 female patients, who were
admitted to the Cardiology Department of the First Hospital of
Kunming Medical University (Kunming, China) between December 2008
and December 2011, were enrolled in this study. The age range of
the patients was 46–78 years (mean, 63±10 years), and the average
body mass index was 23.86 kg/m2. The patients had been
diagnosed with CHD for between 1 and 16 years. Comorbidities
included hypertension (n=19), diabetes mellitus (n=7),
hyperlipidemia (n=4), chronic renal inadequacy (n=3) and colitis
gravis (n=1). One patient had undergone CABG and 12 had undergone
PCI. The remaining patients had not been treated with coronary
revascularization due to poor economic conditions, technical
limitations and religious reasons. Although all the patients had
received standard medical treatment, they all still suffered from
chest discomfort and had poor exercise tolerance; furthermore, all
the patients had been hospitalized >2 times within 1 year due to
myocardial ischemia. Patients with a myocardial infarction within
the past 3 months, cardiac thrombus, chronic obstructive pulmonary
disease, pulmonary embolism, New York Heart Association (NYHA)
heart function IV and malignancy were excluded from this study.
CSWT procedure
The CSWT was performed as described in our previous
report (10). Briefly, patients were
placed in a supine position and rested in a non-agitated state
during the procedure. The electrocardiogram (ECG), blood pressure,
breathing and blood oxygen saturation of each patient were
simultaneously monitored. An ultrasound probe was used to identify
the target myocardial regions, and the water cushion was lowered to
contact the chest. Shockwaves (depth, 150 mm; aperture angle, 48°),
triggered by the R wave on the ECG, were voluntarily released by
the parabolic reflector at the absolute refractory period of
electrical activity. Wave energy was gradually increased in all
patients (maximum, 0.09 mJ/mm2 in patients exhibiting no
chest pain). The regional targeting of shockwave transmission to
the myocardium was finely adjusted using microcontrols. A range of
control levels (+1, +2, +3, 0, −1, −2 and −3) enabled the
incremental modulation of the angle (6°) and distance (2.5 mm)
along the treatment area. Each ischemic region was treated at 9
points (−1, 0, +1 pairs). During the treatment period, the patients
underwent close monitoring for vital signs and symptoms, including
palpitations, chest pain, breathing difficulty and dizziness. The
therapy schedule was devised based on the recommendations of the
Cardiovascular Center of Munich, Germany and the Cardiovascular
Center of the Kyushu University Graduate School of Medical
Sciences, Fukuoka, Japan. The CSWT regimen was conducted over a
3-month period at 3-week intervals. CSWT sessions were administered
during the first week of the month on the first, third and fifth
day for 3 months, for a total of 9 therapies per patient. The
efficacy of CSWT was assessed using the Canadian Cardiovascular
Society (CCS) angina scale, NYHA class, Seattle Angina
Questionnaire (SAQ) scale, 6-min walk test (6MWT) and nitroglycerin
dosage prior to and following 30 days of CSWT (13,14).
Analysis of VEGF, IL-8, SDF-1 and
MMP-9 levels in the peripheral blood (PB)
A 2-ml blood sample was drawn from the arm vein of
all patients 1 day before and 30 days after the CSWT. Blood plasma
was prepared by centrifugation (850 × g) at 4°C for 10 min. The
levels of VEGF, IL-8, SDF-1 and MMP-9 were measured using ELISA
kits from R&D Systems GmbH (Wiesbaden-Nordenstadt,
Germany).
Culture and identification of EPCs
among PB mononuclear cells
PB mononuclear cells were prepared by density
gradient centrifugation using lymphocyte separation liquid
(Sigma-Aldrich, St. Louis, MO, USA) (15). PB mononuclear cells were suspended in
EGM-2-MV microvascular endothelial cell growth medium (Lonza
Walkersville, Inc., Walkersville, MD, USA) following centrifugation
and then inoculated at a density of 5×106 cells/well in
human fibronectin-coated 6-well plates (Corning, Inc., Corning, NY,
USA) and cultured in a 37°C incubator with 5% CO2 and
100% humidity. After 4 days of culture, non-adherent cells were
washed away using phosphate-buffered saline (PBS). Three visual
fields under the inverted phase contrast microscope were randomly
selected to count the EPCs and EPC-colony forming units (EPC-CFUs)
after 7 days of culture. One experimenter and one experienced lab
technician performed the count in a blinded manner to evaluate
repeatability (variation coefficient, <10%). In addition, after
7 days of culture the adherent cells were treated with 2.4 mg/l
Dil-labeled acetylated low-density lipoprotein (Dil-acLDL;
Molecular Probes Life Technologies, Carlsbad, CA, USA) for 1 h at
37°C and fixed using 2% paraformaldehyde. After washing, the EPCs
were incubated with 10 mg/ml fluorescein isothiocyanate-Ulex
europaeus agglutinin-1 (FITCUEA-1; Sigma-Aldrich) at 48°C for
30 min. The incorporation of Dil-acLDL and binding of FITCUEA-1
were detected using a confocal microscope (Leica Microsystems GmbH,
Wetzlar, Germany).
Detection of circulating EPCs
The detection of EPCs in the circulating blood was
performed as described in a previous study (16). In brief, 100 µl blood was
immunostained with monoclonal antibodies against human CD34
(peridinin chlorophyll-conjugated; 10:1; PC1211; Becton Dickinson,
Franklin Lakes, NJ, USA) and against human VEGF receptor 2 (VEGFR2;
50:1; A5441; Sigma-Aldrich), followed by a phycoerythrin-conjugated
secondary antibody (Beckman Coulter, Inc., Brea, CA, USA).
Isotype-identical antibodies served as controls (10:1; AK1035;
Becton Dickinson). Following incubation, cells were lysed, washed
with PBS and fixed in 4% paraformaldehyde; after the exclusion of
debris and platelets, 70,000 events were analyzed using a
FACSCalibur Flow Cytometer (Becton Dickinson).
Clinical follow-up
The patients were followed-up for 4 months after the
last CSWT appointment through outpatient visits, hospital visits
and telephone conservations. During follow-up, the therapy scheme
was selected according to the state of the illness. If the vessels
were not seriously blocked, expectant treatment was administered.
If the vessels was seriously damaged and blocked, surgical
treatment was advised (CABG and PCI).
Ethical approval
This study was authorized by the Medical Ethics
Committee of Kunming Medical University in December 2008. All 26
patients were informed about the details and procedures involved in
the CSWT. Informed written consent was obtained from each patient
prior to CSWT.
Statistical analysis
Continuous variables are expressed as the mean ±
standard deviation and categorical variables are expressed as
percentages. Nonparametric variables are expressed as the median
(interquartile range). The pre-treatment and post-treatment values
were compared using a paired t-test. Statistical analyses were
performed using SPSS 15.0 statistical software (SPSS, Inc.,
Chicago, IL, USA). All statistical tests were two-sided, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Efficacy of CSWT
After 3 months of CSWT, the results of the 6MWT and
the SAQ scale score were found to have increased significantly
(P<0.01). The NYHA class and CCS angina scale score were
observed to have reduced significantly compared with the pre-CSWT
values (P<0.01). The frequency of weekly nitroglycerin use
declined by 50% (1.00 vs. 0.50, pre-treatment and post-treatment)
(Table I).
 | Table I.Evaluation of the clinical curative
effect of CSWT. |
Table I.
Evaluation of the clinical curative
effect of CSWT.
| Time-point | 6MWT (m) | NYHA class | CCS angina
scale | SAQ scale | Nitroglycerin
dosage (doses per week) |
|---|
| Pre-CSWT |
360.69±116.79 | 1.85±0.21 | 1.85±0.15 | 67.58±13.03 | 1.00±0.27 |
| Post-CSWT |
434.15±86.29a |
1.23±0.08a |
1.19±0.08b |
77.54±10.84a |
0.50±0.19a |
Analysis of VEGF, IL-8, SDF-1 and
MMP-9 levels in the PB
As previously observed in vivo (5), SW therapy has been used to upregulate
VEGF expression in a porcine model of chronic myocardial ischemia.
In the present study, the VEGF level in the PB in CHD patients was
>7-fold higher following CSWT compared with the pre-CSWT level
(P<0.01). The post-CSWT IL-8 content also exhibited a marked
increased and was found to be ~7-fold higher than the pre-CSWT
value (P<0.01). By contrast, the levels of SDF-1 and MMP-9 did
not show a significant change following CSWT (P>0.05) (Table II).
 | Table II.Effect of CSWT on the VEGF, IL-8,
SDF-1 and MMP-9 levels in the peripheral blood. |
Table II.
Effect of CSWT on the VEGF, IL-8,
SDF-1 and MMP-9 levels in the peripheral blood.
| Time-point | VEGF (pg/ml) | IL-8 (pg/ml) | SDF-1 (pg/ml) | MMP-9 (ng/ml) |
|---|
| Pre-CSWT |
20.26±19.85 | 21.81±5.94 |
2,750.87±636.74 | 19.66±3.96 |
| Post-CSWT |
155.19±24.67a |
149.70±44.11a |
2,700.47±415.19 | 18.55±3.78 |
PB mononuclear cell culture
The PB mononuclear cells were observed to become
larger and clearer after 3 days' culture in vitro, and rod
and fusiform cells began to form. Several cell clusters appeared,
and colonies were observed in the culture at 7 days. An increased
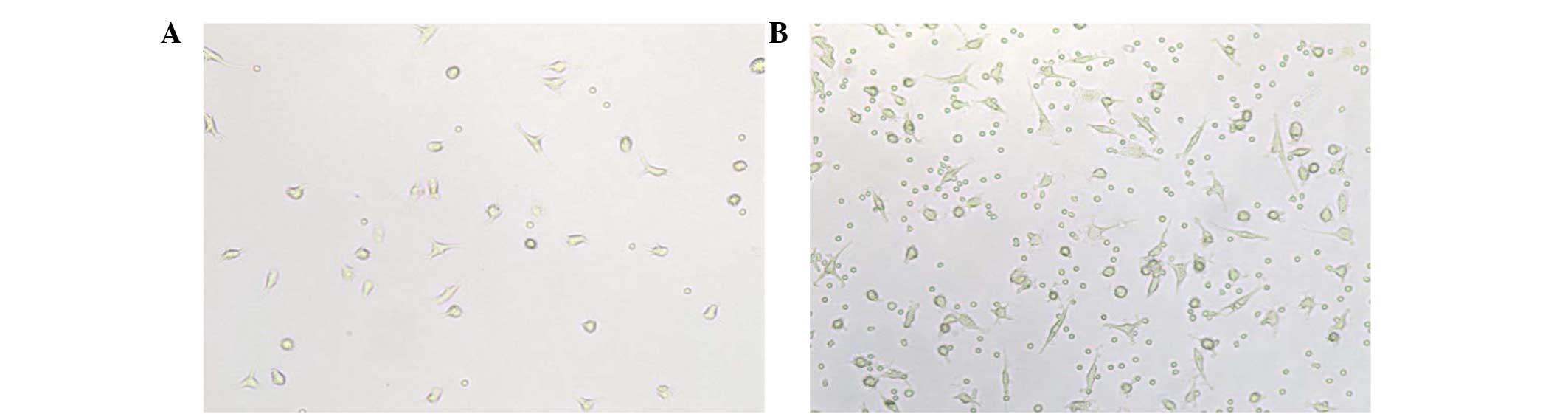
number of differentiating rod and fusiform cells was observed in
patients following CSWT compared with patients before CSWT
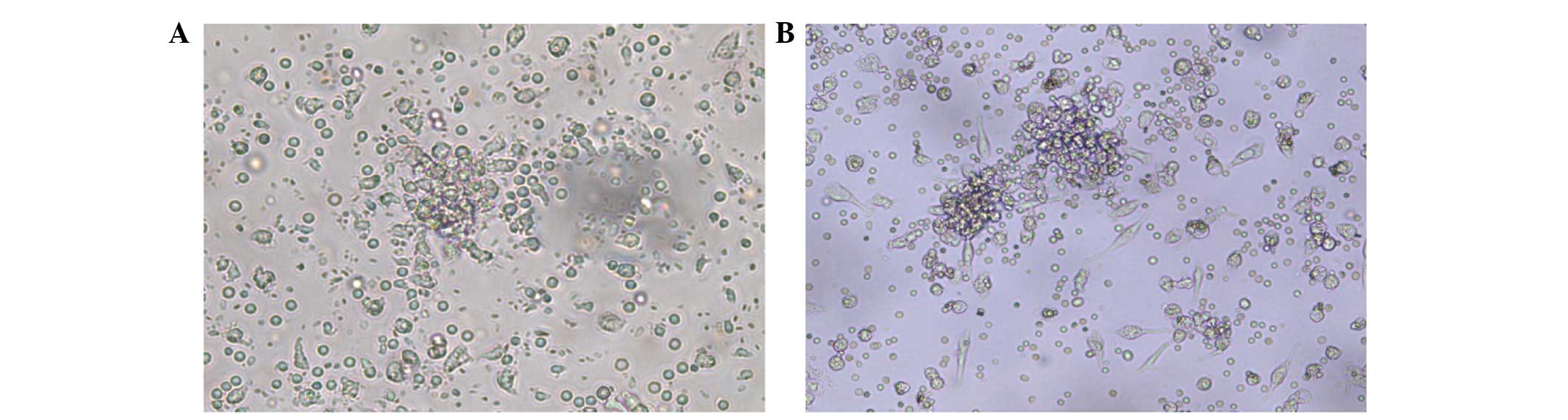
(Fig. 1). Furthermore, an increased
size and frequency of EPC-CFUs were noted in the patients subjected
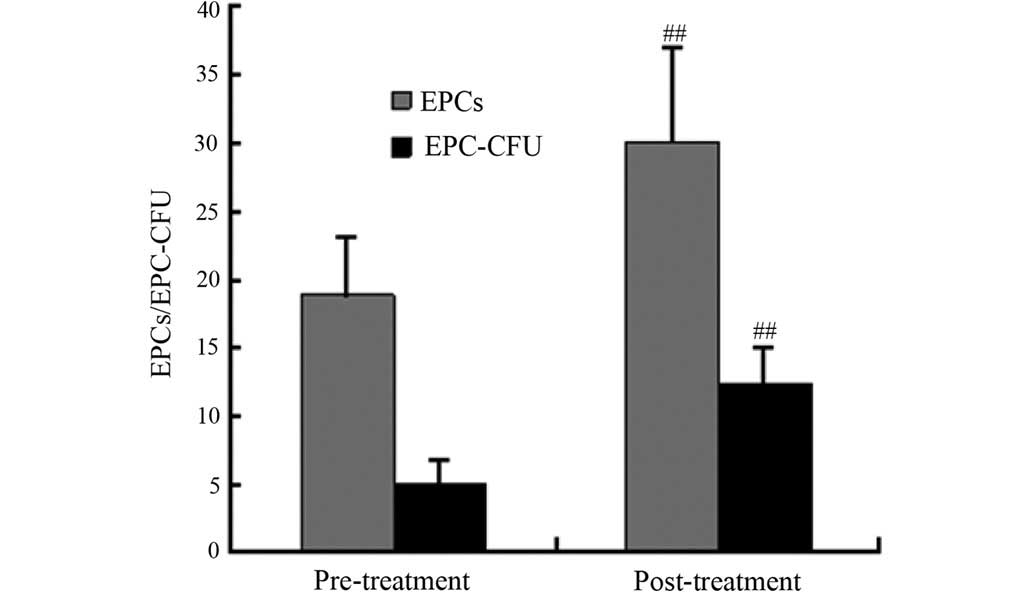
to treatment compared with the pre-treatment observations (Fig. 2). The cell count results showed that
the numbers of EPCs and EPC-CFUs were significantly increased
following CSWT (P<0.001) (Figs. 3
and 4). To evaluate the effect of
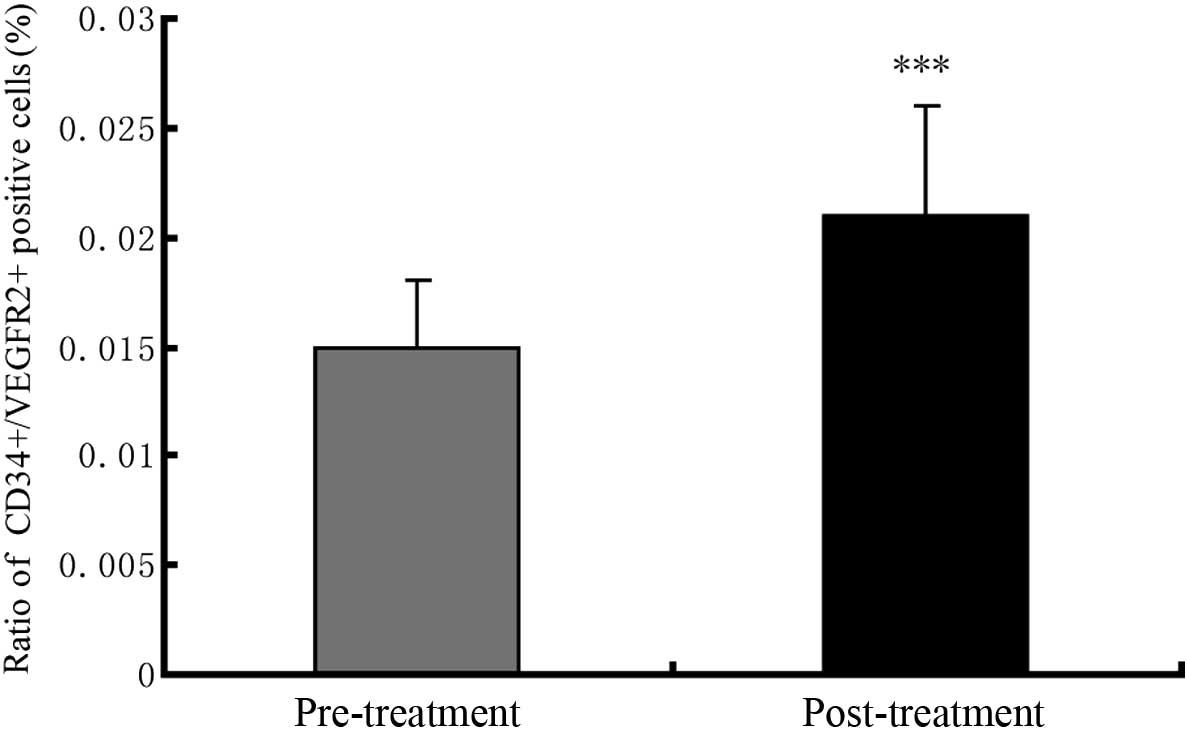
CSWT on the EPCs directly, further comparisons of the circulating
EPCs in the PB from patients prior to and following CSWT were
conducted. The circulating EPCs (represented by the
CD45low/CD34+/VEGFR2+ population)
accounted for 1.5% of the mononuclear cells in the PB before the
CSWT (Fig. 5). After the CSWT, a
0.6% increase in the number of
CD45low/CD34+/VEGFR2+ cells in the
PB was observed (Fig. 5).
 | Figure 1.Identification of EPCs. (A) Red
indicates cells positively marked with Dil-acLDL (magnification,
×200); (B) green indicates cells positively marked with FITCUEA-1
(magnification, ×200); (C) overlap of (A) and (B), with yellow
indicating the differentiating EPCs (magnification, ×200). After 7
days' culure the adherent cells were incubated with Dil-acLDL (2.4
mg/l) for 1 h at 37°C, and the Dil-acLDL uptake was then detected.
If uptake was visible, the cells were fixed using 2%
paraformaldehyde and rinsed with phosphate-buffered saline, prior
to the addition of FITCUEA-1 (10 mg/l) and incubation for another 1
h at 37°C. Images were captured using a confocal laser scanning
microscope. EPC, endothelial progenitor cell; Dil-acLDL,
Dil-labeled acetylated low-density lipoprotein; FITCUEA-1,
fluorescein isothiocyanate-Ulex europaeus agglutinin-1. |
Side effects
All patients completed the 9 treatments of CSWT and
were followed-up successfully. During the course of treatment, no
severe side effects, such as dyspnea, heart failure, heart
palpitations, hemorrhage, syncope, embolism and shock, occurred.
Although several patients complained of a ‘prickly’ pain in the
chest wall during the first CSWT, this uncomfortable sensation
disappeared when the CSWT progressed. Four patients had occasional
premature ventricular contractions within the first week of CSWT;
however, the blood pressure, heart rate and arterial blood oxygen
saturation remained normal. Occasional premature ventricular
contractions did not re-occur in the later stages of treatment.
None of the patients succumbed within 30 days of the last CSWT
appointment, and none of the patients were re-hospitalized due to
heart failure or angina or required PCI or CABG.
Discussion
The finding that CSWT could produce favorable
effects when applied to animal models and patients with CHD was
initially made by Nishida et al (5). Since then, a number of studies have
been conducted to evaluate the efficacy of CSWT in the treatment of
CHD and other diseases (6,7,9,16–20). The
results from the majority of the investigations showed that CSWT
was effective. In the present study, the CCS angina scale, NYHA
class, SAQ scale, nitroglycerin dosage and 6MWT results of the CHD
patients showed improvements following CSWT. Furthermore, the
quality of life and exercise tolerance of the patients were
enhanced, according to the self-descriptions in the follow-up. No
severe negative effects were found during the CSWT or in the
follow-up. The statistical outcomes showed that CSWT was safe and
effective for the treatment of CHD.
EPCs are not only involved in human embryonic
angiogenesis, but also participate in postnatal angiogenesis and
endothelial injury recovery. It has been found that 25% of
endotheliocytes in angiogenesis are derived from EPCs (21). The number and migration ability of
EPCs are negatively associated with vascular risk factors. It has
been reported that EPC numbers in the circulating blood of patients
with CHD decline to ~50% and that the migration ability of the EPCs
is also negatively affected (21,22).
Under multivariate analysis, age and a positive family history of
coronary artery disease remained the only significant independent
predictors of a reduced number of EPCs (23). This result indicated that the number
of EPCs in patients with CHD or other cardiovascular risk factors
may decrease more rapidly with age. Restoring the endothelial
lining to normal is critical for slowing or reversing the
progression of vascular disease. We hypothesized that the number
and function of EPCs could be improved via CSWT in patients with
CHD and that the improvement would aid angiogenesis and endothelial
repair in these patients, ultimately enhancing cardiac function.
The present results showed that the numbers of EPCs and EPC-CFUs
among the PB mononuclear cells were significantly increased
following CSWT, and the differentiation ability of the EPCs was
strengthened. Prior to CSWT, numerous small, characteristic EPCs
were observed in the blood samples of the CHD patients. Those cells
easily metamorphosed and had a poor ability to further
differentiate. In addition, there were few formed EPC colonies.
Following CSWT, the EPCs and EPC-CFUs increased in number, and
differentiation of the rod and fusiform cells increased. These
visible changes were induced by CSWT, and the results were
consistent with the findings of Igarashi et al (24) and Thurston et al (25). The number of circulating EPCs also
increased markedly following CSWT in the present study. By
contrast, Kikuchi et al (17)
found that the number of circulating EPCs in samples from 8
patients with CHD exhibited minimal change following CSWT. It is
possible that these differences were due to the different sampling
time. In the present study, the blood samples were taken 30 days
after the last CSWT, while Kikuchi et al examined the EPC
numbers 24 h after the last CSWT. The circulating EPCs may not have
had time to undergo perceptible changes only 24 h after CSWT in the
study by Kikuchi et al.
Since it was observed in the present study that CSWT
induced EPC differentiation and proliferation, a further
investigation into the reasons underlying the phenomenon would be
beneficial to reveal the mechanism behind the efficacy of CSWT in
the treatment of CHD. The differentiation, proliferation and
function of circulating EPCs are closely associated with growth
factors, including VEGF and other cytokines (26–28). To
evaluate whether the cytokines were involved in the CSWT-induced
EPC differentiation and proliferation, the levels of four
cytokines, including VEGF, IL-8, SDF-1 and MMP-9, were measured in
the PB of CHD patients. Among the growth factors, VEGF is the most
critical factor for vasculogenesis and angiogenesis (29–31), and
the VEGF levels in the PB were found to be significantly increased
following treatment in the present study (Fig. 1). The results were consistent with
the findings in a chronic ischemia pig model and human umbilical
vascular endothelial cells (5,32). The
observed increase in VEGF levels in the PB following CSWT,
accompanied by the increase in the numbers and colonies of EPCs, as
well as the circulating EPC numbers, indicated that CSWT
contributed to the multiplication and differentiation of EPCs.
IL-8, a type of small peptide molecule, promotes
angiogenesis by increasing cell contraction and enlarging gaps
between flanking cells. IL-8 also leads to protein phosphorylation
downstream by activating the Ras and mitogen-activated protein
kinase pathway, which then mediates cell migration, adhesion and
paracrine activity (33–35). IL-8 participates in the recruitment
of EPCs in the myocardium from the circulation and increases their
homing capacity in the ischemic myocardium, thereby promoting
tissue regeneration and favorably affecting cardiac remodeling
(36). In the present study, the
IL-8 levels were significantly increased following CSWT. It is
possible that the increase in IL-8 not only triggered the
proliferation of EPCs, but also resulted in EPC recruitment. The
present findings showed that IL-8 was induced by CSWT in CHD
patients and that, in addition to VEGF, IL-8 was involved in the
increase in EPC multiplication and differentiation caused by
CSWT.
Similarly to IL-8, SDF-1 has been shown to induce
neovascularization through the recruitment of EPCs into ischemic
tissue (36,37). The changes in SDF-1 following CSWT in
the present study were not found to be significant. This may have
been due to the fact that the secreted IL-8 was sufficient to
induce the EPCs, making an increase in SDF-1 unnecessary. Another
reason may be that measurements were not made at the time-point
corresponding to a significant increase in SDF-1. In a study of
patients with acute myocardial infarction, the SDF-1 expression
level was reported to reach a peak in the PB 17 days after CSWT
(38). A perceptible SDF-1
expression change may have been missed in the present study, as the
SDF-1 levels were tested 30 days after CSWT. This requires
confirmation in future studies. The MMP-9 content in the PB also
showed no significant change following CSWT. The MMP-9-induced
migration of EPCs is regulated by SDF-1 (39); since the change in SDF-1 in the
present study was minimal, it is reasonable that the change in
MMP-9 was also found to lack significance.
In conclusion, the clinical symptoms of CHD were
found to be alleviated after 3 months of CSWT in the present study.
The effect of CSWT on CHD was indicated by several aspects of the
study. First, CSWT promoted an increase in the level of VEGF in the
ischemic myocardium, which led to an increase in the number of EPCs
and promoted the survival and migration of EPCs, as well as lumen
formation and conglutination and the synthesis and secretion of
growth factors. Secondly, CSWT induced an increase in the level of
IL-8 in the ischemic myocardium, which induced the recruitment of
EPCs into the ischemic tissue. Notably, although the levels of
SDF-1 and MMP-9 showed little change during the CSWT, the effect of
CSWT on the SDF-1 and MMP-9 expression can not be ruled out, as the
peaks of SDF-1 and MMP-9 expression may have been missed due to the
short therapeutic period and the time limitations of the sampling.
The type of mobilizing factor responsible for inducing the decisive
increase in EPCs remains unclear and warrants further study. In
addition, further research should be conducted into the signal
transduction pathways associated with the growth factors involved
in the promotion of the proliferation and differentiation of the
EPCs.
Acknowledgments
This study was supported by The National Natural
Science Foundation of China (no. 81260027), Technology Department
Program of Yunnan Province (no. 2014FZ023) and the Health
Department Program of Yunnan Province (no. 2012WS0005).
References
|
1
|
Gaziano T, Reddy KS, Paccaud F, Horton S
and Chaturvedi V: Cardiovascular Disease. Disease Control
Priorities in Developing Countries (2nd). (Washington DC). World
Bank. 2006.
|
|
2
|
Bonow RO, Smaha LA, Smith SC Jr, Mensah GA
and Lenfant C: World Heart Day 2002. The international burden of
cardiovascular disease: Responding to the emerging global epidemic
Circulation. 106:1602–1605. 2002.
|
|
3
|
World Health Organization: Program Fact
Sheet no 310. The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/index.htmlAccessed.
January 6–2010
|
|
4
|
Fan L, Ma J, Chen YH and Chen XQ:
Antioxidant and antimicrobial phenolic compounds from Setaria
viridis. Chem Nat Comp. 3:433–437. 2014. View Article : Google Scholar
|
|
5
|
Nishida T, Shimokawa H, Oi K, Tatewaki H,
Uwatoku T, Abe K, Matsumoto Y, Kajihara N, Eto M, Matsuda T, et al:
Extracorporeal cardiac shock wave therapy ameliorates
ischemia-induced myocardial dysfunction in pigs in vivo.
Circulation. 110:3055–3061. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fukumoto Y, Ito A, Uwatoku T, Matoba T,
Kishi T, Tanaka H, Takeshita A, Sunagawa K and Shimokawa H:
Extracorporeal cardiac shock wave therapy ameliorates myocardial
ischemia in patients with severe coronary artery disease. Coron
Artery Dis. 17:63–70. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uwatoku T, Ito K, Abe K, Qi K, Hizume T,
Sunagawa K and Shimokawa H: Extracorporeal cardiac shock wave
therapy improves left ventricular remodeling after acute myocardial
infarction in pigs. Coron Artery Dis. 18:397–404. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shimokawa H, Ito K, Fukumoto Y and Yasuda
S: Extracorporeal cardiac shock wave therapy for ischemic heart
disease. Shock Waves. 17:449–455. 2008. View Article : Google Scholar
|
|
9
|
Khattab AA, Brodersen B,
Schuermann-Kuchenbrandt D, Beurich H, Tölg R, Geist V, Schäfer T
and Richardt G: Extracorporeal cardiac shock wave therapy: First
experience in the everyday practice for treatment of chronic
refractory angina pectoris. Int J Cardiol. 121:84–85. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang P, Guo T, Wang W, Peng YZ, Wang Y,
Zhou P, Luo ZL, Cai HY, Zhao L and Yang HW: Randomized and
double-blind controlled clinical trial of extracorporeal cardiac
shock wave therapy for coronary heart disease. Heart Vessels.
28:284–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ito Y, Ito K, Shiroto T, Tsuburaya R, Yi
GJ, Takeda M, Fukumoto Y, Yasuda S and Shimokawa H: Cardiac shock
wave therapy ameliorates left ventricular remodeling after
myocardial ischemia-reperfusion injury in pigs in vivo. Coron
Artery Dis. 21:304–311. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu M, Sun CK, Lin YC, Wang CJ, Wu CJ, Ko
SF, Chua S, Sheu JJ, Chiang CH, Shao PL, et al: Extracorporeal
shock wave therapy reverses ischemia-related left ventricular
dysfunction and remodeling: Molecular-cellular and functional
assessment. PLoS One. 6:e243422011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schaufelberger M and Swedberg K: Is
6-minute walk test of value in congestive in heart failure? Am
Heart J. 136:371–372. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Spertus JA, Winder JA, Dewhurst TA, Deyo
RA, Prodzinski J, McDonell M and Fihn SD: Development and
evaluation of the Seattle Angina Questionnaire: A new functional
status measure for coronary artery disease. J AM Coll Cardiol.
25:333–341. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen XQ, Wang QQ, Li JM, Wang YQ, Zhang XZ
and Li K: Comparison of simultaneous distillation and extraction,
static headspace and headspace-solid phase microextraction coupled
with GC/MS to measure the flavour components of Tricholoma
matsutake. Asian J Chem. 25:6059–6063. 2013.
|
|
16
|
Nurzynska D, Di Meglio F, Castaldo C,
Arcucci A, Marlinghaus E, Russo S, Corrado B, de Santo L,
Baldascino F, Cotrufo M and Montagnani S: Shock waves activate in
vitro cultured progenitors and precursors of cardiac cell lineages
from the human heart. Ultrasound Med Biol. 34:334–342. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kikuchi Y, Ito K, Ito Y, Shiroto T,
Tsuburaya R, Aizawa K, Hao K, Fukumoto Y, Takahashi J, Takeda M, et
al: Double-blind and placebo-controlled study of the effectiveness
and safety of extracorporeal cardiac shock wave therapy for severe
angina pectoris. Circ J. 74:589–591. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang CJ, Wang FS, Yang KD, Weng LH, Hsu
CC, Huang CS and Yang LC: Shock wave therapy induces
neovascularization at the tendon-bone junction. A study in rabbits.
J Orthop Res. 21:984–989. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Guo T, Cai HY, Ma TK, Tao SM, Sun
S, Chen MQ, Gu Y, Pang JH, Xiao JM, et al: Cardiac shock wave
therapy reduces angina and improves myocardial function in patients
with refractory coronary artery disease. Clin Cardiol. 33:693–699.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Guo T, Ma TK, Cai HY, Tao SM, Peng
YZ, Yang P, Chen MQ and Gu Y: A modified regimen of extracorporeal
cardiac shock wave therapy for treatment of coronary artery
disease. Cardiovasc Ultrasound. 10:352012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vasa M, Fichtlscherer S, Aicher A, Adler
K, Urbich C, Martin H, Zeiher AM and Dimmeler S: Number and
migratory activity of circulating endothelial progenitor cells
inversely correlate with risk factors for coronary artery disease.
Circ Res. 89:E1–E7. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hill JM, Zalos G, Halcox JP, Schenke WH,
Waclawiw MA, Quyyumi AA and Finkel T: Circulating endothelial
progenitor cells, vascular function, and cardiovascular risk. N
Engl J Med. 348:593–600. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schmidt-Lucke C, Rössig L, Fichtlscherer
S, Vasa M, Britten M, Kämper U, Dimmeler S and Zeiher AM: Reduced
number of circulating endothelial progenitor cells predicts future
cardiovascular events: Proof of concept for the clinical importance
of endogenous vascular repair. Circulation. 111:2981–2987. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Igarashi J, Miyoshi M, Hashimoto T, Kubota
Y and Kosaka H: Statins induce S1P1 receptors and enhance
endothelial nitric oxide production in response to high-density
lipoproteins. Br J Pharmacol. 150:470–479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thurston G, Rudge JS, Ioffe E, Zhou H,
Ross L, Croll SD, Glazer N, Holash J, McDonald DM and Yancopoulos
GD: Angiopoietin-1 protects the adult vasculature against plasma
leakage. Nat Med. 6:460–463. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asahara T, Takahashi T, Masuda H, Kalka C,
Chen D, Iwaguro H, Inai Y, Silver M and Isner JM: VEGF contributes
to postnatal neovascularization by mobilizing bone marrow-derived
endothelial progenitor cells. EMBO J. 18:3964–3972. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gehling UM, Ergün S, Schumacher U, Wagener
C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, et
al: In vitro differentiation of endothelial cells from
AC133-positive progenitor cells. Blood. 95:3106–3112.
2000.PubMed/NCBI
|
|
28
|
Hattori K, Dias S, Heissig B, Hackett NR,
Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, et al:
Vascular endothelial growth factor and angiopoietin-1 stimulate
postnatal hematopoiesis by recruitment of vasculogenic and
hematopoietic stem cells. J Exp Med. 193:1005–1014. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Carmeliet P, Ferreira V, Breier G,
Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A,
Harpal K, Eberhardt C, et al: Abnormal blood vessel development and
lethality in embryos lacking a single VEGF allele. Nature.
380:435–439. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ferrara N, Carver-Moore K, Chen H, Dowd M,
Lu L, O'Shea KS, Powell-Braxton L, Hillan KJ and Moore MW:
Heterozygous embryonic lethality induced by targeted inactivation
of the VEGF gene. Nature. 380:439–442. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shalaby F, Rossant J, Yamaguchi TP,
Gertsenstein M, Wu XF, Breitman ML and Schuh AC: Failure of
blood-island formation and vasculogenesis in Flk-1-deficient mice.
Nature. 376:62–66. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gutersohn A, Caspari G and Erbel R:
Upregulation of Vascular endothelial growth factor m-RNA in Human
umbilical vascular endothelial cells via shock waves. Eur J Heart
Fail. 2(Suppl 1): 422000. View Article : Google Scholar
|
|
33
|
Kupatt C, Hinkel R, Pfosser A, El-Aouni C,
Wuchrer A, Fritz A, Globisch F, Thormann M, Horstkotte J, Lebherz
C, et al: Cotransfection of vascular endothelial growth factor-A
and platelet-derived growth factor-B via recombinant
adeno-associated virus resolves chronic ischemic malperfusion role
of vessel maturation. J Am Coll Cardiol. 56:414–422. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Urao N, Inomata H, Razvi M, Kim HW, Wary
K, McKinney R, Fukai T and Ushio-Fukai M: Role of nox2-based NADPH
oxidase in bone marrow and progenitor cell function involved in
neovascularization induced by hindlimb ischemia. Circ Res.
103:212–220. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lund T, Hermansen SE, Andreasen TV, Olsen
JO, Østerud B, Myrmel T and Ytrehus K: Shear stress regulates
inflammatory and thrombogenic gene transcripts in cultured human
endothelial progenitor cells. Thromb Haemost. 104:582–591. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kocher AA, Schuster MD, Bonaros N, Lietz
K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowski P,
Boyle A, et al: Myocardial homing and neovascularization by human
bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J
Mol Cell Cardiol. 40:455–464. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
De Falco E, Porcelli D, Torella AR,
Straino S, Iachininoto MG, Orlandi A, Truffa S, Biglioli P,
Napolitano M, Capogrossi MC and Pesce M: SDF-1 involvement in
endothelial phenotype and ischemia-induced recruitment of bone
marrow progenitor cells. Blood. 104:3472–3482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Huang PH, Chen YH, Wang CH, Chen JS, Tsai
HY, Lin FY, Lo WY, Wu TC, Sata M, Chen JW and Lin SJ: Matrix
metalloproteinase-9 is essential for ischemia-induced
neovascularization by modulating bone marrow-derived endothelial
progenitor cells. Arterioscler Thromb Vasc Biol. 29:1179–1184.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Valenzuela-Fernández A, Planchenault T,
Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini
F, Virelizier JL, Chignard M, et al: Leukocyte elastase negatively
regulates Stromal cell-derived factor-1(SDF-1)/CXCR4 binding and
functions by amino-terminal processing of SDF-1 and CXCR4. J Biol
Chem. 277:15677–15689. 2002. View Article : Google Scholar : PubMed/NCBI
|