Introduction
Thrombocytopenia is a common complication of chronic
liver disease and is considered to be an indicator of an advanced
disease stage (1–3). Thrombocytopenia in patients with
chronic hepatitis, indicated by a platelet count of
<60,000/mm3, has been associated primarily with two
factors. One of these factors is hypersplenism resulting from
splenomegaly in portal hypertension (4). Hypersplenism appears to be the most
common cause of thrombocytopenia associated with liver cirrhosis
and portal hypertension (5). The
second mechanism is associated with the reduced production of
thrombopoietin, a hormone produced by hepatocytes, which regulate
the development of the megakaryocyte (5). In cirrhosis, due to the reduction in
the mass of functioning hepatocytes, there may be a reduction of
thrombopoiesis in the bone marrow, causing thrombocytopenia in the
peripheral blood (6,7).
In numerous situations, patients with
thrombocytopenia that are infected with hepatitis C virus (HCV)
cannot be treated with peginterferon and ribavirin (P-R) due to a
low platelet count, which may jeopardize the treatment. P-R is a
routine treatment in HCV patients with thrombocytopenia and the
only antiviral treatment available to these patients in China, as
protease inhibitors are not approved (8). This is because oral anti-HCV drugs,
such as protease inhibitor, are not typically covered by insurance
schemes in China and are often prohibitively expensive for Chinese
individuals. In the present study, splenectomy and thrombopoietin
were used as two potential treatments to increase platelet count
and assist patients in completing a full course of antiviral
treatment. Furthermore, the negative effects and cost of the two
methods were compared, with the aim of identifying the optimal
therapy for patients in clinical treatment.
Materials and methods
Patients
Between June 2009 and May 2013, a total of 134
inpatients with HCV-associated compensated liver cirrhosis that
were treated at the 302 Hospital of PLA (Beijing, China) were
enrolled in the present study. The enrolled patients had
indications of conditions requiring antiviral treatment, but had
received no previous antiviral therapy. Among the initial 134
patients, 69 patients had a platelet count of
<60,000/mm3, of which 38 patients (Child-Pugh score
A, 32; Child-Pugh score B, 6) were allocated to the research group
and the other 31 patients to the observed group (9). All the patients supplied written
informed consent. Eligible patients that had chronic HCV infection
(defined as the presence of anti-HCV antibodies and detectable
serum HCV RNA levels, as determined using a clinically-available
assay selected by the investigator), compensated liver disease and
thrombocytopenia (defined as a platelet count of
<60,000/mm3) were treated with antiviral treatment
for 48 weeks. Cirrhosis of patients was met according to the
hepatitis C cirrhosis diagnosis standard (10). Patients were excluded if they
exhibited operative contraindication, absolute contraindications of
interferon therapy or thrombocytopenia as a result of hematological
system disease.
Written informed consent was obtained from all
patients and the patient data was anonymized and de-identified
prior to analysis. The study was approved by the Ethics Committee
of the 302 Hospital of PLA (Beijing, China) and was conducted in
accordance with the ethical standards formulated in the Declaration
of Helsinki.
Study design
The present study was conducted as a prospective
randomized controlled trial and was divided into two stages:
Initial treatment phase and antiviral treatment phase, as described
in a previous study (11). A total
of 38 patients that met the eligibility criteria were randomly
assigned to the splenectomy (n=21) and thrombopoietin (3SBio, Inc.,
Shenyang, China) (n=17) groups. A further 31 patients were
allocated to the observed group and treated with glycyrrhizin
injection (100 ml per day; North China Pharmaceutical Co., Ltd.,
Shanghai, China), wuzhi capsule (3 capsules, twice per day; HYGIEN,
Sichuan, China) and thymopentin injection (10 mg per day; Beijing
SL Pharmaceutical Co., Ltd., Beijing, China).
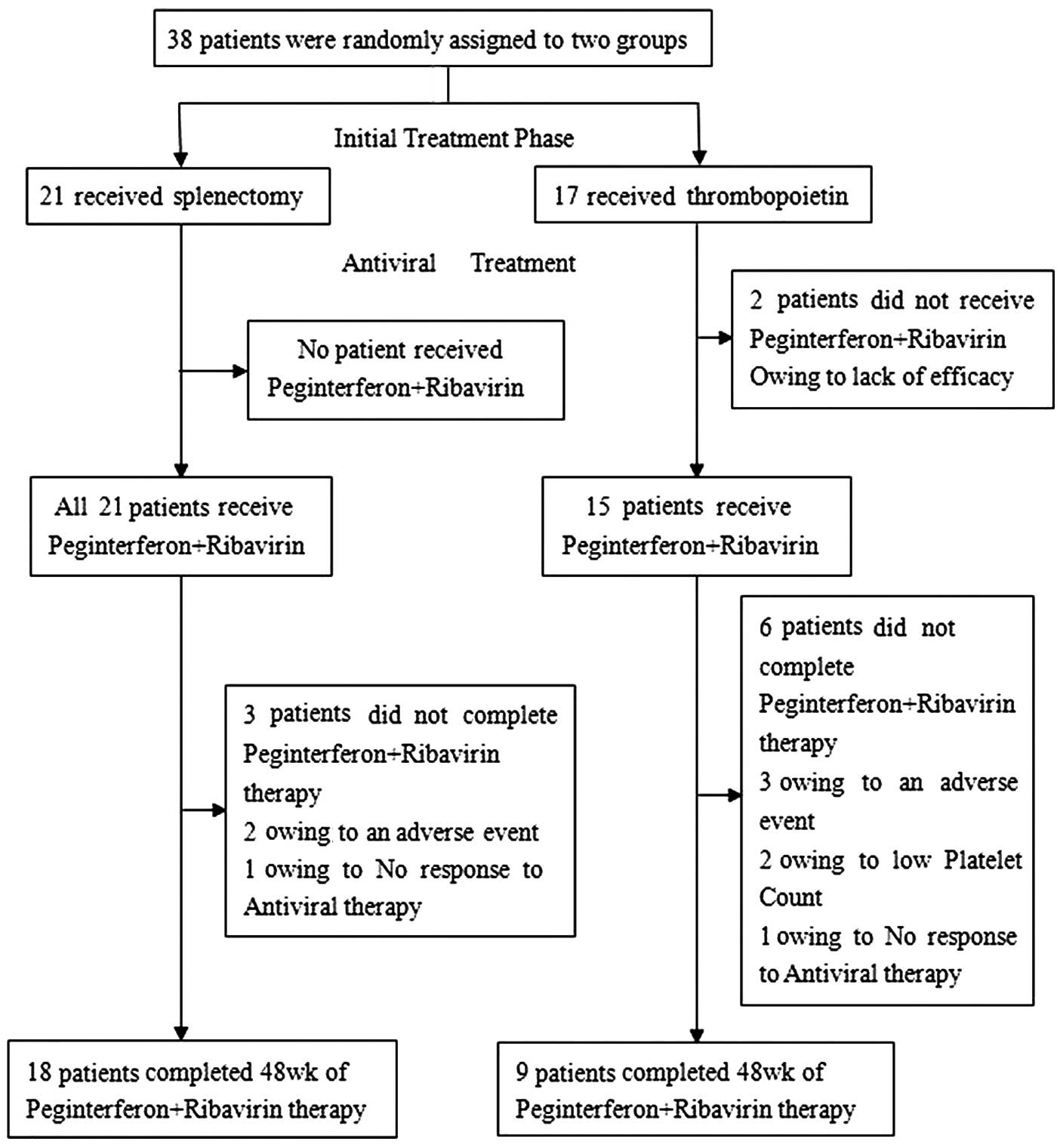
Initial treatment phase
Patients received splenectomy or were administered
thrombopoietin (15,000 units each time; subcutaneous injection),
once per week or as determined by physicians according to patient
platelet levels (Fig. 1). Parameters
assessed to determine safety were prothrombin time, alanine
aminotransferase, aspartate amino transferase, blood albumin,
neutrophil count, creatinine clearance rate and hemoglobin, using
an XE2100 hematology analyzer (Sysmex Shanghai Ltd., Shanghai,
China) and an AU5800 biochemical analyzer (Beckman Coulter, Inc.,
Tokyo, Japan). Tolerability indices included fatigue, insomnia and
depression. Patients that completed the initial treatment phase
were subsequently administered antiviral treatment with
peginterferon (Pegasys; Roche Diagnostics, Basel, Switzerland) and
ribavirin (Biejing SL Pharmaceutical Co., Ltd., Beijing, China) if
they attained a predefined platelet count of
≥60,000/mm3. The control group included 31 patients (23
men and 8 women) with an average age of 55 years (age range, 39–70
years). Patients in the control group did not receive treatments
such as thrombopoietin and P-R.
Antiviral treatment phase
Peginterferon (180 µg peginterferon alfa-2a per
week) and ribavirin (15 mg/kg body weight per day) were
administered for 48 weeks concomitantly with hematological,
biochemical and other safety assessments. If the treatment was
insufficiently effective or caused severe side effects, antiviral
treatment was immediately discontinued. Treatment follow-up was
scheduled for 24 weeks after the final P-R treatment. Throughout
the antiviral treatment phase, in accordance with the product
labels for these approved therapies, the dose of P-R was reduced by
50% if the platelet count had decreased to
25,000–50,000/mm3 and was discontinued completely if the
platelet count was <25,000/mm3. Data were obtained in
the form of questionnaire, including work income during
hospitalization and lost income of accompanying family members,
food and accommodation costs.
Statistical analysis
All statistical analyses were conducted using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA). The primary
efficacy end-point was defined as an increase in the platelet count
from the original value to ≥60,000/mm3 following the
4-week initial treatment phase. Secondary end-points included those
associated with safety, tolerability and the ability to continue
P-R therapy during the antiviral treatment phase. The analyses
included all patients that were randomly assigned to the
splenectomy and thrombopoietin groups and received at least one
treatment course of P-R. However, data for 2/69 patients (3%) that
were enrolled into the study were excluded from the analysis of the
primary end-point, since their platelet count remained at
≤60,000/mm3 after treatment.
The primary end-point was analyzed with the use of
multiple logistic-regression analysis. Research group patients were
compared with the observed group patients using a closed testing
procedure. The global null hypothesis of no significant difference
was tested between the two groups. The criterion for stopping the
study early was a two-sided P-value ≤0.0001, based on the
O'Brien-Fleming adjustment for a group sequential design, from an
interim analysis of the efficacy data (12). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
A total of 69 patients were included in the study
between 2009 and 2013, among which 38 patients were randomly
assigned to the research group. Among these 38 patients, 21
underwent a splenectomy and 17 received thrombopoietin (Fig. 1). A further 31 patients were
allocated to the observed group and treated with glycyrrhizin,
legalon, Chinese herbs and thymic peptide. The demographic and
baseline clinical characteristics of the research groups were
well-balanced, with no statistically significant differences
between the groups (Table I). The
median patient ages were 52 years (range, 40–66 years) and 57 years
(range, 42–63 years) for the splenectomy and thrombopoietin groups,
respectively, and >80% of the patients were men.
 | Table I.Baseline characteristics of the
patients. |
Table I.
Baseline characteristics of the
patients.
| Characteristic | Splenectomy
(n=21) | Thrombopoietin
(n=17) |
|---|
| Age, years |
|
|
|
Median | 52 | 57 |
|
Range | 40–66 | 42–63 |
| Gender, n (%) |
|
|
| Male | 17 (81) | 14 (82) |
|
Female | 4 (19) | 3 (18) |
| Platelet count,
/mm3 |
|
|
|
Median | 39,000 | 39,000 |
|
Range | 21,000–59,000 | 19,000–57,000 |
| Liver stiffness,
kPa |
|
|
|
Median | 19.9 | 19.7 |
|
Range | 12.6–31.2 | 13.4–29.7 |
| HCV RNA load, IU/ml
HCV genotype, n (%) |
5.1×105±2.3×103 |
4.9×105±2.4×103 |
| 1b | 16 (76) | 12 (71) |
| 2a | 5 (24) | 5 (29) |
| Alanine
aminotransferase, IU/l | 48.3±19.6 | 47.9±15.3 |
| Aspartate
aminotransferase, IU/l | 50.7±22.6 | 52.1±16.3 |
| Total bilirubin,
µmol/l | 21.1±12.4 | 19.8±13.5 |
| Child-Pugh score, n
(%) |
|
|
| A | 17 (81) | 15 (88) |
| B | 4 (19) | 2 (12) |
The median original platelet count was
39,000/mm3 (range, 19,000–59,000/mm3) and all
platelet counts were <60,000/mm3 (Table I). However, in 2/38 patients, the
platelet count was not successfully increased to
≥60,000/mm3 following treatment, which was considered a
violation of the study inclusion criteria. Therefore, the data for
these 2 patients were excluded from the analysis of the antiviral
treatment phase; however, they were included in the safety analysis
as the patients received the various study therapies. The observed
group (control group), that received no treatments such as
thrombopoietin and P-R, had a platelet count of
36,000/mm3 (range, 23,000–56,000/mm3). During
the 72-week observed period, the results showed 5 cases of
mortality, 4 cases of upper digestive tract bleeding, 1 cases of
hepatic encephalopathy and 3 cases of hepatocellular carcinoma.
Efficiency
Initial treatment phase
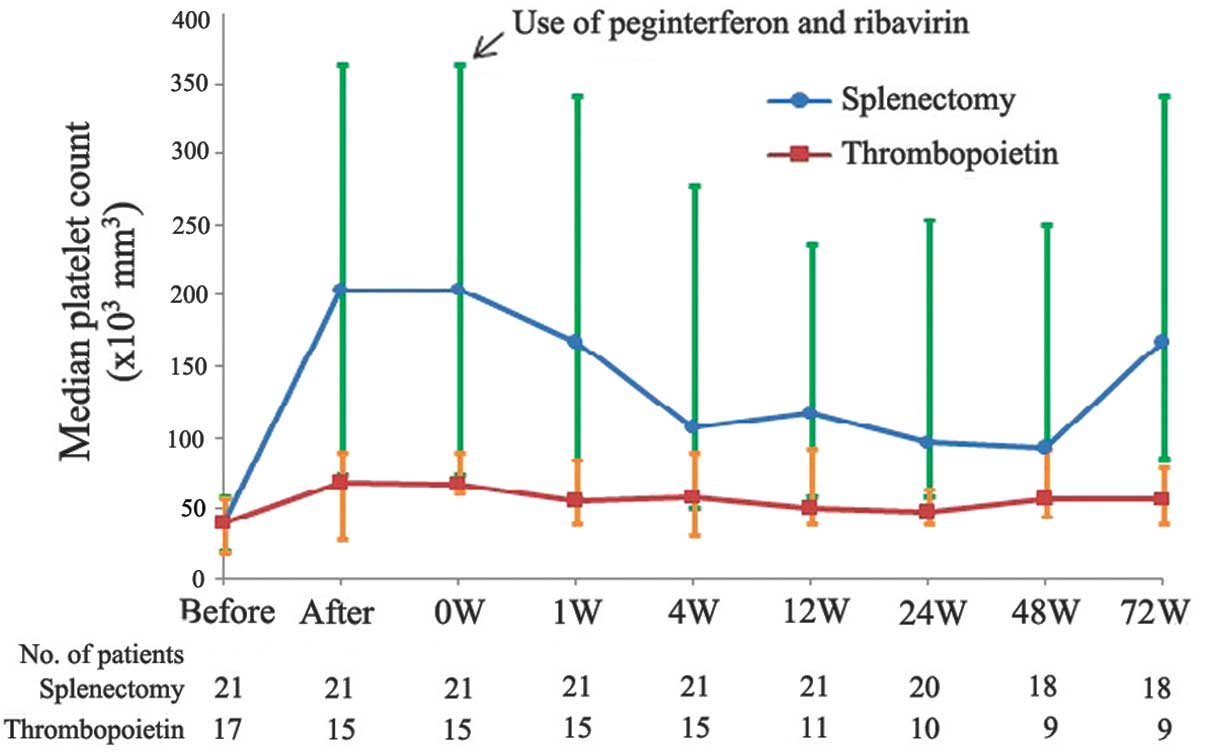
The receipt of splenectomy or thrombopoietin
increased platelet counts to ≥65,000/mm3 at week 4 in
all patients, with the exception of two patients that received
thrombopoietin (P<0.005 for overall treatment effect; Table II). A total of 18 patients (86%)
that underwent a splenectomy had platelet counts of
≥100,000/mm3 at all time points during the initial
treatment phase, thus follow-up to the antiviral treatment required
cautious observation.
 | Table II.Median platelet counts at the initial
treatment phase and the end of the antiviral treatment phase. |
Table II.
Median platelet counts at the initial
treatment phase and the end of the antiviral treatment phase.
| A, End of initial
treatment phase |
|
|
|---|
|
|---|
| Variable | Splenectomy
(n=21) | Thrombopoietin
(n=17) |
|---|
| Platelet
counta,/mm3 |
|
|
|
Median | 204,000 | 68,000 |
|
Range | 74,000–365,000 | 29,000–89,000 |
| Difference from
baselinea |
|
|
| Median,
/mm3 | 155,000 | 25,000 |
| Range,
/mm3 | 33,000–335,000 | 6,000–42,000 |
|
≥60,000/mm3, n
(%) | 21/21 (100) | 15/17 (88) |
| Responders/total
patients, n (%) |
|
|
|
≥100,000/mm3 | 18/21 (86) | 0/17
(0)a |
|
| B, End of antiviral
treatment phase |
|
|
|
| Variable | Splenectomy
(n=21) | Thrombopoietin
(n=17) |
|
| Platelet count |
|
|
| No.
patients, n (%) | 18 (86) | 9 (53) |
| Median,
/mm3 | 96,000 | 56,000 |
| Range,
/mm3 | 54,000–251,000 | 44,000–92,000 |
| Difference from
baseline, /mm3 |
|
|
|
Median | 68,000 | 16,000 |
|
Range | −3,000–198,000 | −7,000–60,000 |
Antiviral treatment phase
Overall, 36/38 patients proceeded to the antiviral
treatment phase, including 21/21 patients (100%) that received a
splenectomy and 15/17 (88%) that received thrombopoietin (Fig. 1). The platelet counts of these 36
patients all attained the prespecified threshold for entry into the
antiviral treatment phase (≥60,000/mm3) and therefore
P-R-based therapy was initiated (Fig.
1).
On an intention-to-treat basis, the first 12 weeks
of antiviral therapy were completed by 21/21 patients that
underwent a splenectomy (100%) and 11/15 patients that received
thrombopoietin (73%; Fig. 2).
Platelet counts in the splenectomy and thrombopoietin groups
decreased during the antiviral treatment phase, but remained
consistently above baseline values, with a nadir of
>60,000/mm3 (Fig. 2).
In total, 18/21 patients (86%) from the splenectomy group and 9/11
patients (82%) from the thrombopoietin treatment group completed
the 48-week antiviral treatment phase. At all time points during
the antiviral treatment phase, platelet counts in the splenectomy
group were high and remained elevated compared with the level at
which a reduction in the P-R dose is recommended
(<50,000/mm3). During the 72-week observed period,
the percentage of completed antiviral treatment was 85.7%
(P=0.0636), the rapid virological response was 28.6% (P=0.3879),
the early virological response was 76.2% (P=0.0272) and the
sustained virologic response (SVR) was 61.9% (P=0.0416) in
splenectomy group, whereas the values in the thrombopoietin group
were 52.9, 11.8, 35.3 and 23.5%, respectively. In addition, the
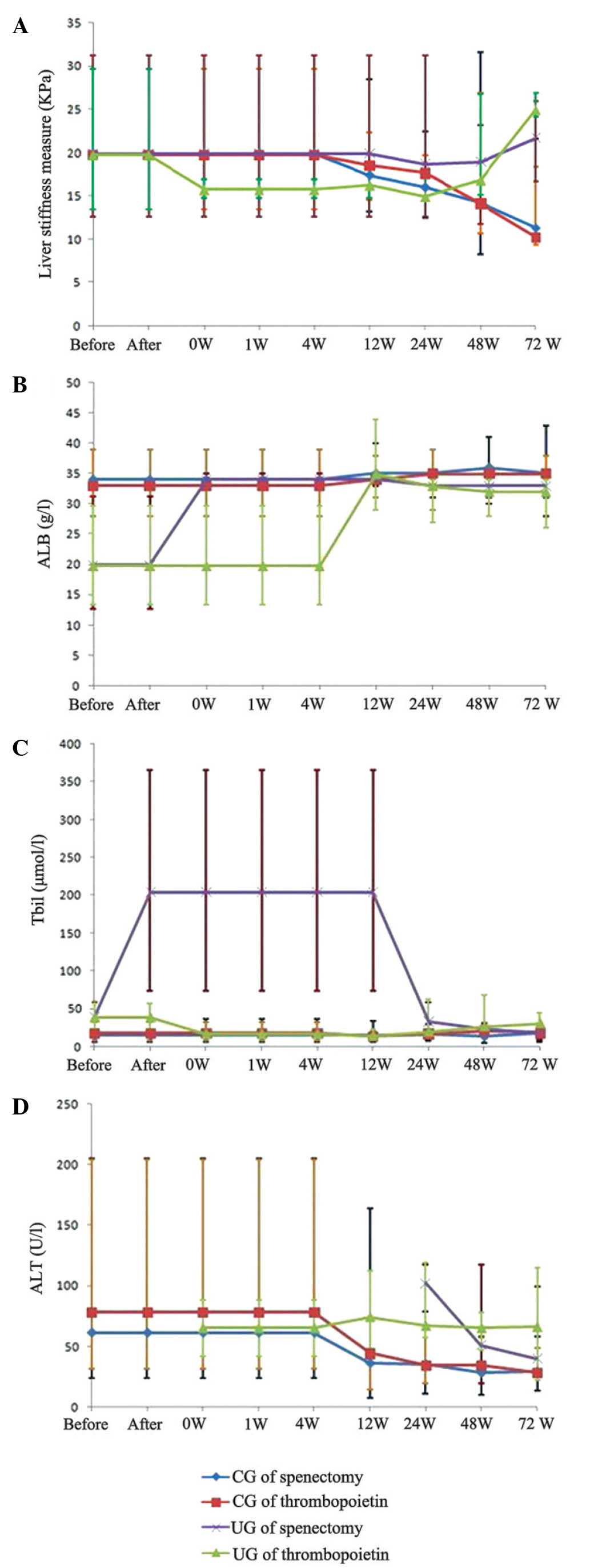
differences in liver stiffness measure, albumin, total bilirubin
and alanine aminotransferase are presented in Fig. 3.
Safety and tolerability
During the 4-week initial treatment phase, headache
was reported in 5 and 24% of patients that underwent splenectomy or
received thrombopoietin, respectively (Table III). Other common adverse events in
the two research groups were upper abdominal pain, pyrexia, rash
and nausea. During the subsequent antiviral treatment phase, the
incidence of adverse events was similar between the two research
groups. The adverse events most commonly reported during this phase
were influenza-like illness, anemia, fatigue, pyrexia and
irritability, all of which are known side-effects of P-R-based
therapy (13,14).
 | Table III.Common adverse events during the
initial treatment phase and the antiviral phase [n (%)]. |
Table III.
Common adverse events during the
initial treatment phase and the antiviral phase [n (%)].
| Events | Splenectomy
(n=21) | Thrombopoietin
(n=17) |
|---|
| Initial treatment
phase |
|
|
|
Any | 16 (76) | 12 (71) |
|
Headache | 1 (5) | 4
(24) |
| Upper
abdominal pain | 5
(24) | 0 (0) |
|
Pyrexia | 6
(29) | 4
(24) |
|
Nausea | 4
(19) | 1 (6) |
|
Rash | 0 (0) | 3
(18) |
| Antiviral treatment
phase |
|
|
|
Any | 20 (95) | 14 (93) |
|
Influenza-like illness | 13 (62) | 11 (73) |
|
Fatigue | 8
(38) | 6
(40) |
|
Headache | 3
(14) | 9
(60) |
|
Arthralgia | 3
(14) | 4
(27) |
|
Depression | 2
(10) | 2
(13) |
|
Myalgia | 3
(14) | 2
(13) |
|
Nausea | 3
(14) | 4
(27) |
|
Anemia | 12 (57) | 10 (67) |
|
Pyrexia | 8
(38) | 10 (67) |
|
Diarrhea | 4
(19) | 3
(20) |
|
Irritability | 5
(24) | 4
(27) |
|
Pruritus | 3
(14) | 5
(33) |
|
Rash | 2
(10) | 7
(47) |
During the entire study, 24 patients reported
adverse events and 5 patients reported serious adverse events.
These serious events included ascites and depression in the
splenectomy group, as well as severe asthenia, rash and upper
gastrointestinal bleeding in the thrombopoietin group, resulting in
the patients discontinuing therapy. Among these patients, 3
patients discontinued the P-R treatment, 1 due to ineffective
treatment and 2 due to serious adverse effects, including fatigue,
skin itching or severe skin rash. Furthermore, 6 patients in the
thrombopoietin group stopped treatment, 1 due to inefficacy, 2 due
to severe fatigue of and 3 due to low platelet counts. Furthermore,
1 patient in the splenectomy group developed liver cancer following
SVR and ceased therapy 7 months later. In addition, the Child-Pugh
score of 4 patients (splenectomy, 3; thrombopoietin, 1) changed
from A to B after the 48-week therapy, and from B to A in another 3
patients in the thrombopoietin group prior to antiviral treatment.
The causes for patients discontinuing the treatment are presented
in Table IV.
 | Table IV.Reasons for not completing therapy [n
(%)]. |
Table IV.
Reasons for not completing therapy [n
(%)].
| Variable | Splenectomy
(n=21) | Thrombopoietin
(n=17) |
|---|
| Patients | 3
(14) | 8
(47) |
| Wuxiao | 1 (5) | 1 (6) |
| Platelet count | 0 (0) | 4
(24) |
| Fatigue | 1 (5) | 2
(12) |
| Depression | 1 (5) | 0 (0) |
| Rash | 0 (0) | 1 (6) |
Cost
As the enzyme inhibitor drugs are not typically
covered by insurance schemes in China and are often prohibitively
expensive, splenectomy, thrombopoietin and internal medicine are
the three primary methods used for the treatment of compensatory
cirrhosis associated with HCV and complicated with thrombocytopenia
in China. Cost and efficacy are crucial factors for patients when
selecting a treatment option. During the initial treatment phase
the median cost of splenectomy was 59,000 RMB (range, 49,000–78,000
RMB) and the rate of effectiveness was 100%. During the antiviral
treatment phase, the median cost was 85,000 RMB (range,
78,000–93,000 RMB) and the effectiveness was 86% (Table V). A total of 31 patients in the
observed group were uncertain factors such as ascites and bleeding
caused by the treatment with glycyrrhizin, legalon, Chinese herbs
and thymic peptide. The median cost of treatment was 95,000 RMB
(range, 37,000–190,000 RMB). However, follow-up analysis of the
observed group after 72-week revealed that 6 patients exhibited
decompensation, 3 patients had large ascites, 2 patients presented
upper gastrointestinal bleeding, 1 case had hepatic encephalopathy
and 3 cases had developed hepatocellular carcinoma. In addition,
the Child-Pugh Score of 1 patient changed from A to B, and those of
another 6 patients turned from B to A. The cost included all
expenses incurred during the hospitalization period, including the
living expenses of the patient and accompanying persons.
 | Table V.Cost-effectiveness analysis at the
percent of initial treatment and the end of the antiviral
treatment. |
Table V.
Cost-effectiveness analysis at the
percent of initial treatment and the end of the antiviral
treatment.
| Variable | Splenectomy
(n=21) |
| Thrombopoietin
(n=17) |
|---|
| Initial treatment
phase |
|
|
|
| Cost,
RMB (Yuan) | 59,000
(49,000–78,000) |
| 16,000
(9,800–31,000) |
|
Effectiveness, % | 100 |
| 88 |
|
Incremental
cost-effectiveness, ΔC/ΔE |
| 3,583 |
|
| Antiviral treatment
phase |
|
|
|
| Cost,
RMB (Yuan) | 85,000
(78,000–93,000) |
| 126,000
(102,000–157,000) |
|
Effectiveness, % | 86 |
| 53 |
|
Incremental
cost-effectiveness, ΔC/ΔE |
| 879 |
|
Discussion
Thrombocytopenic patients with chronic HCV infection
are poor candidates for antiviral treatment with P-R (15); however, there is little information
concerning the eligibility for treatment of patients with platelet
counts of <60,000/mm3. In the present study, 36/38
patients exhibited a response to splenectomy or thrombopoietin,
with an increase in platelet count to ≥60,000/mm3 during
the initial treatment phase. These patients completed 48 weeks of
antiviral treatment with P-R and 24-week follow-up, during which
time their platelet count was monitored.
Splenectomy is a safe, efficacious and
cost-effective intervention for patients with compensatory
cirrhosis associated with HCV. The International Consensus panel
and the revised American Society of Hematology guidelines suggest
deferral of splenectomy for as long as possible or to at least
until 12 months of disease duration in patients with severe disease
(16,17). This caution is based on several
concerns, including the high spontaneous remission rate, long-term
risk for overwhelming post-splenectomy infection, increasing
available medical alternatives (such as thrombopoietin agonists),
and more recent concerns for vascular or hypercoagulable delayed
complications (16–21).
Thrombopoietin, which is also known as megakaryocyte
growth and development factor, is a protein that is encoded by the
thrombopoietin gene in humans (22).
Patients with cirrhosis usually exhibit thrombocytopenia in
discrete levels. The mechanism of thrombocytopenia is hypothesized
to involve the splenic sequestration and destruction of platelets,
impaired bone marrow generation and diminished hepatic
thrombopoietin synthesis (23).
The most commonly observed side effects of
splenectomy and thrombopoietin during the initial treatment phase
were headache, upper abdominal pain, pyrexia, nausea and rash;
these effects were of insufficient severity to require
discontinuation of the antiviral treatment (24). In the present study, no evidence of a
dose-response association with respect to the occurrence of adverse
events was observed in the antiviral treatment phase, during which
the reported side effects were consistent with those associated
with P-R-based therapy (11).
During the initial treatment phase, significant
increases in platelet count were observed in each research group as
compared with the original count. The primary end-point (platelet
count of ≥60,000/mm3 at week 4) was attained in 100 and
88% of patients in the splenectomy and thrombopoietin groups,
respectively. During the subsequent antiviral phase, platelet
counts decreased, which may be due to the antiplatelet effect of
P-R; however, the platelet counts remained consistently above the
baseline levels.
The results of the present study indicate that
splenectomy is more effective treatment for thrombocytopenia
compared with thrombopoietin for facilitating the subsequent
completion of antiviral therapy in patients with compensatory
cirrhosis associated with HCV. In the splenectomy group, more
patients were able to receive a full course of antiviral therapy
and achieved an SVR. Follow-up demonstrated that the patients were
in good condition. Side effects were observed in the two groups due
to the P-R treatment or other factors, depending on each
individual's physical condition. However, the expenses incurred by
patients in the splenectomy group were higher than those in the
thrombopoietin group due to the surgery fees, artificial nursing
fees and other associated costs. In general, the therapeutic
efficiency of the treatment is the highest priority for patients,
and cost is the second priority, as long as it is within acceptable
levels. Currently, splenectomy is considered a good
thrombocytopenia treatment for assisting the completion of
antiviral therapy in patients with compensatory cirrhosis
associated with HCV. However, for patients that are able to afford
the expenses associated with, the application of ribavirin therapy
may result in higher efficacy with reduced risk.
Acknowledgements
This study was supported by the Chinese Medical
Association Foundation (project no. 13071110496: Polyethylene
Glycol Interferon-α-2b plus Ribavirin for Treatment of Hepatitis C
in Patients with Cirrhosis). The authors would like to thank all
participants who volunteered for this study, and particularly thank
Professor George Ka Kit Lau (Li Ka Shing Faculty of Medicine,
University of Hong Kong, Hong Kong, China).
References
|
1
|
Bashour FN, Teran JC and Mullen KD:
Prevalence of peripheral blood cytopenias (hypersplenism) in
patients with nonalcoholic chronic liver disease. Am J
Gastroenterol. 95:2936–2939. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Peck-Radosavljevic M: Thrombocytopenia in
liver disease. Can J Gastroenterol. 14(Suppl D): 60D–66D.
2000.PubMed/NCBI
|
|
3
|
Giannini EG: Review article:
Thrombocytopenia in chronic liver disease and pharmacologic
treatment options. Aliment Pharmacol Ther. 23:1055–1065. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moreno A, Bárcena R, Blázquez J, Quereda
C, Gil-Grande L, Sánchez J, Moreno L, Perez-Elías MJ, Antela A,
Moreno J, et al: Partial splenic embolization for the treatment of
hypersplenism in cirrhotic HIV/HCV patients prior to pegylated
interferon and ribavirin. Antivir Ther. 9:1027–1030.
2004.PubMed/NCBI
|
|
5
|
de Oliveira AC: Treatment options in the
management of thrombocytopenia in patients infected with HCV. Braz
J Infect Dis. 11(Suppl 1): 71–72. 2007. View Article : Google Scholar
|
|
6
|
Fried MW, Shiffman ML, Reddy KL, Smith C,
Marinos G, Gonçales FL Jr, Häussinger D, Diago M, Carosi G,
Dhumeaux D, et al: Peginterferon alfa-2a plus ribavirin for chronic
hepatitis C virus infection. N Engl J Med. 347:975–982. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Manns MP, McHutchison JG, Gordon SC,
Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M
and Albrecht JK: Peginterferon alfa-2b plus ribavirin compared with
interferon alfa-2b plus ribavirin for initial treatment of chronic
hepatitis C: A randomised trial. Lancet. 358:958–965. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bussel JB and Marks KM: How effective is
eltrombopag for the treatment of thrombocytopenia in patients with
HCV infection? Nat Clin Pract Gastroenterol Hepatol. 5:424–425.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cholongitas E, Papatheodoridis GV, Vangeli
M, Terreni N, Patch D and Burroughs AK: Systematic review: The
model for end-stage liver disease - should it replace Child-Pugh's
classification for assessing prognosis in cirrhosis? Aliment
Pharmacol Ther. 22:1079–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lackner C, Struber G, Bankuti C, Bauer B
and Stauber RE: Noninvasive diagnosis of cirrhosis in chronic
hepatitis C based on standard laboratory tests. Hepatology.
43:378–379. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McHutchison JG, Dusheiko G, Shiffman ML,
Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC,
Campbell FM, Theodore D, et al: TPL102357 Study Group: Eltrombopag
for thrombocytopenia in patients with cirrhosis associated with
hepatitis C. N Engl J Med. 357:2227–2236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
O'Brien PC and Fleming TR: A multiple
testing procedure for clinical trials. Biometrics. 35:549–556.
1979. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manns M, Wedemeyer H and Cornberg M:
Treating viral hepatitis C: Efficacy, side effects and
complications. Gut. 55:1350–1359. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gutfreund K and Bain V: Chronic viral
hepatitis C: Management update. CMAJ. 162:827–833. 2000.PubMed/NCBI
|
|
15
|
Ikezawa K, Naito M, Yumiba T, Iwahashi K,
Onishi Y, Kita H, Nishio A, Kanno T, Matsuura T, Ono A, et al:
Splenectomy and antiviral treatment for thrombocytopenic patients
with chronic hepatitis C virus infection. J Viral Hepat.
17:488–492. 2010.PubMed/NCBI
|
|
16
|
Neunert C, Lim W, Crowther M, Cohen A,
Solberg L Jr and Crowther MA: American Society of Hematology: The
American society of hematology 2011 evidence-based practice
guideline for immune thrombocytopenia. Blood. 117:4190–4207. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Provan D, Stasi R, Newland AC, Blanchette
VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB,
Godeau B, et al: International consensus report on the
investigation and management of primary immune thrombocytopenia.
Blood. 115:168–186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bussel JB, Buchanan GR, Nugent DJ, Gnarra
DJ, Bomgaars LR, Blanchette VS, Wang YM, Nie K and Jun S: A
randomized, double-blind study of romiplostim to determine its
safety and efficacy in children with immune thrombocytopenia.
Blood. 118:28–36. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tiede MP, Ahn ER, Jy W, Scagnelli T, Bidot
CJ, Horstman LL, Jimenez JJ and Ahn YS: Life-threatening
hypercoagulable state following splenectomy in ITP: Successful
management with aggressive antithrombotic therapy and danazol. Clin
Appl Thromb Hemost. 11:347–352. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crary S and Buchanan G: Vascular
complications after splenectomy for hematologic disorders. Blood.
114:2861–2868. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Blanchette V and Bolton-Maggs P: Childhood
immune thrombocytopenic purpura: Diagnosis and management. Hematol
Oncol Clin North Am. 24:249–273. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Deutsch VR and Tomer A: Megakaryocyte
development and platelet production. Br J Haemotol. 134:453–466.
2006. View Article : Google Scholar
|
|
23
|
Temel T, Cansu DU, Temel HE and Ozakyol
AH: Serum thrombopoietin levels and its relationship with
thrombocytopenia in patients with cirrhosis. Hepat Mon.
14:e185562014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ES, Lyons RM, Larson RA, Gandhi S,
Liu D, Matei C, Scott B, Hu K and Yang AS: A randomized,
double-blind, placebo-controlled phase 2 study evaluating the
efficacy and safety of romiplostim treatment of patients with low
or intermediate-1 risk myelodysplastic syndrome receiving
lenalidomide. J Hematol Oncol. 5:712012. View Article : Google Scholar : PubMed/NCBI
|