Introduction
The normal liver obtains its blood supply from two
sources, namely the portal vein, which provides 70% of the supply,
and the hepatic artery, which provides 30% (1). However, primary liver cancer, also
known as hepatoma or hepatocellular carcinoma (HCC), obtains blood
exclusively from the hepatic artery (2,3). Since
Yamada et al first reported transcatheter arterial
embolization (TAE) treatment for HCC in 1983 (4), transcatheter arterial chemoembolization
(TACE) has become widely used for the treatment of unresectable
HCC, with the direct infusion of chemotherapeutic drugs to the
tumor through the hepatic artery. The advantage of TACE is that
greater concentrations of drug can be delivered directly to the
tumor to reduce systemic toxicity and increase the efficacy of
treatment (5,6).
With the development of TACE, physicians have found
that the main hepatic artery is not the only artery that supplies
blood to hepatic tumors (7–9). Superselective TACE requires proper
identification of tumor feeding arteries (10). Tumors are commonly supplied by
extrahepatic collateral vessels, irrespective of the patency of the
hepatic artery; indeed, most tumors fed from extrahepatic arteries
(EHAs) are found in patients with a patent hepatic artery (11,12).
Investigators now consider that location of a tumor adjacent to the
suspensory ligaments and bare area of the liver, direct invasion of
or adhesion to adjacent organs, adhesions induced by previous
abdominal surgery and recurrent tumor at the resection margin are
the causes of EHA development (13–16).
In China, due to imbalances of economic development,
many patients are diagnosed late with HCC and lose the opportunity
for surgical treatment. TACE is an effective way to prolong the
life of such patients. In practice, HCCs supplied by EHAs are often
encountered even when the hepatic arteries are patent (17–19).
Moreover, the development of EHAs interferes with effective
treatment of the tumor with TACE. Although there are 401,000
patients in China diagnosed with liver cancer annually (20), few studies have focused on the
formation of EHAs feeding hepatic tumors in Chinese patients.
The purpose of this study was to assess the
occurrence of EHAs feeding HCCs, to determine the spectrum of
extrahepatic collateral vessels and the causes of EHA formation,
and to study the imaging characteristics of EHAs on routine
radiological examinations such as enhanced magnetic resonance
imaging (MRI) and computed tomography (CT), to improve the efficacy
and avoid complications in Chinese patients undergoing TACE of
EHAs.
Materials and methods
Patients
This retrospective study was approved by the ethics
committee of Zhongshan Hospital of Fudan University (Shanghai,
China). Informed consent that the associated clinical information
would possibly be used in future retrospective studies was obtained
from all patients before TACE was performed. Each year, TACE is
performed on ~6,000 patients from different regions of China in the
Department of Interventional Radiology at Zhongshan Hospital of
Fudan University. There are three completely independent clinical
groups in this interventional radiological center, and each
independent group treats ~2,000 TACE cases each year. Therefore, in
this study the clinical data of the patients in these three
independent clinical groups were compared to evaluate the
extrahepatic collateral arteries in HCC and considered that the
data may be representative of the occurrence of EHAs in Chinese
patients with HCC.
A total of 942 patients were diagnosed with HCC by
enhanced CT or MRI in the hospital from November 2011 to September
2012. The patients were treated in three independent groups of 285,
301 and 356 patients, respectively. These patients were underwent
routine history taking, physical examination, blood tests, liver
function and serum α-fetoprotein (AFP) tests, ultrasonography, and
abdominal enhanced CT or enhanced MRI. HCC was staged according to
the Barcelona Clinic Liver Cancer (BCLC) criteria (21).
Inclusion and exclusion criteria
Inclusion criteria
The related clinical information of the patients
treated with TACE was reviewed. Patients included in this
retrospective study had unresectable BCLC stage B HCC, with <50%
tumor volume in the liver, Child stage A or B and a patent portal
vein.
Exclusion criteria
Patients with BCLC stage B/C HCC with chronic renal
failure, congestive heart failure, encephalopathy, previous upper
gastrointestinal bleeding, severe coronary artery disease and
portal vein occlusion were not included in this retrospective
study.
Indications of EHAs in HCC
Enhanced CT or MRI was performed prior to TACE to
confirm the location and size of tumors and the retention of
iodized oil. Maximum tumor diameter was measured to indicate the
tumor size. Results were evaluated by three radiologists. The
levels of AFP, a tumor marker, were determined prior to TACE.
Certain findings suggested that it was necessary to
search for EHAs by selective angiography. These were cases where:
Tumors were in a peripheral or subcapsular location; there was
exophytic tumor growth; a peripheral iodized-oil retention defect
was exhibited within the tumor; a peripherally located portion of
viable tumor was observed on a follow-up CT scan after TACE;
hypertrophied arteries other than the hepatic artery were visible
around the tumor on a CT scan; and a persistent elevation of the
serum AFP level even after successful chemoembolization via the
hepatic arteries.
Identification of arteries supplying tumors by
initial TACE
Angiography or TACE was performed through the right
femoral artery using a 4F or 5F catheter and a 0.035-inch J-shaped
guide wire. Celiac axis arteriography was first undertaken to
identify the location and number of tumors. Subsequently, selective
cannulation was achieved by placing the catheter in the artery
feeding the tumor.
In cases where celiac axis arteriography did not
image the entire liver or there was a contrast staining defect in
part of the liver, angiography of at least one of the superior
mesenteric artery, inferior phrenic artery, omental branch, adrenal
artery, intercostal artery, internal mammary artery, renal capsular
artery, gastric artery or lumbar artery was conducted to determine
the blood vessels supplying the tumor.
During TACE, in cases where the lipiodol emulsion
did not cover the entire tumor or there was a defect in the tumor
in addition to the deposition of lipiodol, other potential tumor
supplying arteries were sought.
Chemoembolization
The emulsion comprised iodized oil (Lipiodol; Andre
Guerbet, Aulnay-sous-Bois, France) and a chemotherapeutic drug, and
was made by mixing, with stirring, 1–15 ml iodized oil and 10–30 mg
doxorubicin hydrochloride or 50–150 mg oxaliplatin. The emulsion
was then infused into the selected arteries. When the flow slowed
or ceased and tiny portal venules were seen, TACE was stopped to
avoid emulsion reflux and non-target embolization.
Follow-up for the presence of tumor-supplying
EHAs
The patients underwent MRI or CT and measurement of
AFP 4–6 weeks after TACE, to detect primary or recurrent tumors
supplied by EHAs. In cases where the presence of a tumor other than
the lipiodol-covered lesion or tumor recurrence was suspected,
angiography was conducted to identify the EHAs supplying the tumor.
Superselective cannulation using a microcatheter and microguide
wire was performed to reach as far as possible into the target
arteries and avoid non-target organ embolization. Chemoembolization
of the EHAs in these patients was performed in the same manner as
that through the hepatic artery. When sustained elevation of serum
AFP was observed, even in the absence of tumor on MRI or CT, the
presence of EHAs was suspected. In such cases, repeat TACE was
performed to identify EHAs supplying a tumor.
Statistical analysis
All data are expressed as the mean ± the standard
error of the mean and n represents the number of patients per
clinical. Data processing and analysis were conducted using SPSS
version 10.0 (SPSS Inc., Chicago, IL, USA). Statistical comparisons
between the groups were performed using the rank sum test.
Differences were considered significant at P<0.05.
Results
Detection of EHAs
A total of 942 patients with HCC underwent TACE. In
these patients, 698 EHAs were found in addition to the hepatic
artery. The finding of elevated AFP following TACE usually implied
that EHAs supplying the tumor had formed or existed. The presence
of EHAs was often demonstrated by superselective angiography in
addition to celiac artery angiography. In this study, 92.1% of EHAs
were identified by arteriography and 7.9 % were initially present
in the HCC cases and were diagnosed by enhanced MRI or CT prior to
angiography and chemoembolization (Fig.
1). In addition, 67.3% of patients, EHAs were indicated by
persistantly increased levels of AFP. Hepatic artery occlusion is
not a major cause of EHAs; in the present study, 84.5% of patients
with EHAs had widely patent hepatic arteries.
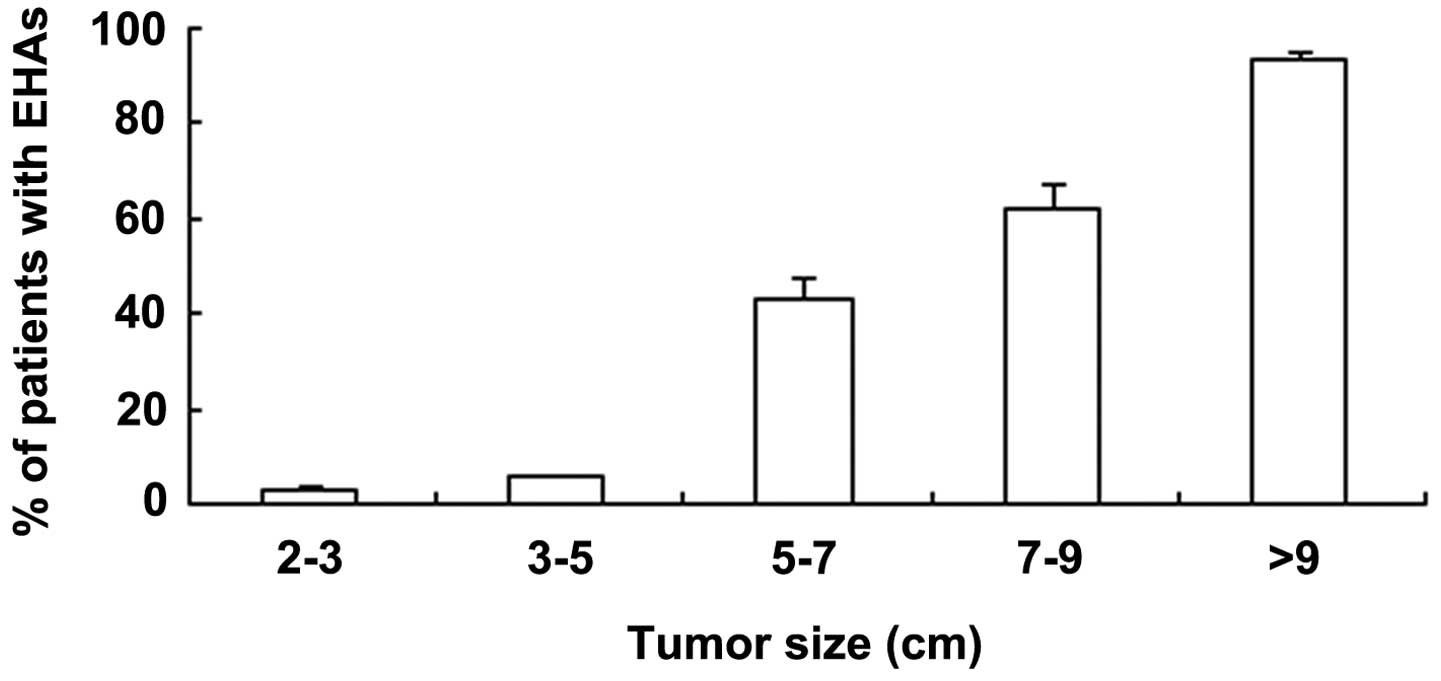
Tumor size is associated with the
formation of extrahepatic collateral arteries
The present study shows that the prevalence of an
extrahepatic collateral supply to HCC was closely associated with
tumor size. When the tumor was <5 cm in diameter, the prevalence
of EHAs at the initial TACE session was <3.0±0.4%; when the
tumor was >5 cm in diameter, the prevalence was 43.3±4.0%. In
patients with primary tumors >5 cm in diameter, the majority of
the EHAs fed the primary tumor.
The cumulative probability of EHAs in patients with
a large tumor (≥5 cm) was significantly higher than that in those
with a small tumor (<5 cm; P<0.05). In patients who initially
had a large primary tumor, EHAs supplying the primary tumor were
usually present, whereas patients with a small tumor usually had
EHAs supplying a recurrent tumor following several TACE sessions.
The percentages of patients with EHAs were 2.7±3.0, 5.5±0.5,
43.2±4.0, 61.8±5.2 and 93.4±1.8% with tumors of 2–3, 3–5, 5–7, 7–9
and >9 cm, respectively (Fig. 2).
The difference between any two tumor size groups was significant
(P<0.05).
Tumor location
There were 159±19 EHAs feeding tumors in a
peripheral location in the liver and 48.7±6.8 feeding tumors in a
central location. The number of the former was significantly higher
than that of the latter (P<0.05; Fig.
3). Tumors located in the bare area were found to be associated
with a higher prevalence of EHAs than tumors not in the bare area.
It was also observed that some tiny tumor foci located in the
periphery of the liver were initially supplied by EHAs, and that
EHAs supported local tumor progression as the primary tumor grew to
reach a subcapsular location or exophytically invaded adjacent
organs. The right inferior phrenic artery accounted for half of all
of the observed EHAs and supplied the tumors in any liver location
(Fig. 4).
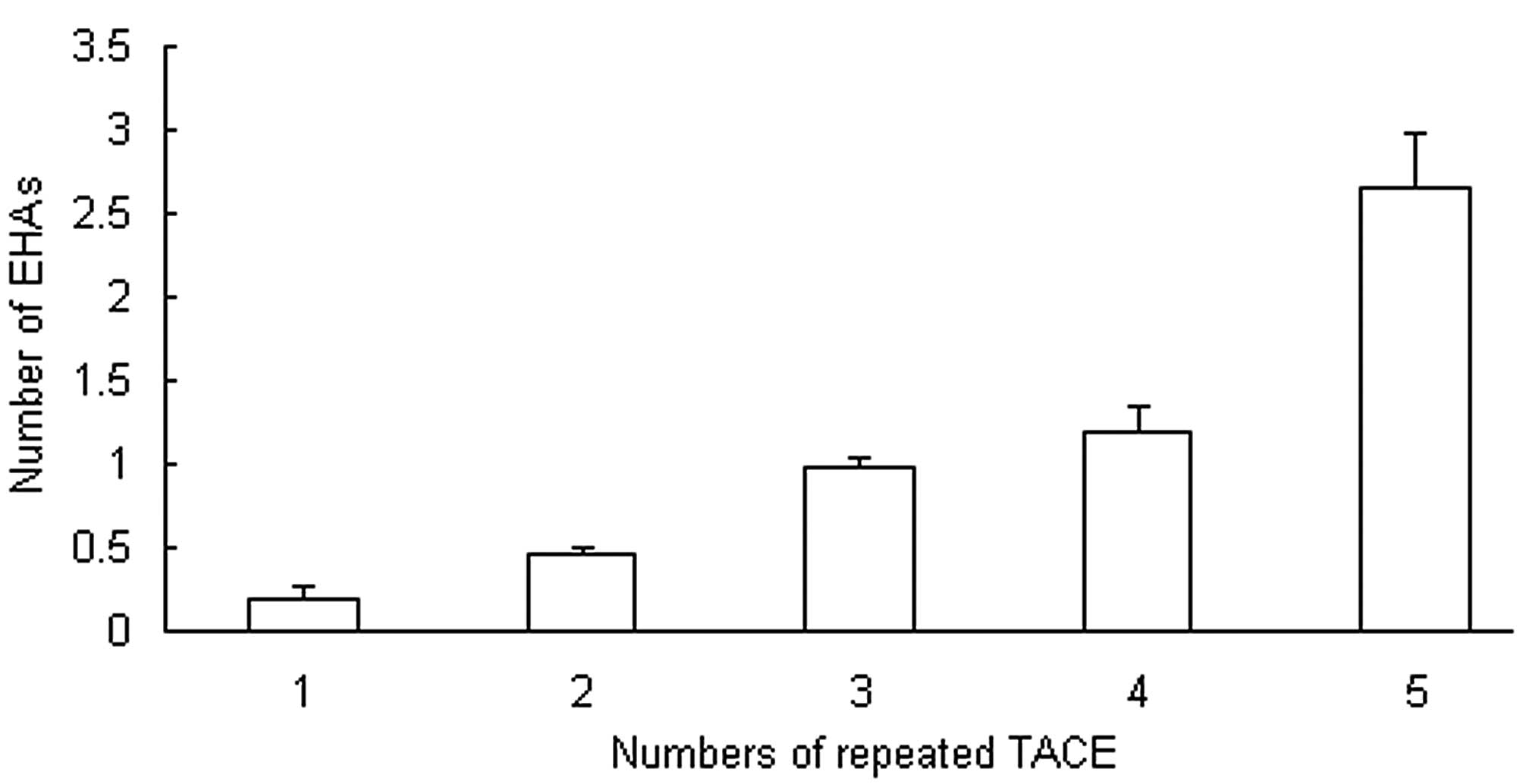
Number of TACE sessions correlates
with the cumulative probability of EHAs
It was observed that the majority of the EHAs
supplied the recurrent small tumors (<5 cm) that appeared after
repeated TACE. Peripheral hepatic artery attenuation or occlusion
subsequent to multiple TACE sessions contributed to the development
of EHAs. In the present study of 942 cases, EHAs were detected from
the first to the fifth TACE session; 18.3±8.5% of the study
subjects had EHAs at the initial session, 47.0±3.5% at the second
session and 98.0±5.6% at the third session, and all had EHAs at the
fourth and fifth sessions. In 46.1% of the 942 patients, EHAs
supplied the primary tumors that were present at TACE. In the other
patients, EHAs supplied recurrent tumors. The cumulative
probability of the presence of EHAs is shown in Fig. 5. As the number of TACE sessions
increased, the cumulative probability of EHAs also increased. From
the initial TACE to the fifth TACE, the numbers of EHAs were
0.18±0.08, 0.47±0.04, 0.98±0.06, 1.19±0.16 and 2.66±0.31,
respectively (Fig. 5).
Adhesion and invasion
In 19.2% of the patients in this study, omental
arteries were found to feed tumors that were adjacent to the
stomach or colon and growing exophytically. The development of
collateral vessels to a tumor could also be triggered by omental
adhesion caused by exophytic growth, extracapsular HCC
infiltration, abdominal postoperative omental or peritoneal
adhesion, or recurrent tumor at the resection margin.
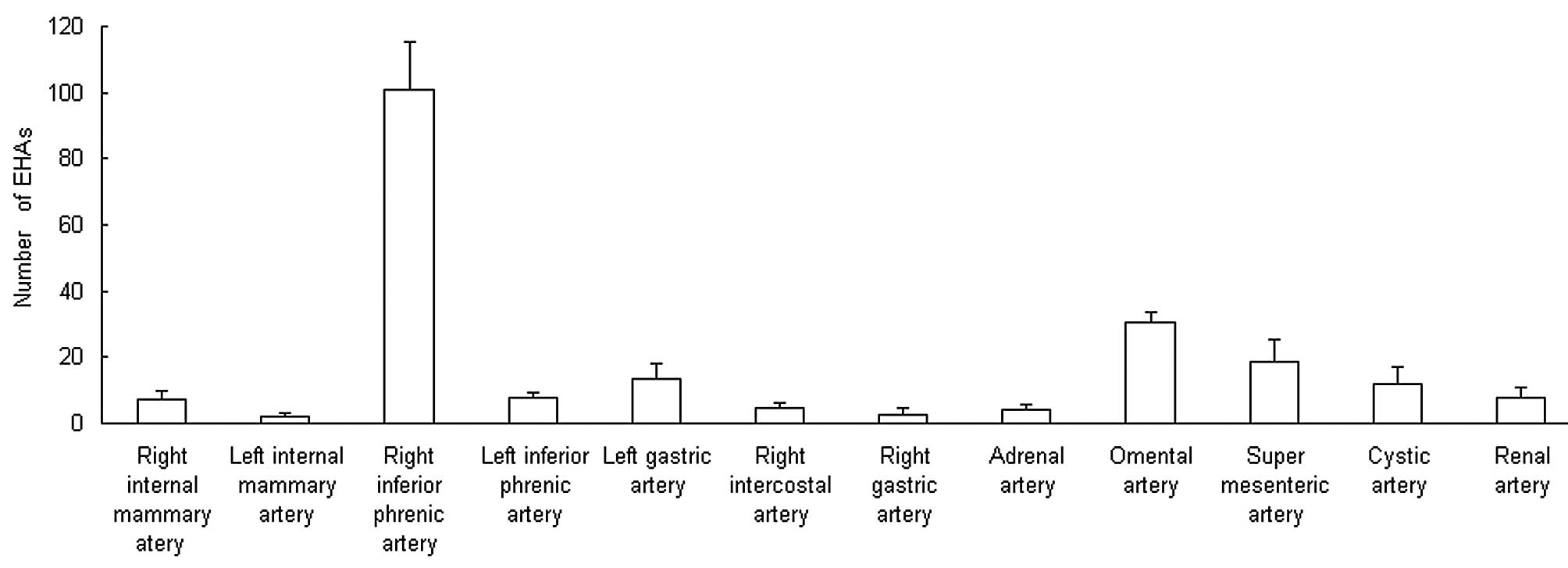
Types of EHA
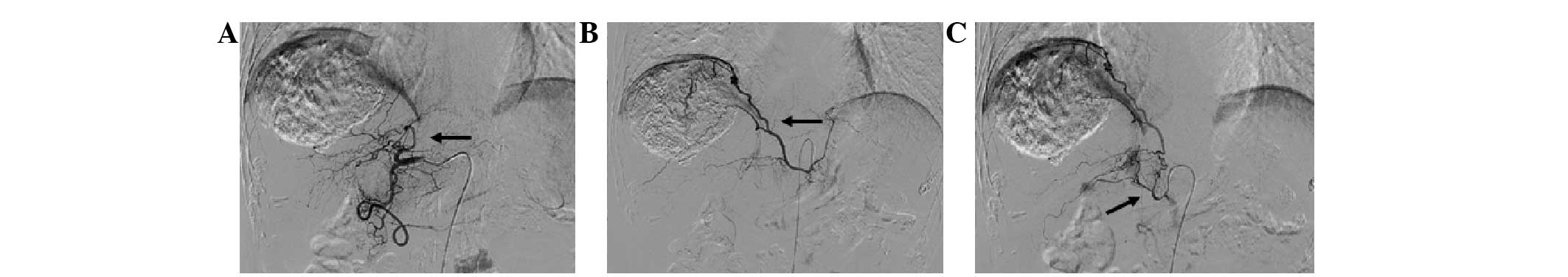
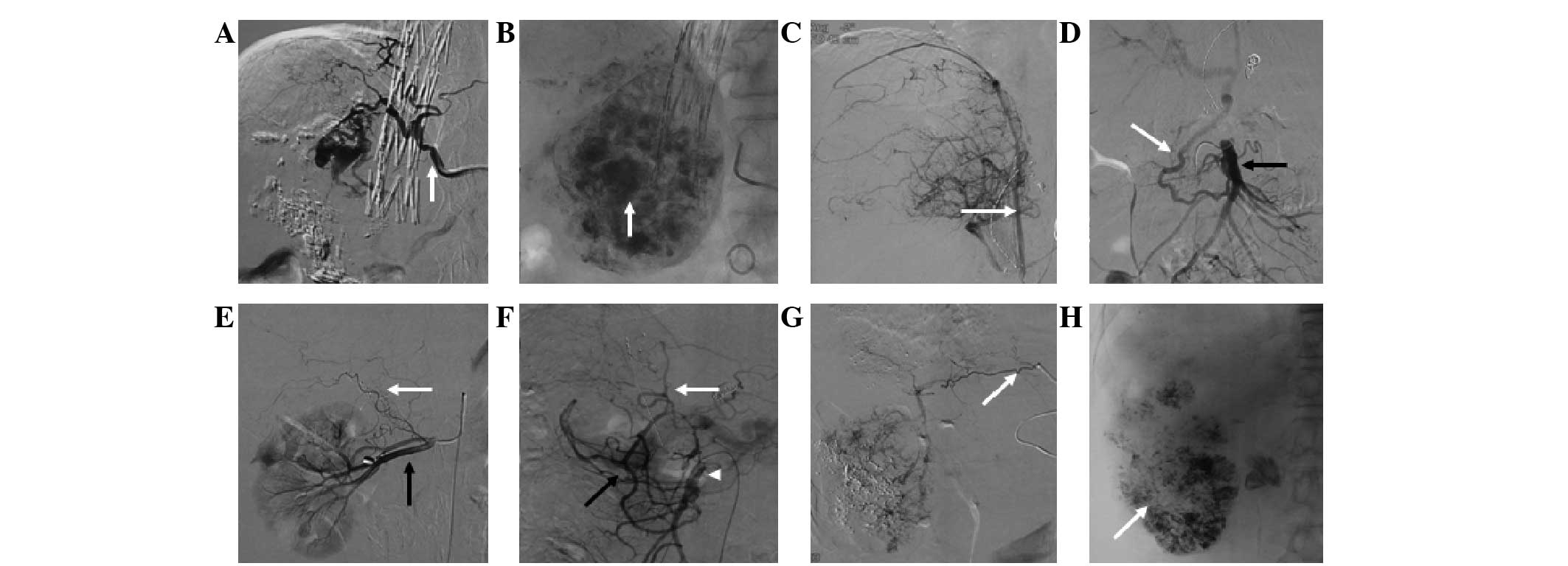
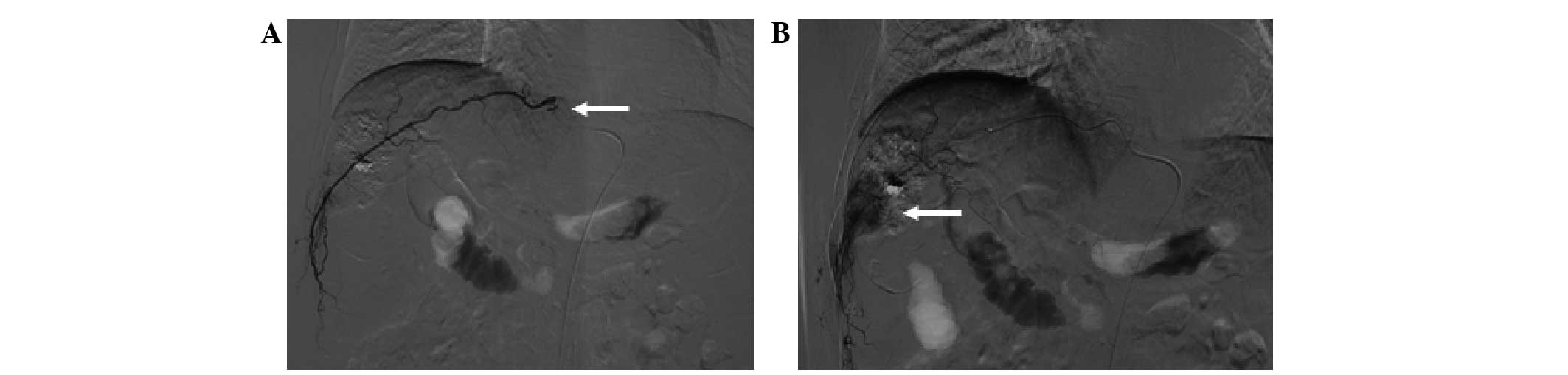
The most common EHA was the inferior phrenic artery,
with 101.0±14.1 (Figs. 6–8), followed by the omental artery
(30.3±3.1; Fig. 9) and adrenal
artery (4.0±1.7; Figs. 7–8). Other EHAs included the right
intercostal artery (4.7±1.5; Fig.
10), renal artery (7.7±3.1; Fig.
11), left inferior phrenic artery (7.7±1.5), right internal
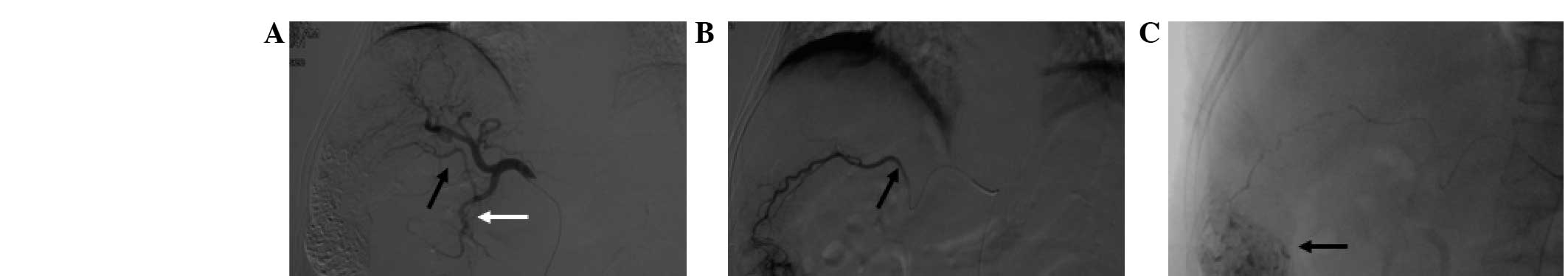
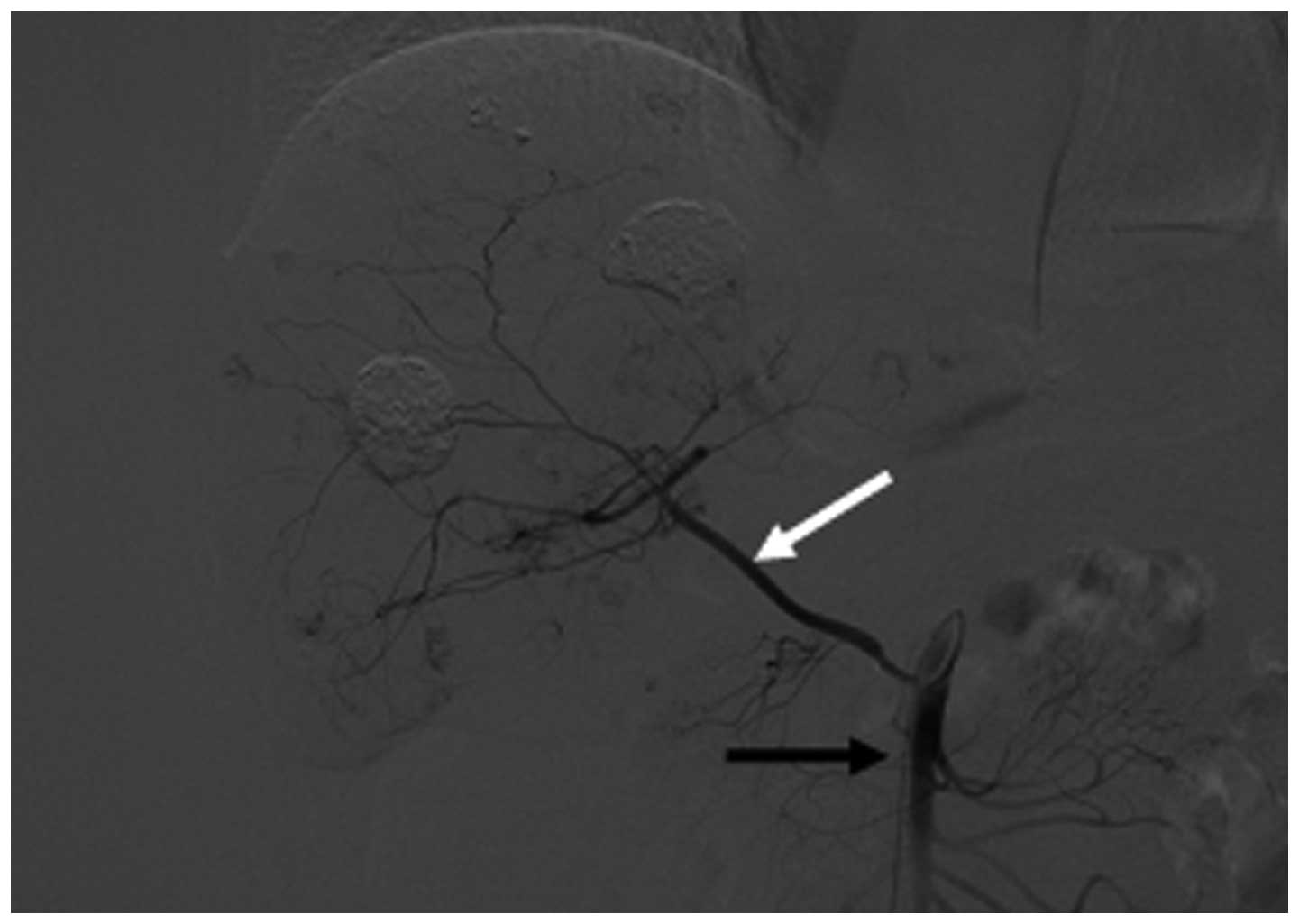
mammary artery (7.3±2.5; Fig. 1),
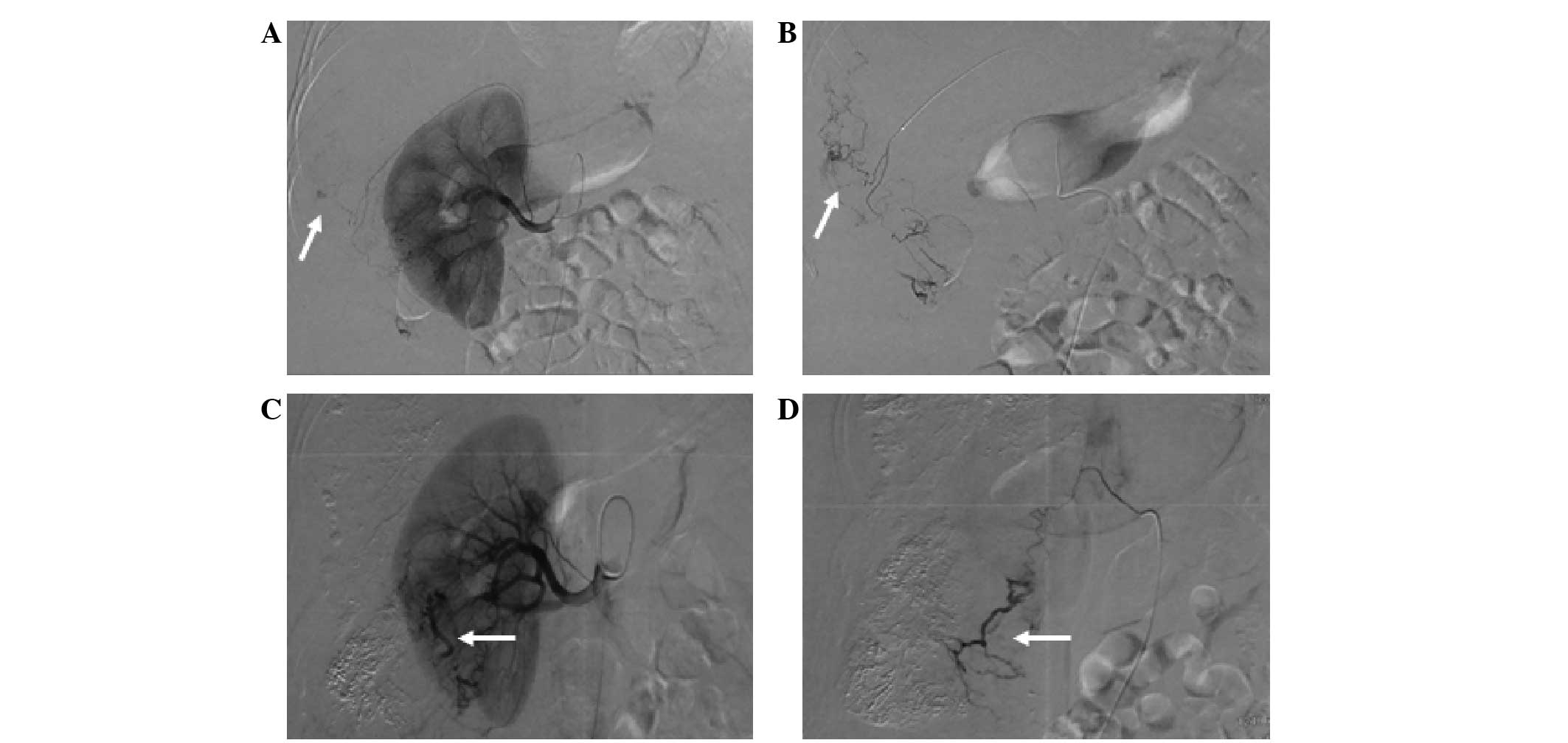
left gastric artery (13.3±4.6; Figs.
7 and 12), cystic artery
(12.0±5.0; Fig. 13), super
mesenteric artery (18.7±6.4; Fig.
14), right gastric artery (2.3±2.3) and left internal mammary
artery (2.0±1.0), as shown in Fig.
4.
Complications of EHA embolization
Complications were observed in 32 patients.
Complications were usually associated with the location of the
embolized lesions and included hiccup, acute gastroduodenal mucosal
ischemic ulceration, cholecystitis, aggressive hepatic failure,
hepatic artery spasm, hepatic artery constriction, hepatic artery
occlusion, dissection, hepatic encephalopathy, left
face-neck-shoulder pain, pleural effusion and lung infection.
Discussion
In this retrospective study, the radiological
findings and imaging of HCCs supplied by EHAs were evaluated. The
results demonstrated that the formation of EHAs in HCC is closely
associated with the anatomical location of the tumor, tumor size,
multiple TACE procedures, postsurgical adhesions and exophytic
tumor growth. EHAs supplying HCCs require embolization; however,
these arteries should be treated carefully and superselectively to
avoid complications such as vascular dissection, spasm and
non-target tissue or organ embolization.
There are various strategies to treat hepatic tumors
by the transcatheter arterial approach (22–24).
Familiarity with the blood supply that feeds hepatic tumors is
essential not only to appreciate the limitations of these
strategies but also to improve their therapeutic efficacy. The
development of EHAs in HCC is a situation that limits the efficacy
of TACE (25–27). In a study of TACE, Kim et al
observed 2,104 extrahepatic collateral routes in 1,622 sessions in
860 patients and performed TACE via 1,556 extrahepatic collateral
vessels in 732 patients (7).
Chemoembolization through EHAs can be attempted to
improve the therapeutic efficacy of TACE. In this situation,
selective catheterization should be achieved by manipulating the
guide wire or catheter tip into the branches of the EHAs that are
feeding the tumor, in order to prevent tumor progression or
recurrence.
The main cause of EHAs was once considered to be
hepatic artery occlusion by surgical ligation (which is no longer
performed), mechanical injury or multiple TACE procedures. Certain
studies have reported interruption or dissection of the hepatic
artery by multiple TACE procedures to be the principal cause of the
formation of extrahepatic collateral vessels (28,29). A
previous study showed that only 4% of patients with HCC suffered
proximal hepatic artery occlusion and the majority of patients with
a collateral supply had a patent hepatic artery (7).
The present study indicates a close association
between the prevalence of an extrahepatic collateral supply to HCC
and tumor size. It was found that the occurrence of an extrahepatic
collateral supply to HCC was closely associated with tumor size.
These data are consistent with the literature (7–13).
The majority of the EHAs were found to supply the
recurrent small (<5 cm) tumors that appeared after repeated
TACE. Peripheral hepatic artery attenuation or occlusion following
multiple TACE sessions contributed to the development of EHAs. In
other studies, researchers have included only patients who
underwent TACE ≤5 times, due to the fact that patients treated with
TACE >5 times had advanced disease and a poor prognosis
(7,13). As the number of TACE sessions
increased, the cumulative probability of EHAs also increased,
particularly for EHAs supplying recurrent tumors (7,11).
It has been hypothesized that EHAs develop early at
the bare area of the liver because the diaphragm and liver are in
direct contact without any capsular barrier, and the blood supply
to the diaphragm can thus reach the liver by adherence (30). A surface tumor location is a
prerequisite for and the most important factor associated with the
formation of EHAs (7,31). EHAs develop to supply the peripheral
zone of the liver parenchyma, with the subsequent recurrence of
tumor at remote sites in the peripheral zone supplied by the EHAs
(32,33). In the present study, it was found
that tumors located in the bare area may be associated with a
higher prevalence of EHAs than tumors not in the bare area.
The development of collateral vessels to a tumor
could be triggered by omental adhesion caused by exophytic growth,
extracapsular HCC infiltration, abdominal postoperative omental or
peritoneal adhesion, or recurrent tumor at the resection margin. In
cases of multiple TACE, hepatic infarction in peripheral zones
could trigger omental or peritoneal adhesion leading to the
development of extrahepatic collateral arteries (34). Direct contact with or invasion into
other organs, including the stomach (35), colon (36), adrenal gland (37) and kidney (38), may create extrahepatic collateral
arteries to the tumor from these organs.
The inferior phrenic artery (29), omental branch (38), adrenal artery, intercostal artery,
internal mammary artery, renal capsular artery (39), left gastric artery (40), gastroduodenal artery, cystic artery
and mesenteric superior artery are among the EHAs known to feed
tumors (41). Considering the broad
contact between the liver and the diaphragm, it may be expected
that diaphragmatic blood supplies, including the inferior phrenic,
internal mammary and intercostal arteries, are major sources of
collateral circulation.
When collateral vessels are chemoembolized, there is
a risk of embolizing non-target tissues or organs. Hiccups were
usually the result of embolization through right inferior phrenic
arteries (42). Acute gastroduodenal
mucosal ischemic ulceration or necrosis typically occurs following
embolization through the branches of the gastroduodenal artery
(43). Cholecystitis is frequently
caused by the embolization of the cystic artery (44). Aggressive hepatic failure may occur
as a result of multiple embolization through the hepatic artery and
EHA branches (45). Hepatic artery
spasm, hepatic artery constriction and hepatic artery occlusion are
typically caused by the repeated stimulation of the hepatic artery
with a 5F catheter or by careless actions (46). Pleural effusion is common when tumors
adjacent to the diaphragm are treated (47). Lung infection usually results from an
overdose of the iodized oil infusion (48).
Strategies to avoid these complications include
superselective catheterization of the specific branch supplying the
tumor, avoiding reflux of embolization material into non-target
vessels, and use of coils and microparticles to occlude normal
vessels before chemoembolization is performed (49). However, the risk in patients with
advanced HCC is worthwhile if the EHAs of tumors are carefully
occluded with a microcatheter.
Selective angiography of EHAs was performed in
patients in whom it was suspected that there was a blood supply to
a tumor. However, unsuspected EHAs may have been missed in some
patients, because some tumor-feeding arteries are difficult to
identify as a result of insufficient tumor vascularity or
overlapping vessels. The development of alternative methods for
identifying EHAs, in addition to routine examinations such as MRI,
enhanced CT and digital subtraction angiography, is required. In
this study, it was not possible to chemoembolize every detected
extrahepatic collateral vessel because of potential complications
or difficulties in superselective catheterization. In cases where
the EHA supply could not be accessed, the tumor lesion was treated
with local ethanol injection or radiofrequency ablation guided with
sonography or CT.
In conclusion, the number of extrahepatic collateral
arteries feeding tumors was positively associated with tumor size,
peripheral location of the tumor in the liver, and the number of
TACE sessions. In addition to the main hepatic artery, the most
common EHAs were the right inferior phrenic artery and the omental
artery. Knowledge of changes in the hepatic artery can help when
performing repeated TACE and reduce the time required for the
procedure, and may help to improve the outcome of patients with
HCC.
References
|
1
|
Sergio A, Cristofori C, Cardin R, et al:
Transcatheter arterial chemoembolization (TACE) in hepatocellular
carcinoma (HCC): The role of angiogenesis and invasiveness. Am J
Gastroenterol. 103:914–921. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lee HS, Kim JS, Choi IJ, et al: The safety
and efficacy of transcatheter arterial chemoembolization in the
treatment of patients with hepatocellular carcinoma and main portal
vein obstruction. A prospective controlled study. Cancer.
79:2087–2094. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Takayasu K, Arii S, Ikai I, et al:
Prospective cohort study of transarterial chemoembolization for
unresectable hepatocellular carcinoma in 8510 patients.
Gastroenterology. 131:461–469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamada R, Sato M, Kawabata M, et al:
Hepatic artery embolization in 120 patients with unresectable
hepatoma. Radiology. 148:397–401. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yoon CJ, Chung JW, Park JH, et al:
Transcatheter arterial chemoembolization with paclitaxel-lipiodol
solution in rabbit VX2 liver tumor. Radiology. 229:126–131. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsimberidou AM, Letourneau K, Fu S, et al:
Phase I clinical trial of hepatic arterial infusion of paclitaxel
in patients with advanced cancer and dominant liver involvement.
Cancer Chemother Pharmacol. 68:247–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HC, Chung JW, Lee W, Jae HJ and Park
JH: Recognizing extrahepatic collateral vessels that supply
hepatocellular carcinoma to avoid complications of transcatheter
arterial chemoembolization. Radiographics. 25(Suppl 1): S25–S39.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyayama S, Matsui O, Taki K, et al:
Extrahepatic blood supply to hepatocellular carcinoma: Angiographic
demonstration and transcatheter arterial chemoembolization.
Cardiovasc Intervent Radiol. 29:39–48. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park SI, Lee DY, Won JY and Lee JT:
Extrahepatic collateral supply of hepatocellular carcinoma by the
intercostal arteries. J Vasc Interv Radiol. 14:461–468. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shibata T, Kojima N, Tabuchi T, Itoh K and
Konishi J: Transcatheter arterial chemoembolization through
collateral arteries for hepatocellular carcinoma after arterial
occlusion. Radiat Med. 16:251–256. 1998.PubMed/NCBI
|
|
11
|
Chung JW, Kim HC, Yoon JH, et al:
Transcatheter arterial chemoembolization of hepatocellular
carcinoma: Prevalence and causative factors of extrahepatic
collateral arteries in 479 patients. Korean J Radiol. 7:257–266.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YL, Li MH, Cheng YS, Shi HB and Fan
HL: Influential factors and formation of extrahepatic collateral
artery in unresectable hepatocellular carcinoma. World J
Gastroenterol. 11:2637–2642. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim HC, Chung JW, Park JH, et al:
Transcatheter arterial chemoembolization for hepatocellular
carcinoma: Prospective assessment of the right inferior phrenic
artery with C-arm CT. J Vasc Interv Radiol. 20:888–895. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim HC, Chung JW, Jae HJ, et al:
Hepatocellular carcinoma: Prediction of blood supply from an
internal mammary artery with multi-detector row CT. J Vasc Interv
Radiol. 19:1419–1426. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HC, Chung JW, Choi SH, et al: Internal
mammary arteries supplying hepatocellular carcinoma: Vascular
anatomy at digital subtraction angiography in 97 patients.
Radiology. 242:925–932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim HC, Chung JW, Jae HJ, et al:
Hepatocellular carcinoma: Transcatheter arterial chemoembolization
of the gonadal artery. J Vasc Interv Radiol. 17:703–709. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng ZW, Guo RP, Zhang YJ, et al: Hepatic
resection versus transcatheter arterial chemoembolization for the
treatment of hepatocellular carcinoma with portal vein tumor
thrombus. Cancer. 118:4725–4736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xi T, Lai EC, Min AR, et al: Adjuvant
transarterial chemoembolization after curative resection of
hepatocellular carcinoma: A non-randomized comparative study.
Hepatogastroenterology. 59:1198–1203. 2012.PubMed/NCBI
|
|
19
|
Luo XJ, Tan WF, Yi B, et al: Surgery of
hepatocellular carcinoma complicated with cancer thrombi in bile
duct: Efficacy for criteria for different therapy modalities.
Langenbecks Arch Surg. 394:1033–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu Q and Qin SK: Features and treatment
options of Chinese hepatocellular carcinoma. Chin Clin Oncol.
2:382013.PubMed/NCBI
|
|
21
|
Llovet JM, Fuster J and Bruix J:
Barcelona-Clínic Liver Cancer Group: The Barcelona approach:
Diagnosis, staging, and treatment of hepatocellular carcinoma.
Liver Transpl. 10(2 Suppl 1): S115–S120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lencioni R, Chen XP, Dagher L and Venook
AP: Treatment of intermediate/advanced hepatocellular carcinoma in
the clinic: How can outcomes be improved? Oncologist. 15(Suppl 4):
42–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Forner A, Reig ME, de Lope CR and Bruix J:
Current strategy for staging and treatment: The BCLC update and
future prospects. Semin Liver Dis. 30:61–74. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Welker MW, Zangos S, Kriener S, et al:
Sequential therapy of transarterial chemoembolisation and sorafenib
in intermediate stage hepatocellular carcinoma. J Gastrointest
Cancer. 41:149–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miyayama S, Yamashiro M, Okuda M, et al:
The march of extrahepatic collaterals: Analysis of blood supply to
hepatocellular carcinoma located in the bare area of the liver
after chemoembolization. Cardiovasc Intervent Radiol. 33:513–522.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Won JY, Lee DY, Lee JT, et al:
Supplemental transcatheter arterial chemoembolization through a
collateral omental artery: Treatment for hepatocellular carcinoma.
Cardiovasc Intervent Radiol. 26:136–140. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kimura S, Okazaki M, Higashihara H, et al:
Clinico-roentogenologic findings in hepatocellular carcinoma fed by
the right inferior phrenic artery at the initial chemoembolization.
Hepatogastroenterology. 56:191–198. 2009.PubMed/NCBI
|
|
28
|
Vaidya S, Dighe M, Bhargava P and Dick AA:
Chronic hepatic artery occlusion with collateral formation: Imaging
findings and outcomes. Transplant Proc. 43:1770–1776. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang L, Zhang XM, Ren YJ, Miao ND, Huang
XH and Dong GL: The features of extrahepatic collateral arteries
related to hepatic artery occlusion and benefits in the
transarterial management of liver tumors. Radiol Res Prac.
2013:5352722013.
|
|
30
|
Miyayama S, Yamashiro M, Yoshie Y, et al:
Inferior phrenic arteries: Angiographic anatomy, variations and
catheterization techniques for transcatheter arterial
chemoembolization. Jpn J Radiol. 28:502–511. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guan YS, Zheng XH, Zhou XP, et al:
Multidetector CT in evaluating blood supply of hepatocellular
carcinoma after transcatheter arterial chemoembolization. World J
Gastroenterol. 10:2127–2129. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kodama Y, Shimizu T, Endo H, et al:
Spontaneous rupture of hepatocellular carcinoma supplied by the
right renal capsular artery treated by transcatheter arterial
embolization. Cardiovasc Intervent Radiol. 25:137–140. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eurvilaichit C: Outcome of transcatheter
oily chemoembolization in patients with hepatocellular carcinoma.
Hepatogastroenterology. 51:20–24. 2004.PubMed/NCBI
|
|
34
|
Paul SB, Gamanagatti SR, Mukund A, Abbas
SZ and Acharya SK: Transarterial chemoembolization for
hepatocellular carcinoma: significance of extrahepatic collateral
supply. Indian J Cancer. 48:339–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu ML, Tai WC, Chuah SK, et al: Gastric
metastasis of hepatocellular carcinoma via a possible existing
retrograde hematogenous pathway. J Gastroenterol Hepatol.
25:408–412. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zech CJ, Bilzer M, Mueller-Lisse UG, et
al: Perforation of the colon: A rare complication of hepatocellular
carcinoma. Acta Radiol. 47:538–542. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Uchino K, Tateishi R, Shiina S, et al:
Hepatocellular carcinoma with extrahepatic metastasis: Clinical
features and prognostic factors. Cancer. 117:4475–4483. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi JW, Kim HC, Chung JW, et al:
Chemoembolization via branches from the splenic artery in patients
with hepatocellular carcinoma. Cardiovasc Intervent Radiol.
35:90–96. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baba Y, Miyazono N, Inoue H, et al: Small
hepatocellular carcinoma supplied by the right renal capsular
artery. A case report. Acta Radiol. 40:449–450. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Jeon UB, Lee JW, Baik SK, et al:
Hepatocellular carcinoma supplied from the short gastric artery:
Treatment with chemoembolization. Cardiovasc Intervent Radiol.
35:1512–1514. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miyayama S, Yamashiro M, Okuda M, et al:
Hepatocellular carcinoma supplied by the right lumbar artery.
Cardiovasc Intervent Radiol. 33:53–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shin SW, Do YS, Choo SW, et al:
Diaphragmatic weakness after transcatheter arterial
chemoembolization of inferior phrenic artery for treatment of
hepatocellular carcinoma. Radiology. 241:581–588. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Nakamura H, Hashimoto T, Oi H, Sawada S
and Furui S: Prevention of gastric complications in hepatic
arterial chemoembolization. Balloon catheter occlusion technique.
Acta Radiol. 32:81–82. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shah RP and Brown KT: Hepatic arterial
embolization complicated by acute cholecystitis. Semin Intervent
Radiol. 28:252–257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jeon SH, Park KS, Kim YH, et al: Incidence
and risk factors of acute hepatic failure after transcatheter
arterial chemoembolization for hepatocellular carcinoma. Korean J
Gastroenterol. 50:176–182. 2007.(In Korean). PubMed/NCBI
|
|
46
|
Sueyoshi E, Hayashida T, Sakamoto I and
Uetani M: Vascular complications of hepatic artery after
transcatheter arterial chemoembolization in patients with
hepatocellular carcinoma. AJR Am J Roentgenol. 195:245–251. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Negrini S, Zoppoli G, Andorno E, Picciotto
A and Indiveri F: Iodized oil pleural effusion in a patient
previously treated with transarterial chemoembolization for
hepatocellular carcinoma. Chest. 138:193–195. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Naorungroj T, Naksanguan T and
Chinthammitr Y: Pulmonary lipiodol embolism after transcatheter
arterial chemoembolization for hepatocellular carcinoma: A case
report and literature review. J Med Assoc Thai. 96(Suppl 2):
270–275. 2013.
|
|
49
|
Poggi G, Pozzi E, Riccardi A, et al:
Complications of image-guided transcatheter hepatic
chemoembolization of primary and secondary tumours of the liver.
Anticancer Res. 30:5159–5164. 2010.PubMed/NCBI
|