Introduction
Very small intracranial aneurysms have been
associated with intracranial hemorrhage following rupture. Although
ruptured very small aneurysms can be treated by direct surgical
clipping or endovascular embolization, these treatments are often
highly complex and are associated with a high risk of complication
(1). Currently, there is no
consensus with regard to the definition of very small intracranial
aneurysms; however, in general, aneurysms with a diameter of ≤3 mm
are generally defined as very small (2). Recent advances in neuroradiological
techniques, particularly the widespread use of three-dimensional
(3D) cerebral angiography, have increasingly improved the diagnosis
rate for very small aneurysms. However, the treatment of small
aneurysms remains a considerable challenge for neurosurgeons
(1).
Endovascular coiling has emerged as a potential
treatment option for intracranial aneurysms. A number of studies
have demonstrated that Solitaire AB stent-assisted coiling
embolization is a safe and effective treatment for wide-necked or
complex intracranial aneurysms (3–6). In
addition, Zhao et al reported that Solitaire AB stents are
effective for the treatment of very small (≤3 mm) and small (3–10
mm) ruptured and unruptured intracranial aneurysms (7). However, the use of Solitaire AB stents
for the treatment of ruptured very small intracranial aneurysms has
not been reported in the literature. The present retrospective
study assessed nine patients with ruptured very small intracranial
aneurysms that underwent Solitaire AB stent-assisted coiling
embolization between July 2010 and December 2012. The aim of the
current study was to investigate and evaluate the efficacy and
safety of Solitaire AB stent-assisted coiling embolization for the
treatment of ruptured very small intracranial aneurysms.
Materials and methods
Patients
The retrospective study was approved by the Ethics
Committees of Qilu Hospital (Jinan, China) and Qingdao Municipal
Hospital (Qingdao, China). Written informed consent was obtained
from each subject or their representative. The subject population
consisted of nine patients (male, 7; female, 2) with ruptured very
small intracranial aneurysms that underwent Solitaire AB
stent-assisted coiling embolization at the Department of
Neurosurgery at Qingdao Municipal Hospital between July 2010 and
December 2012. The average patient age was 55±4.9 years (range,
46–66 years). All patients had a history of spontaneous
subarachnoid hemorrhage (SAH). The study inclusion criteria were as
follows: i) History of SAH confirmed by a head computed tomography
examination, which showed the hemorrhage from the aneurysms; ii)
very small aneurysms with a size of ≤3 mm in diameter diagnosed by
digital subtraction angiography (DSA); iii) no other aneurysms in
the brain; and iv) aneurysms meeting the indication for Solitaire
AB stent-assisted coiling embolization (4–6).
Exclusion criteria were as follows: i) No history of SAH; ii)
intracranial hemorrhage not associated with the aneurysms; iii)
intracranial hematoma with a volume of ≥30 ml; and iv) parent
arteries that were tortuous or exhibited severe stenosis, which
indicated that Solitaire AB stent-assisted coiling embolization was
not suitable. A total of 10 aneurysms were identified among the
nine patients, including eight patients with a single aneurysm and
one patient with two aneurysms. The aneurysms were located in the
ophthalmic branch of the internal carotid artery (n=2, including
the case with two aneurysms), the posterior communicating branch of
the internal carotid artery (n=4), the top of the basilar artery
(n=1) and the middle cerebral artery (n=2).
Procedure
All patients underwent systemic heparinization under
general anesthesia (500 mg/ml propofol and 200 µg/50 ml
dexmedetomidine). A 6F arterial sheath (Terumo Holding Co. Ltd.,
Tokyo, Japan) was inserted following a puncture to the femoral
artery using the Seldinger technique. Routine DSA with 3D
reconstruction was performed for each patient to evaluate the
aneurysm size and the proximal and distal diameters of the parent
arteries, in order to clearly determine the anatomical association
between the aneurysm and the parent artery and its branches
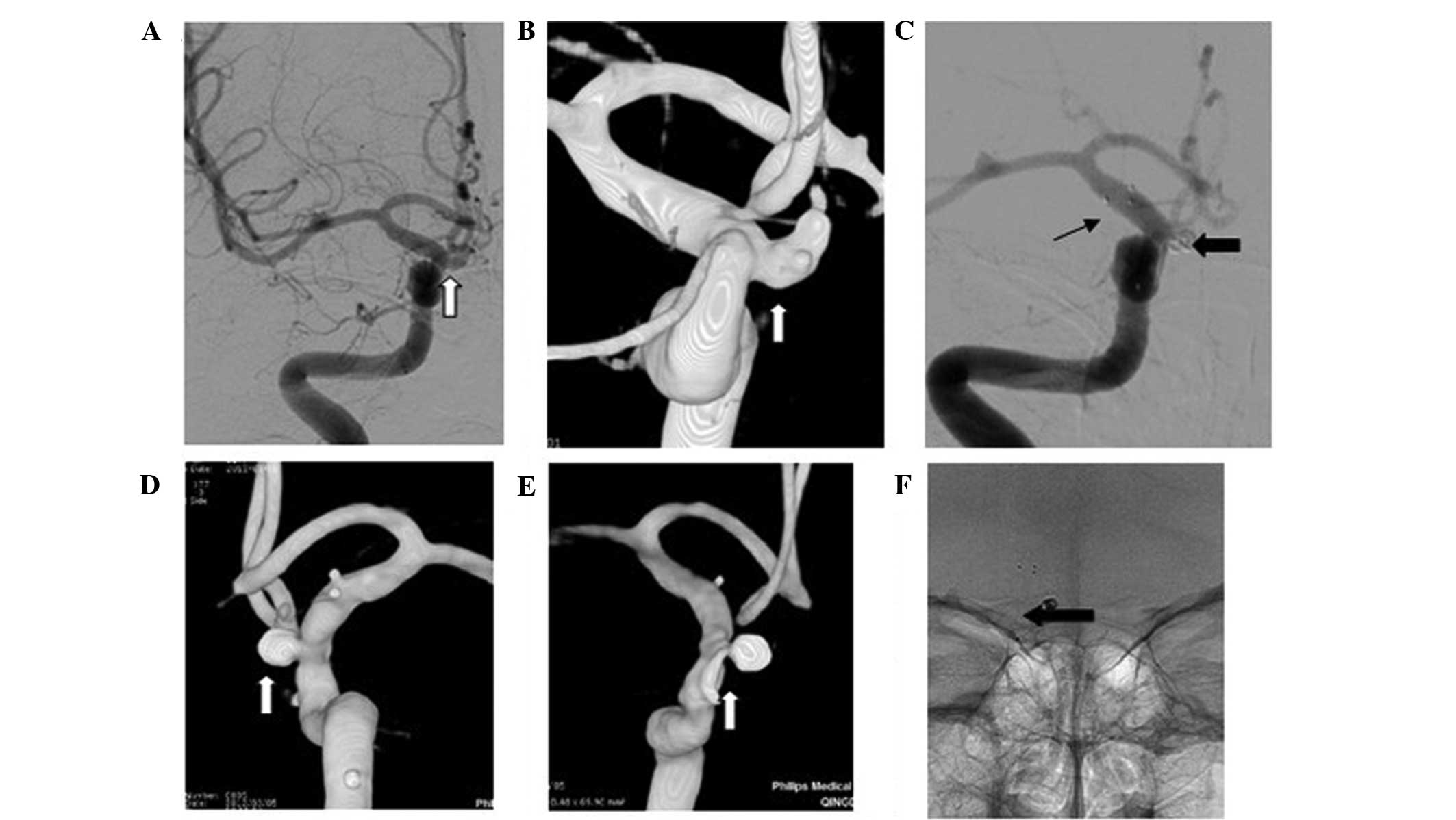
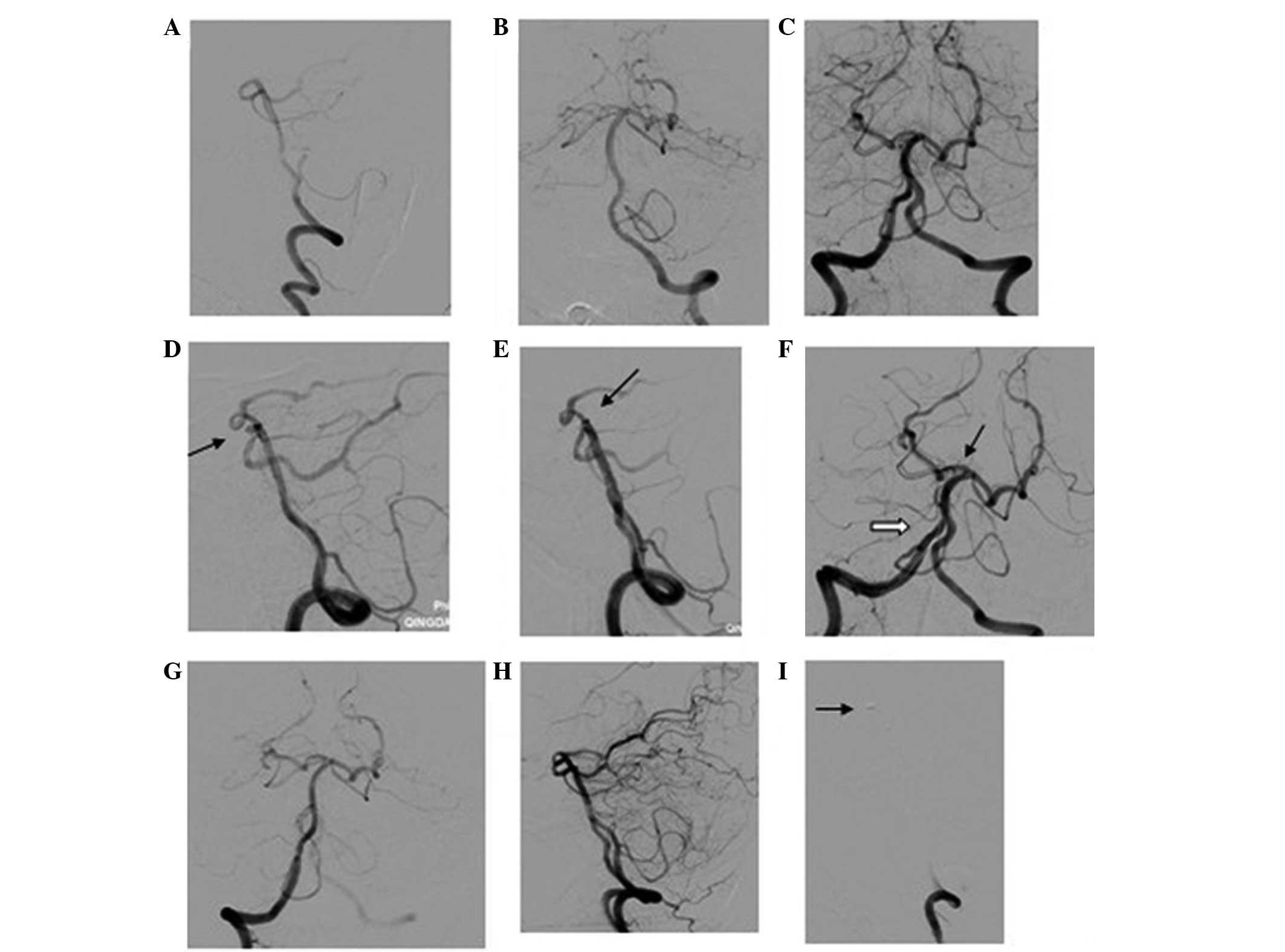
(Figs. 1–3). Based on the DSA results, the
appropriate surgical approach, operating angle, coil and stent were
selected for each patient. A 6F ENVOY guiding catheter (Cordis
Neurovascular, Inc., Miami Lakes, FL, USA) was inserted into the
internal carotid artery at the level of the second cervical
vertebra. A Rebar microcatheter (eV3 Endovascular, Inc., Plymouth,
MN, USA) was conducted through a Traxcess guidewire (MicroVention,
Inc., Tustin, CA, USA) and positioned in the parent artery past the
aneurysmal neck. The Solitaire AB stent was advanced through the
Rebar microcatheter and placed at the cephalic end of the
microcatheter; however, the stent was not released. An Echelon 10
microcatheter (eV3 Endovascular, Inc.) with a very small ‘L’-shaped
tip was inserted into the aneurysmal sac or neck. Aneurysmal
coiling was performed using an Axium 3D coil (eV3 Endovascular,
Inc.) of appropriate diameter and length. After one or two coil
loops were released to fill the aneurysmal cavity, the stent was
slowly released, and the release was stopped after the
microcatheter was fixed and the coil did not pulsate. The
aneurysmal cavity was continuously filled with the coil until 1–2
cm of the coil remained inside the parent artery. The microcatheter
was removed after the guidewire achieved stability. After the
position of the coil was stabilized, the stent was completely
released and the coil was isolated.
All patients received 300 mg aspirin and 300 mg
Plavix (Sanofi Winthrop, Paris, France) orally or intragastrically
prior to the intervention. Following the intervention, patients
were treated with 100 mg aspirin and 75 mg Plavix per day. Plavix
administration was terminated after 1 month, while aspirin
treatment was stopped after 6 months. A lumbar puncture was
performed daily, starting on the first day after the intervention,
until no blood was observed in the cerebrospinal fluid. For
patients with acute hydrocephalus, routine ventricular drainage was
performed.
Postoperative evaluation and
follow-up
The degree of occlusion was evaluated immediately
post-procedure (Figs. 1–3). Occlusion degree was graded according to
the modified Raymond scale (8) as
follows: Grade I, complete occlusion (no contrast agents observed
in the aneurysm); grade II, neck remnant (contrast agents observed
in the aneurysmal neck); and grade III, partial occlusion (contrast
agents observed in the aneurysmal cavity). All patients underwent
follow-up via telephone and hospital visits at 1, 3, 6 and 12
months following the intervention. Patients underwent a follow-up
DSA at ~6 months after the intervention (Figs. 1–3).
Clinical outcome was evaluated according to the modified Rankin
scale (mRS) (9): Grade 0, no
symptoms; grade 1, limited symptoms, but with no significant
disability (able to perform daily duties and activities); grade 2,
minor disability (unable to perform previous activities, but able
to perform own bodily activities without assistance); grade 3,
moderate disability (requiring limited assistance, but able to walk
independently); grade 4, moderate to severe disability (unable to
walk without assistance and unable to attend to own bodily
activities without assistance); and grade 5, severe disability
(bedridden, incontinent and requiring nursing care and
attention).
Results
Surgical outcomes
The Hunt-Hess classification (10) of SAH was used to describe the
severity of preoperative bleeding. Among the nine patients, 22.2%
(n=2) were Hunt-Hess grade I, 22.2% (n=2) were grade II, 44.4%
(n=4) were grade III and 11.1% (n=1) was grade IV. All patients
underwent 3D-DSA immediately following the surgery, and the degree
of occlusion was evaluated according to the Raymond scale. Among
the nine patients, six patients exhibited complete occlusion at
Raymond grade I and three patients exhibited occlusion at Raymond
grade II.
No intraoperative hemorrhage due to aneurysm rupture
was observed in any patient, and no acute thrombosis formed in the
parent arteries. In addition, no problems were encountered in the
deployment of stents and coils in any of the nine patients, and no
operation-associated mortality or morbidity occurred.
Complications
Vasospasms occurred in one patient with an aneurysm
in the middle cerebral artery (preoperative Hunt-Hess grade I).
During surgery, 5 ml Nimotop (Bayer Schering Pharma, Berlin,
Germany) in 15 ml saline was administered via the guiding catheter
at a rate of 5 ml/30 min. DSA was conducted after each 5-ml
infusion of Nimotop. Nimotop administration was ended if the
vasospasm was relieved. No evident cerebral ischemia occurred
postoperatively. All patients underwent a neurological examination,
and no patients exhibited evident neurological dysfunction. A total
of two patients with hydrocephalus underwent was drainage for a
limited period (<7 days), one patient with Hunt-Hess grade III
and one patient was Hunt-Hess grade IV. One of the two patients
(Hunt-Hess grade IV) subsequently underwent ventriculoperitoneal
shunting.
Follow-up
All nine patients underwent follow-up for a period
of 8–13 months (average, 10.6 months). A total of two patients (one
patient with Hunt-Hess grade III and one patient with Hunt-Hess
grade IV) exhibited mRS grade 2, while the other seven patients
were classified as mRS grade 1. Of the nine patients, seven
patients underwent follow-up DSA at 5–10 months post-intervention.
All seven of these patients exhibited complete occlusion at Raymond
grade I. No recurrence or enlargement of the aneurysms occurred and
no stenosis or occlusion were observed in the parent arteries.
Discussion
Very small aneurysms reportedly account for ~7% of
all ruptured aneurysms (11).
However, very small aneurysms are rarely identified during clinical
practice due to the intracranial bleeding from the rupture of these
aneurysms, possibly due to the low resolution of DSA or infrequent
use of 3D-DSA. Advances in neuroradiological techniques have
significantly improved the detection rate of very small aneurysms.
In particular, 3D neuroradiological imaging of aneurysms is able to
improve the safety of surgery for the treatment of very small
aneurysms. However, the surgical treatment of very small aneurysms
with direct clipping and endovascular embolization presents
considerable difficulty. For example, during the clipping of very
small aneurysms (1), the aneurysmal
neck may easily break, since very small aneurysms have a thin wall
and parent arteries typically exhibit a thick wall with
calcification. With regard to endovascular embolization of very
small aneurysms, maneuvering coils inside the sac of the very small
aneurysm is technically difficult, and the walls of the aneurysms
can be easily punctured (12). For
wide-necked very small aneurysms, despite assistance with stents,
coils inside the aneurysms migrate easily, potentially leading to
cerebral infarction (1). Brinjikji
et al (12) performed a
meta-analysis of published studies on endovascular treatment for
very small intracranial aneurysms and reported that ~61% of the
aneurysms ruptured and ~39% did not rupture. The procedure rupture
rate for very small aneurysms was 8.3%, and the associated
mortality rate was 2.4%. By contrast, the morbidity rate due to
thromboembolic complication was 1.9%, and subarachnoid hemorrhage
within 1 month post-procedure occurred in 1.6% of cases. Thus, the
meta-analysis indicated that endovascular embolization is feasible
and effective for treating >90% of very small aneurysms;
however, the technique is associated with a high risk of
procedure-associated aneurysm rupture (12). Previous studies have indicated that
the rupture rate following endovascular embolization of very small
aneurysms is ~4% (10,13), which is considerably lower compared
with the 8.3% reported by Brinjikji et al (12). In addition, numerous studies have
suggested that endovascular embolization is safe and effective for
the treatment of very small aneurysms (13–15). In
accordance with these studies, no procedure-associated rupture or
thromboembolic complications were observed in the nine patients
included in the present study, and the follow-up DSA examinations
presented complete occlusion of the aneurysms. Similarly, Zhao
et al reported that Solitaire AB stent-assisted coiling
embolization effectively treated saccular and dissected
intracranial aneurysms, including ruptured and unruptured very
small aneurysms (7). Furthermore,
Zhou et al hypothesized that coils should remain inside the
aneurysms, not in the parent arteries, to prevent hemodynamic
alterations in the parent arteries. In addition, the authors
proposed that stents should be used to assist coiling to fix coils
within the aneurysms, improve the coil packing density in the neck
and increase the embolization rate (16).
To date, a number of types of stent have been used
in clinical practice, including Neuroform, light-emitting diode,
Enterprise and Solitaire stents (3).
In addition, numerous stent deployment techniques have been
developed for the treatment of aneurysms, including jailing,
semi-releasing, stenting after coiling, coil-through, coil-stent,
Y-stenting, horizontal-stenting, and single- or multiple-stenting
techniques. Stenting after coiling techniques and semi-jailing
techniques are the most widely employed in clinical practice
(3,5,17,18). The
Solitaire AB stent is a self-expanding electric detachable stent
with one open end and a closed-mesh design. This design affords
specific advantages for the Solitaire AB stent. Firstly, the
open-end characteristics facilitate the passing of the stent
through tortuous vessels. Secondly, the closed-mesh design
increases the radial strength of the stent and its resistance to
bending, and makes the stents also favorable for vessels with 2–3
folds overlapping the stent, leading to lower stent porosity
(7,19). Thirdly, the Solitaire AB stent is
fully retrievable prior to complete deployment. This is the most
prominent feature of the Solitaire stent and permits accurate
deployment or redeployment of the stent during the procedure
(7,19).
In the present study, coil migration into the parent
artery following the complete release of the stent was observed in
a single case. As the pore in the stent was larger than the
diameter of the coil, the coil and stent were retrieved, and
subsequently redeployed and released, resulting in stabilization of
the coil. Stabilization of the coil following redeployment may be
due to the increased stent porosity following retrieval or the
positioning of the stent in the aneurysmal neck to block coil
movement. Since Solitaire AB stents are fully retrievable, for all
patients in the present study, a semi-releasing method was adopted,
which allowed for the adjustment of the position of the
microcatheter as required during surgery. Thus, based on the
coil-filling condition, the time at which the microcatheter was
able to be retrieved and the length of the coil outside the
aneurysm were determinable. In this manner, the length of the coil
may be adjusted to avoid aneurysm rupture due to excessive tension
inside the aneurysm and to prevent puncture of the aneurysmal wall
by the microcatheter following coil detachment. In addition, the
use of Solitaire AB stents permits compression of the coil within
the aneurysms, particularly in the aneurysmal neck, which leads to
an increased coil packing density in the aneurysm. In the present
study, no aneurysm-associated hemorrhage was observed in any of the
nine patients, and of the three patients with Raymond grade II
occlusion after the surgery, two patients reached Raymond grade I
during the follow-up period.
Filling two or more coils in very small aneurysms is
difficult and potentially harmful; thus, based on the understanding
of the size and anatomy of the aneurysms from 3D-DSA results, one
properly selected coil is hypothesized to be appropriate for very
small aneurysms. A 3D spherical coil with a diameter less than or
equal to the aneurysmal diameter should be the first choice coil
for very small aneurysms. This type of coil can be maintained in an
aneurysm with less possibility of migration out of the aneurysm,
while reducing the possibility of an aneurysm rupture due to the
increased compression on the aneurysmal wall from the coil after
release of the stent. If a coil in the program is considered to be
inappropriate, the coil should be discarded and replaced
immediately with an appropriate coil. In addition, a small section
of the coil may be left in the parent artery, which is compressed
onto the parent artery wall by the stent, to prevent coil prolapse
into the parent artery and subsequent cerebral ischemia. This
method may facilitate the embolization of very small aneurysms.
In conclusion, endovascular treatment of very small
aneurysms presents considerable difficulties and high risk for
neurosurgeons. Preoperative determination of the 3D structure of an
aneurysm and intraoperative selection of an appropriate coil are
important for the success of the surgery. The Solitaire AB stent
may be fully retrieved a number of times, and the stent porosity is
adjustable, which promotes the success rate of the stent-assisted
coiling embolization technique for the treatment of very small
aneurysms. In the present study, Solitaire AB stent-assisted
coiling embolization was effective and safe for the treatment of
ruptured very small intracranial aneurysms in nine patients.
However, the sample size of the present study was limited. Future
studies with a larger sample size are required to investigate the
reopening of aneurysms and the stenosis of parent arteries, in
addition to the long-term safety and efficacy of Solitaire AB
stent-assisted coiling embolization for the treatment of very small
aneurysms.
References
|
1
|
Zhang HQ: Is it necessary to treat the
mini unruptured intracranial aneurysms? Chin J Cerebrovasc Dis.
10:1–3. 2013.
|
|
2
|
van Rooij WJ, Sprengers ME, de Gast AN,
Peluso JP and Sluzewski M: 3D rotational angiography: The new gold
standard in the detection of additional intracranial aneurysms.
AJNR Am J Neuroradiol. 29:976–979. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gross BA and Frerichs KU: Stent usage in
the treatment of intracranial aneurysms: Past, present and future.
J Neurol Neurosurg Psychiatry. 84:244–253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Almekhlafi MA, Hockley A, Wong JH and
Goyal M: Temporary Solitaire stent neck remodeling in the coiling
of ruptured aneurysms. J Neurointerv Surg. 5(Suppl 3): iii76–iii78.
2013.PubMed/NCBI
|
|
5
|
Gory B, Klisch J, Bonafé A, et al:
Solitaire AB stent-assisted coiling of wide-necked intracranial
aneurysms: Short-term results from a prospective, consecutive,
European multicentric study. Neuroradiology. 55:1373–1378. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clajus C, Sychra V, Strasilla C and Klisch
J: Stent-assisted coil embolization of intracranial aneurysms using
the Solitaire™ AB Neurovascular Remodeling Device: Initial and
midterm follow-up results. Neuroradiology. 55:629–638. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao KJ, Zhang YW, Xu Y, et al:
Reconstruction of saccular and dissected intracranial aneurysms
using Solitaire™ AB stents. PLoS One. 8:e572532013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roy D, Milot G and Raymond J: Endovascular
treatment of unruptured aneurysms. Stroke. 32:1998–2004. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bonita R and Beaglehole R: Recovery of
motor function after stroke. Stroke. 19:1497–1500. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hong B, Yang PF, Zhao R, et al:
Endovascular treatment of ruptured tiny intracranial aneurysms. J
Clin Neurosci. 18:655–660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gupta V, Chugh M, Jha AN, Walia BS and
Vaishya S: Coil embolization of very small (2 mm or smaller) berry
aneurysms: Feasibility and technical issues. AJNR Am J Neuroradiol.
30:308–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brinjikji W, Lanzino G, Cloft HJ,
Rabinstein A and Kallmes DF: Endovascular treatment of very small
(3 mm or smaller) intracranial aneurysms: Report of a consecutive
series and a meta-analysis. Stroke. 41:116–121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu J, Liu JC, Wang LJ, Qi P and Wang DM:
Tiny intracranial aneurysms: Endovascular treatment by coil
embolisation or sole stent deployment. Eur J Radiol. 81:1276–1281.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fang C, Li MH, Zhu YQ, et al: The
effectiveness and feasibility of endovascular coil embolization for
very small cerebral aneurysms: mid- and long-term follow-up. Ann
Vasc Surg. 24:400–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Im SH, Han MH, Kwon OK, et al:
Endovascular coil embolization of 435 small asymptomatic unruptured
intracranial aneurysms: Procedural morbidity and patient outcome.
AJNR Am J Neuroradiol. 30:79–84. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou C, Hong QH, Zhao R, Xu Y, Hong B and
Zhao WY: Stent-assisted coil embolization of ruptured anterior
communicating artery tiny aneurysms. Chin J Cerebrovasc Dis.
10:9–12. 2013.
|
|
17
|
Martínez-Galdámez M, Saura P, Saura J,
Martínez A, De Campos JM and Pérez A: Y-stent-assisted coil
embolization of anterior circulation aneurysms using two Solitaire
AB devices: A single center experience. Interv Neuroradiol.
18:158–163. 2012.PubMed/NCBI
|
|
18
|
Sychra V, Klisch J, Werner M, et al:
Waffle-cone technique with Solitaire™ AB remodeling device:
Endovascular treatment of highly selected complex cerebral
aneurysms. Neuroradiology. 53:961–972. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Klisch J, Clajus C, Sychra V, et al: Coil
embolization of anterior circulation aneurysms supported by the
Solitaire AB Neurovascular Remodeling Device. Neuroradiology.
52:349–359. 2010. View Article : Google Scholar : PubMed/NCBI
|