Introduction
Basketball is considered one of the most popular
team sports worldwide and has gained the fascination of countless
spectators due to its dynamic characteristics. The overall duration
of a typical basketball match is 40–48 min, in which an athlete
carries out a combination of multidirectional movements, such as
running, jumping and dribbling at variable velocities. Even though
both aerobic and anaerobic systems are activated in order to
execute these movements, previous studies have demonstrated that
the anaerobic metabolism is the primary energy pathway activated in
basketball players (1–4).
Elite basketball athletes undergo heavy training and
competition throughout the season, continously playing numerous
difficult and demanding matches. It has been well established that
intense exercise increases the production of free radicals, which
may lead to a pathophysiological condition known as oxidative
stress (5,6) that has been implicated in the oxidative
damage to macromolecules (e.g., lipids, proteins and DNA) (7), immune dysfunction (8), muscle damage (9) and fatigue (10). It is well known that oxidative stress
frequently occurs in muscle tissue exposed to reactive oxygen
species (ROS) production (8). During
intense exercise, there is a high rate of O2 consumption
in skeletal muscle, which, due to the incomplete O2
reduction and electron leakage from the electron transfer chain,
then leads to the accumulation of mitochondrial-derived ROS
(8). These, in combination with the
extra-mitochondrial produced ROS may cause oxidative stress
(11). These effects in turn result
in muscle fatigue, cell damage and apoptosis (11,12). A
previous study demonstrated that the intensity of a basketball game
varies depending on the level of the competition and the playing
position (13). In addition, other
studies have indicated the induction of oxidative stress in elite
team-sport athletes over the course of an athletic season,
particularly in periods of intense competition (14,15).
In the present study, an elite European basketball
professional team was monitored at the beginning and end of an
athletic season to examine the differences in the redox status in
players between the beginning of the season and following a series
of highly competitive matches at the end of the season. In order to
monitor the redox status of the athletes, conventional oxidative
stress markers, such as thiobarbituric acid reactive substances
(TBARS), glutathione (GSH) levels, catalase (CAT) activity, protein
carbonyl (CARB) levels and total antioxidant capacity (TAC) were
measured. Moreover, a novel method based on the measurement of
oxidation-reduction potential (ORP) was used for assessing
oxidative stress. ORP is an integrated measure of the balance
between total oxidants [e.g., oxidized thiols, superoxide radicals,
hydroxyl radicals, hydrogen peroxide (H2O2),
nitric oxide, peroxynitrite and transition metal ions] and total
reductants (e.g., free thiols, ascorbate, α-tocopherol, β-carotene
and uric acid), as well as other unknown markers (16). In previous studies, we demonstrated
that the measurement of ORP using the RedoxSYS diagnostic system
was an effective method for assessing oxidative stress induced by
strenuous exercise, such as a mountain marathon race and eccentric
exercise (17,18).
Subjects and methods
Subjects
A total of 14 adult male basketball players (age,
26.8±1.2 years; height, 1.99±0.02 m; weight, 101.6±2.63 kg)
participated in the present study. All players were members of an
elite European professional basketball club that participates in
both European and national basketball leagues. All experimental
procedures were carried out in accordance with the European Union
Guidelines laid down in the 1964 Declaration of Helsinki and were
approved by the Ethics Committee of the University of Thessaly
(Larissa, Greece).
Training load
The measurements were carried out on 2 different
selected time points of a basketball championship season (32
weeks), which presented differences in the tiredness and fatigue of
the athletes. Thus, blood collections were made at 2 different
phases, at the beginning of the regular season (phase 1; November)
and at the end of the season (phase 2; May) following a series of
high-level competitive matches. In phase 1, the team had already
played 9 matches over the course of 1 month, having 2 matches per
week. In phase 2, the team had played 59 matches, while over the
last 10 days prior to blood sampling, the players had participated
in 4 consecutive and very intense games.
Blood collection
The blood samples were collected by the Health
Centre ‘Hartografoi Ygeias’ (Athens, Greece), stored in
ethylenediaminetetraacetic acid (EDTA) or heparin tubes and
centrifuged at 1,370 × g for 10 min at 4°C to divide the
erythrocytes from the plasma. The packed erythrocytes were lysed
with 1:1 (v/v) distilled water, inverted vigorously and centrifuged
at 4,020 × g for 15 min at 4°C. The plasma and erythrocyte lysates
were then stored at −80°C until use in biochemical analysis.
Blood assays
The static ORP (sORP) marker was determined using
the RedoxSYS diagnostic system (Luoxis Diagnostics, Inc.,
Englewood, CO, USA) as previously described (17,18).
This value is indicative of the integrated balance of oxidants and
reductants in a specimen and is presented in mV. Using this
innovative method, 20 µl of plasma were applied to disposable
sensors designed by Luoxis Diagnostics, Inc., which were inserted
into the RedoxSYS diagnostic system and the sORP value was reported
within 4 min.
For the determination of the levels of TBARS, an
assay was used based on the study by Keles et al (19). TBARS is a commonly and frequently
used method to determine the lipid peroxidation (20). In accordance with this method, 100 µl
of plasma were mixed with 500 µl of 35% trichloroacetic acid (Merck
KGaA, Darmstadt, Germany) and 500 µl of Tris-HCl (Sigma-Aldrich,
St. Louis, MO, USA; 200 mmol/l, pH 7.4) followed by incubation for
10 min at room temperature. A total of 1 ml of 2 M sodium sulfate
and 55 mmol/l TBA solution were added and the samples were then
incubated at 95°C for 45 min. The samples were cooled on ice for 5
min and were vortexed following the addition of 1 ml of 70% TCA.
The samples were centrifuged at 15,000 × g for 3 min and the
absorbance of the supernatant was read at 530 nm using a
spectrophotometer (Hitachi U-1900; serial no. 2023-029; Hitachi,
Tokyo, Japan). A baseline absorbance was taken into account by
running a blank along with all samples during the measurement. The
calculation of the TBARS concentration was based on the molar
extinction co-efficient of malondialdehyde.
The GSH concentration was measured as previously
described in the study by Reddy et al (21). A total of 20 µl of erythrocyte lysate
treated with 5% TCA was mixed with 660 µl of 67 mmol/l sodium
potassium phosphate (pH 8.0) and 330 µl of 1 mmol/l
5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB). The samples were then
incubated in the dark at room temperature for 45 min and the
absorbance was read at 412 nm using a spectrophotometer (Hitachi
U-1900; serial no. 2023-029; Hitachi). The GSH concentration was
calculated on the basis of calibration curves made using commercial
standards.
The concentration of CARB, an index of protein
oxidation, was determined based on the method described in the
study by Patsoukis et al (22). In this assay, 50 µl of 20% TCA were
added to 50 µl of plasma and this mixture was then incubated in an
ice bath for 15 min and centrifuged at 15,000 × g for 5 min at 4°C.
The supernatant was discarded and 500 µl of 10 mmol/l
2,4-dinitrophenylhydrazine (DNPH; in 2.5 N HCl) for the sample, or
500 µl of 2.5 N HCl for the blank, were added to the pellet. The
samples were incubated in the dark at room temperature for 1 h with
intermittent vortexing every 15 min and were centrifuged at 15,000
× g for 5 min at 4°C. The supernatant was discarded and 1 ml of 10%
TCA was added, vortexed and centrifuged at 15,000 × g for 5 min at
4°C. The supernatant was discarded and 1 ml of ethanol-ethyl
acetate (1:1 v/v) was added, vortexed and centrifuged at 15,000 × g
for 5 min at 4°C. This washing step was repeated twice. The
supernatant was discarded and 1 ml of 5 M urea (pH 2.3) was added,
vortexed and incubated at 37°C for 15 min. The samples were
centrifuged at 15,000 × g for 3 min at 4°C and the absorbance was
read at 375 nm using a spectrophotometer (Hitachi U-1900; serial
no. 2023-029; Hitachi). The calculation of the CARB concentration
was based on the molar extinction co-efficient of DNPH. Total
plasma protein was assayed using Bradford reagent (Sigma, Hamburg,
Germany).
The determination of TAC was based on the method
described in the study by Janaszewska and Bartosz (23). Briefly, 20 µl of plasma were added
respectively to 480 µl of 10 mmol/l sodium potassium phosphate (pH
7.4) and 500 µl of 0.1 mmol/l 1,1-diphenyl-1-picrylhydrazyl (DPPH)
and the samples were incubated in the dark for 60 min at room
temperature. The samples were then centrifuged for 3 min at 20,000
× g and the absorbance was read at 520 nm using a spectrophotometer
(Hitachi U-1900; serial no. 2023-029; Hitachi).
The measurement of CAT activity was carried out as
previously described by Aebi (24).
In particular, 4 µl οf erythrocyte lysate (diluted 1:10) were added
to 2,991 µl οf 67 mmol/l sodium potassium phosphate (pH 7.4) and
the samples were incubated at 37°C for 10 min. A total of 5 µl of
30% H2O2 was added to the samples and the
change in absorbance was immediately read at 240 nm [using a
spectrophotometer (Hitachi U-1900; serial no. 2023-029; Hitachi)]
for 130 sec. The calculation of CAT activity was based on the molar
extinction co-efficient of H2O2. Each assay
was performed twice in triplicate.
Statistical analysis
For statistical analysis, data were analyzed by
one-way ANOVA followed by Dunnett's test for multiple pairwise
comparisons. The correlation between different oxidative stress
markers was examined by Spearman's correlation analysis. A value of
P<0.05 was considered to indicate a statistically significant
difference. For all statistical analyses, SPSS version 13.0
software (SPSS, Inc., Chicago, IL, USA) was used. Data are
presented as the means ± standard error of the mean.
Results
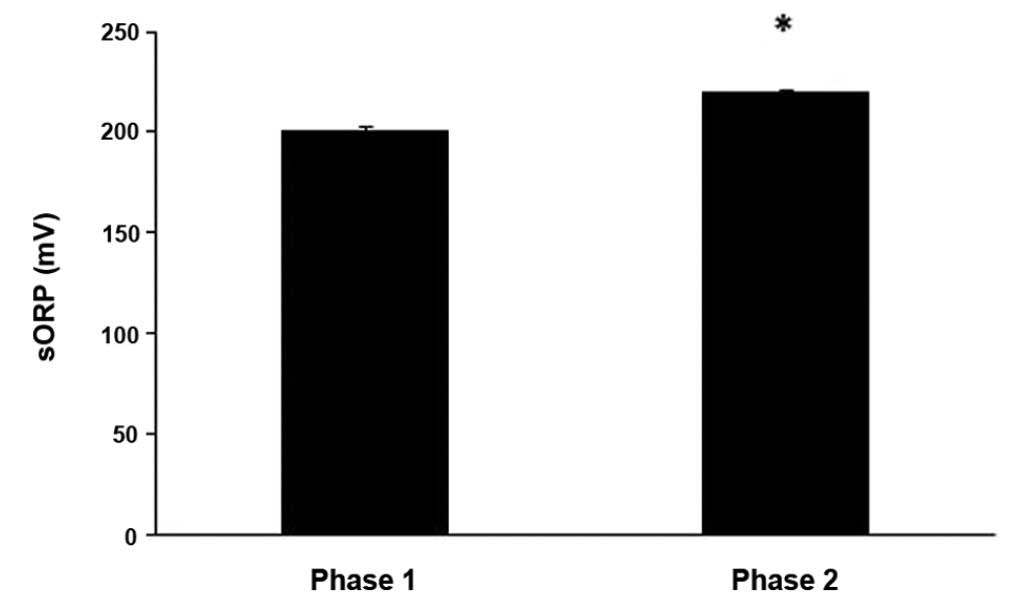
The results revealed that the sORP value, which is
indicative of the current redox status, increased significantly
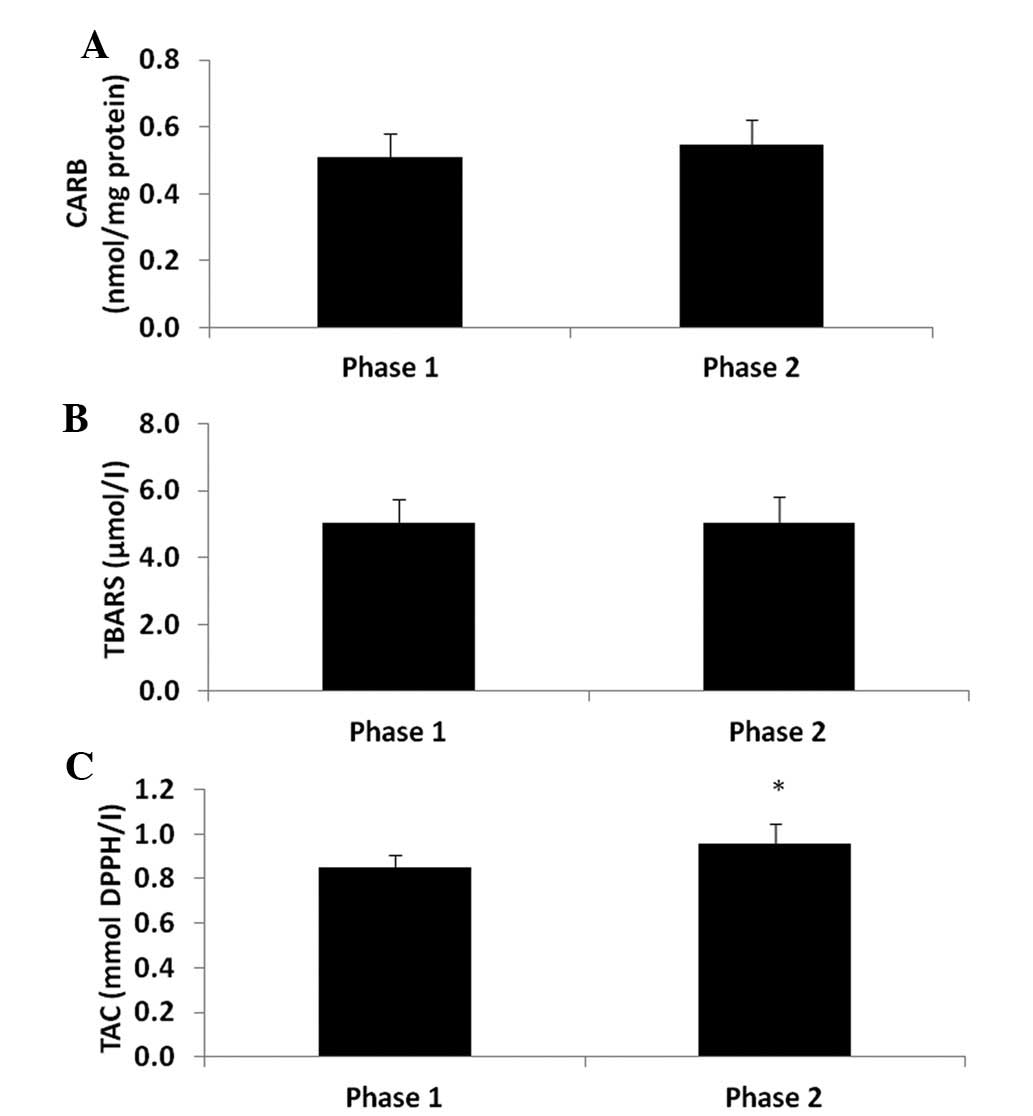
(P<0.05) by 9.6% at phase 2 compared to phase 1 (Fig. 1). As regards the other oxidative
stress markers, the CARB and TBARS levels which are indicative of
protein oxidation and lipid peroxidation, respectively were not
significantly affected (Fig. 2A and
B). However, TAC was significantly increased (P<0.05) by
12.9% (Fig. 2C) at phase 2 compared
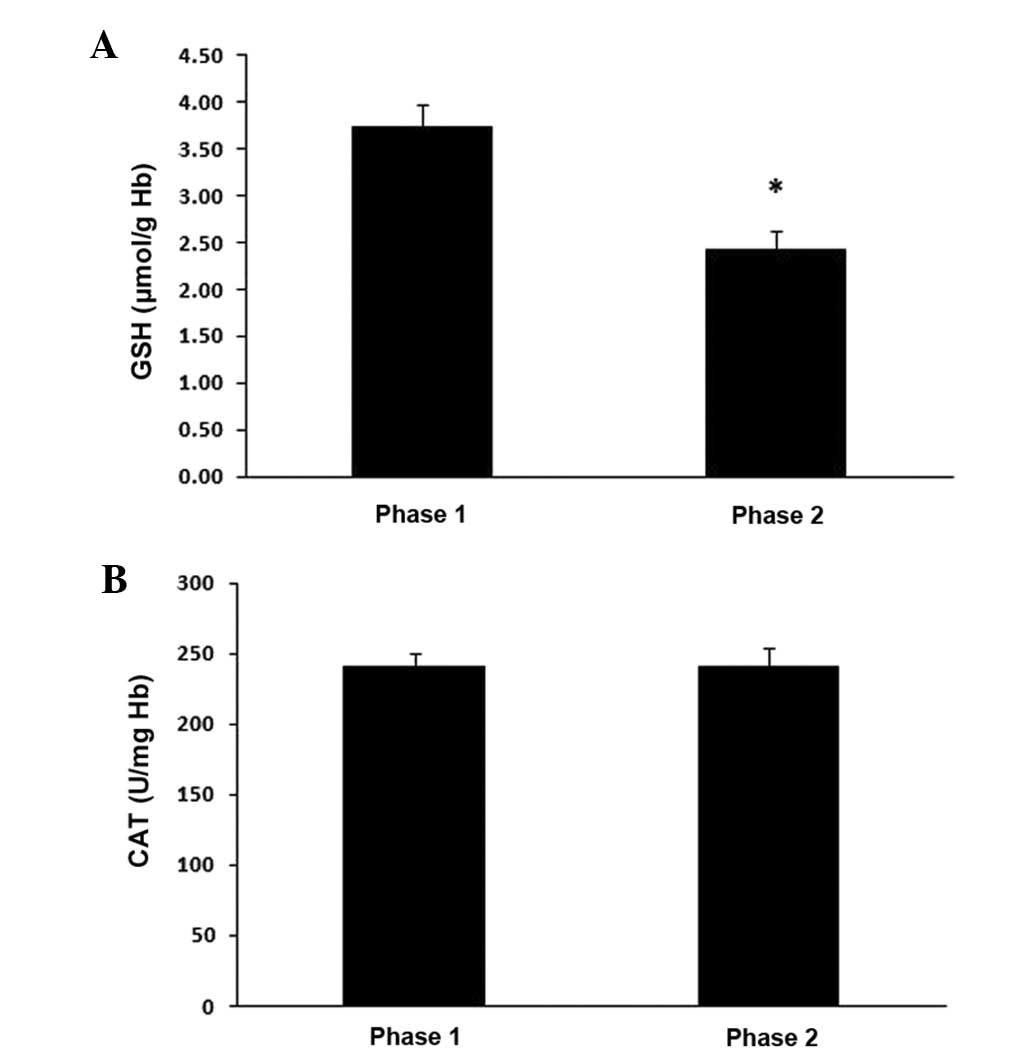
to phase 1. In addition, the GSH levels in erythrocytes were
significantly decreased (P<0.05) by 35% at phase 2 compared to
phase 1 (Fig. 3A), whereas CAT
activity was not affected (Fig.
3B).
Since there is growing evidence provided by ours and
previous studies that there is a marked heterogeneity in responses
between different individuals to exercise-induced oxidative stress
(17,25–27), in
this study, we examined the changes occurring in oxidative stress
markers in each individual player between phase 1 and 2 of the
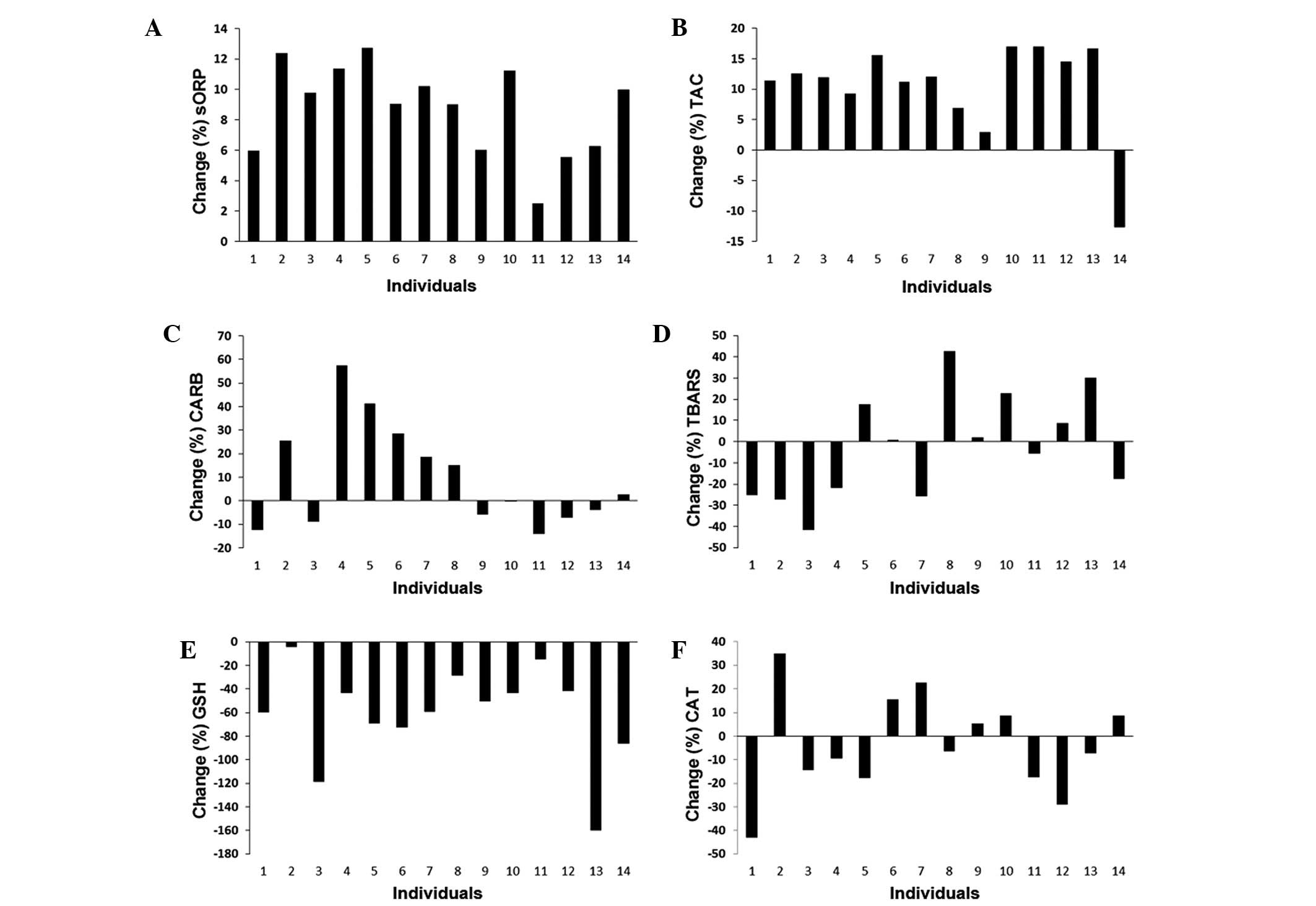
athletic season (Fig. 4). We found
that in all players, the sORP values were higher at phase 2
compared to phase 1 (Fig. 4A).
Similar to the sORP values, the TAC values were altered in a
similar manner in almost all players at phase 2 compared to phase
1, apart from one player (Fig. 4B).
However, the changes in the levels of 2 other markers measured in
plasma, CARB and TBARS, exhibited marked variations between the
players (Fig. 4C and D).
Specifically, half of the players exhibited an increase in CARB and
TBARS levels at phase 2 compared to phase 1, whereas the remaining
players exhibited a decrease (Fig. 4C
and D). As regards the markers measured in erythrocytes, the
GSH concentration was decreased in all players at phase 2 compared
to phase 1 (Fig. 4E). On the
contrary, the changes in CAT activity presented marked differences
between players, since in 6 players, there was an increase in
enzymatic activity at phase 2 compared to phase 1, while in 8
players there was a decrease (Fig.
4F).
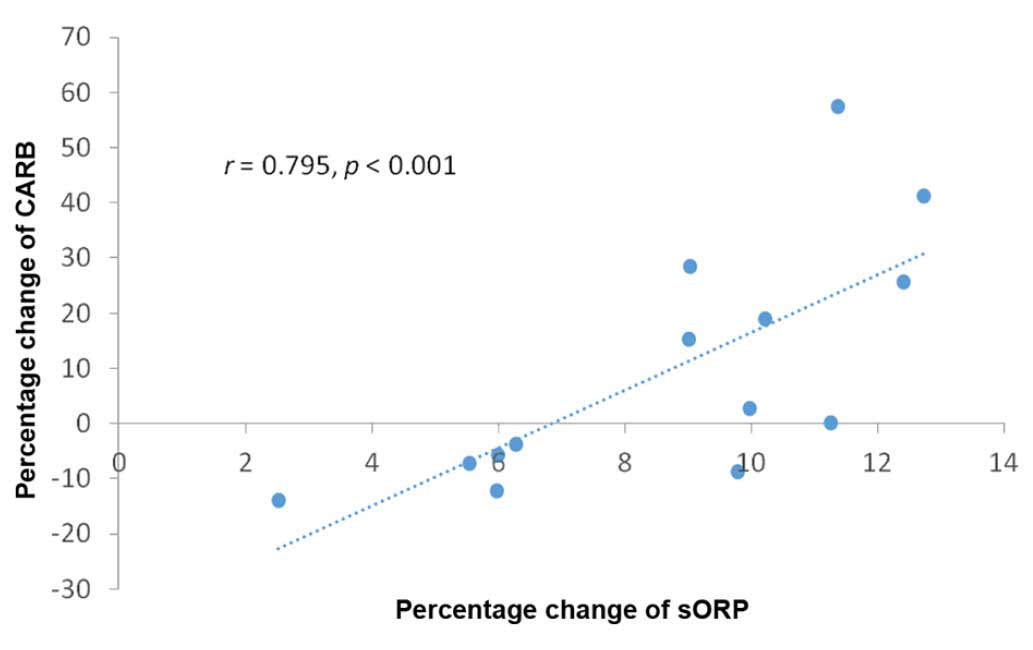
Spearman's correlation analysis between percentage
changes of the oxidative stress markers revealed that there was
only a statistically significant (P<0.001) high correlation
(r=0.798) between the sORP and CARB markers (Table I and Fig.
5).
 | Table I.Correlation co-efficient (r) of
percentage change (i.e., change between phase 1 and phase 2)
between the sORP, TAC, CARB, TBARS and CAT markers. |
Table I.
Correlation co-efficient (r) of
percentage change (i.e., change between phase 1 and phase 2)
between the sORP, TAC, CARB, TBARS and CAT markers.
| Markers | sORP | TAC | TBARS | CARB | GSH | CAT |
|---|
| sORP |
| 0.011 | −0.191 |
0.798a | −0.064 |
0.455 |
| TAC |
|
|
0.209 | −0.213 |
0.182 | −0.165 |
| TBARS |
|
|
|
0.055 |
0.051 | −0.125 |
| CARB |
|
|
|
| −0.007 |
0.477 |
| GSH |
|
|
|
|
|
0.095 |
Discussion
To the best of our knowledge, there are only a few
studies available to date on the assessment of the redox status of
basketball players, particularly over the course of a season
(28,29). In this study, the redox status of 14
elite basketball athletes was examined at 2 different time points,
at the beginning of a season (phase 1) and at the end of a season
(phase 2). At phase 1, the players had only played 9 matches.
However, at phase 2, the players had played 59 competitive matches,
a number which is considered extremely high for a European
team.
Thus, the excessive amounts of exercise due to the
high number of matches at phase 2 resulted in more severe oxidative
stress compared to phase 1. Specifically, the GSH levels in
erythrocytes were significantly lower in the athletes at phase 2
than in phase 1. It has previously been reported that acute aerobic
exercise results in a decrease in GSH levels due in part to the
inactivation of free radicals for regenerating ascorbic acid and
α-tocopherol (14). Of note, a
similar study conducted by Zembron-Lacny et al (28), in professional players of the Polish
Basketball Extraleague, demonstrated that the GSH levels were also
decreased at the end of the playoff period. This increase in GSH
levels was accompanied by an increase in the levels of
anti-inflammatory interleukin-6 (IL-6) and pro-inflammatory tumor
necrosis factor-α (TNF-α) cytokines (28). In general, it has been proposed that
the exercise-induced increase in ROS activates adaptive responses
through signaling pathways regulated by the thiol status (i.e.,
reduced and oxidized GSH levels) (28–31).
Changes in the thiol status induce the expression of the
transcription factors, nuclear factor-κB and activator protein-1
(AP-1), which in turn increase the levels of the cytokines, IL-6
and TNF-α (30–32). Finally, IL-6 and TNF-α play crucial
role in muscle regeneration and in the development of tolerance
following ROS-induced muscle damage (33). In this study, in contrast to the GSH
levels, the activity of CAT in erythrocytes did not differ between
the 2 phases. However, a recent study reported that CAT activity
increased after a basketball game (34). In general, the mechanisms through
which exercise affects CAT activity are not clear, although studies
have indicatd that CAT activity is not increased after exercise
(35–38).
Moreover, in this study, the oxidative stress which
occurred at phase 2 was not so severe in order to cause lipid
peroxidation and protein oxidation, as evidenced by the unaltered
TBARS and CARB levels. Similarly, Zembron-Lacny et al
(28) did not observe any increase
in the TBARS and CARB levels in basketball players at the end of
the playoff round. The hypothesis accounting for this absence of
lipid peroxidation and protein oxidation is that exercise-induced
ROS generation increases antioxidant defense mechanisms through
changes in the thiol status as mentioned above (28).
The above mentioned results of the present study
demonstrating a decrease in GSH levels were also confirmed by the
increase in the values of the sORP marker. This marker as measured
by the RedoxSYS diagnostic system, was used in order to evaluate
oxidative stress in basketball players. sORP is the standard
potential between a working electrode and a reference electrode
with no driving current (or extremely small current) which is
proportional to the balance of reductants and oxidants in plasma
(17). Low sORP values mean that the
biological sample is in the normal range of redox status, while
high sORP values mean that the biological sample is in a higher
state of oxidative stress. We have previously reported that the
sORP value was increased in athletes following a mountain marathon
race, consequently indicating the induction of oxidative stress
(18). Similarly, in this study, the
increase in the values of the sORP marker at phase 2 compared to
phase 1 suggested an increase in oxidative stress. Moreover, the
percentage change in the values of the sORP marker between phase 1
and 2 had a high correlation with the changes in the CARB levels,
although as mentioned above, there was no statistically significant
difference in the CARB levels at phase 2 compared to phase 1. This
finding suggests that the higher the oxidative stress, the higher
the probability for protein oxidation in athletes and also supports
the use of sORP as a novel marker of oxidative stress.
The other marker which was used to assess the total
redox status was TAC. TAC was higher in the athletes at phase 2
compared to phase 1, indicating an enhancement of the antioxidant
mechanisms at the end of the season. This result seems intriguing,
particularly when compared to the induction of oxidative stress
suggested by other biomarkers. Other studies, including ours have
also reported an increase in TAC post-exercise (18,39–41).
This apparent contradiction is explained if we bear in mind that
TAC actually assesses the total amount of molecules acting as
antioxidants. Indeed, previous studies have reported that exercise
enhances the antioxidant mechanisms (42,43).
However, as we have previously noted (18), the increase in TAC following exercise
may indicate that this method is inappropriate for assessing the
in vivo redox status. The weakness of TAC as a method is
that it is based on the reduction of a free radical (e.g., DPPH) by
the antioxidant molecules in plasma. Thus, this method evaluates
only the reductants in plasma. However, the redox status is
determined not only by the amount of reductants, but also by the
amount of oxidants in plasma. Thus, sORP may be a better marker
than TAC for assessing the total oxidative stress in vivo,
since its assessment is based on both the amount of reductants and
oxidants in plasma.
In a previous study, we also examined the use of ORP
markers for assessing eccentric exercise-induced oxidative stress
and found a marked variation in the changes of oxidative stress
markers between different individuals (17). In general, the issue of examining not
only average group responses to exercise-induced oxidative stress,
but also individual responses is of great interest, since previous
research has also recently reported great inter-individual
variability regarding the changes in oxidative stress markers
following exercise (26). The
present findings also exhibited great inter-individual variations
in the changes of oxidative stress markers in athletes between
phase 1 and 2. Thus, the 6 tested oxidative stress markers could be
divided into 2 categories based on their changes at phase 2
compared to phase 1.
The first category includes the 3 oxidative stress
markers (i.e., sORP, TAC and GSH) in which all (or almost all) the
athletes exhibited the same directional variations at phase 2
compared to phase 1. Thus, the sORP value was increased in all
athletes, GSH levels were decreased in all athletes and TAC was
increased in all but one athlete at phase 2 compared to phase 1.
The increase in the sORP value in all athletes indicated that
oxidative stress occurred in all athletes due to excessive exercise
at phase 2. However, there was great inder-individual variability
in the percentage change of sORP at phase 2 compared to phase 1
(the least percentage change was 2.5% and the highest was 12.7%).
The increase in TAC in all but one athlete at phase 2 compared to
phase 1 conformed with the increase in the sORP value, since it
suggested an increase in antioxidant molecules (i.e., reductants)
due to adaptive response to oxidative stress induced at phase 2.
Although the concurrence of the increase in TAC with oxidative
stress is intriguing, it may explained by the fact that oxidative
stress occurs when there is an imbalance between oxidants and
reductants in favour of the former (44). Thus, a stimulus may cause an increase
in the amount of both oxidants and reductants, but if the former
are higher than the latter, then oxidative stress will occur. For
this reason, we believe that the sORP marker is more effective than
TAC for assessing the redox status, since it is based on the
evaluation of the difference between oxidants and reductants.
Similar to the sORP value, TAC also exhibited great
inder-individual variability in the percentage change (the least
percentage increase was 2.9% and the highest was 17.0%). The
decrease in TAC in 1 athlete at phase 2 compared to phase 1 may be
explained by his inability to effectively activate adaptive
responses to oxidative stress. The decrease in the GSH levels in
all athletes at phase 2 compared to phase 1 may indicate the
crucial role that this antioxidant molecule plays in
exercise-induced oxidative stress. As mentioned above, there is
evidence that oxidative stress induced by exercise activates
adaptive responses through signaling pathways regulated by the
thiol status (30,31).
The second category includes the 3 oxidative stress
markers (i.e., TBARS, CARB and TAC) that exhibited either a
decrease or increase among different athletes at phase 2 compared
to phase 1. The marked variation in the levels of these markers may
be explained by the high complexity of the regulation of redox
homeostasis in humans. Previous studies have reported that
different factors, such as genetic, physiological, biochemical and
dietary factors affect the final effects of oxidative stress
(27,41).
In conclusion, the findings of the present study
demonstrated that oxidative stress is induced in basketball players
at the beginning and end of a season due to excessive exercise. The
most important effect was the increase in the sORP value and TAC
and the decrease in GSH levels in the athletes. The decrease in GSH
levels occurs particularly when exercise induces muscle damage
(45). Basketball athletes are
subjected to muscle injury due to the high numbers of jumps and
sprints during a basketball game (46). Muscle-damaging exercise induces
inflammation which at low levels, in turn, helps the muscle
regeneration (41). However,
exercise-induced inflammation at high levels can cause health
problems and affect the performance of athletes (47). Adaptive responses are also induced
due to this oxidative stress as shown by an increase in TAC.
Moreover, the sORP marker has been shown to be effective for
monitoring the redox status in basketball athletes, as previously
demonstrated for other types of exercise (18). On the other hand, there was a great
variation in the oxidative damage to biological macromolecules
(e.g., protein oxidation, lipid peroxidation and CAT) between the
different athletes. From the above-mentioned findings, it can be
inferred that the optimal intervention approach with which (e.g.,
antioxidant supplementation) to reduce the detrimental effects of
exercise-induced oxidative stress on human health is the
individualized examination of the redox status in athletes using
different markers. This would allow the identification of athletes
affected by severe oxidative stress and inflammation, and they
would thus be administered the appropriate antioxidant
supplementation. On the other hand, athletes who present low levels
of exercise-induced oxidative stress and inflammation may not
require antioxidant intervention in order for the antioxidant
adaptive mechanisms to be activated and exert their beneficial
effects.
Our research group has performed the individualized
monitoring of exercise-induced oxidative stress in athletes for
several years. We believe that currently a crucial point regarding
exercise-induced oxidative stress is to determine the optimal
threshold of the oxidative stress level above which the appropriate
antioxidant supplementation should be used in order to help each
athlete improve his health status and performance.
Glossary
Abbreviations
Abbreviations:
|
CAT
|
catalase
|
|
EDTA
|
ethylenediaminetetraacetic acid
|
|
GSH
|
glutathione
|
|
H2O2
|
hydrogen peroxide
|
|
ROS
|
reactive oxygen species
|
|
sORP
|
static oxidation-reduction
potential
|
|
TAC
|
total antioxidant capacity
|
|
TBA
|
thiobarbituric acid
|
|
TBARS
|
thiobarbituric acid reactive
substances
|
|
TCA
|
trichloroacetic acid
|
References
|
1
|
Ciuti C, Marcello C, Macis A, Onnis E,
Solinas R, Lai C and Concu A: Improved aerobic power by detraining
in basketball players mainly trained for strength. Sports Med Train
Rehabil. 6:325–335. 1996. View Article : Google Scholar
|
|
2
|
Balčiūnas M, Stonkus S, Abrantes C and
Sampaio J: Long term effects of different training modalities on
power, speed, skill and anaerobic capacity in young male basketball
players. J Sports Sci Med. 5:163–170. 2006.PubMed/NCBI
|
|
3
|
Crisafulli A, Melis F, Tocco F, Laconi P,
Lai C and Concu A: External mechanical work versus oxidative energy
consumption ratio during a basketball field test. J Sports Med Phys
Fitness. 42:409–417. 2002.PubMed/NCBI
|
|
4
|
McInnes SE, Carlson JS, Jones CJ and
McKenna MJ: The physiological load imposed on basketball players
during competition. J Sports Sci. 13:387–397. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veskoukis AS, Nikolaidis MG, Kyparos A,
Kokkinos D, Nepka C, Barbanis S and Kouretas D: Effects of xanthine
oxidase inhibition on oxidative stress and swimming performance in
rats. Appl Physiol Nutr Metab. 33:1140–1154. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nikolaidis MG, Jamurtas AZ, Paschalis V,
Kostaropoulos IA, Kladi-Skandali A, Balamitsi V, Koutedakis Y and
Kouretas D: Exercise-induced oxidative stress in G6PD-deficient
individuals. Med Sci Sports Exerc. 38:1443–1450. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mylonas C and Kouretas D: Lipid
peroxidation and tissue damage. Vivo. 13:295–309. 1999.
|
|
8
|
Schneider BSP and Tiidus PM: Neutrophil
infiltration in exercise-injured skeletal muscle: how do we resolve
the controversy? Sports Med. 37:837–856. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nikolaidis MG, Kyparos A, Hadziioannou M,
Panou N, Samaras L, Jamurtas AZ and Kouretas D: Acute exercise
markedly increases blood oxidative stress in boys and girls. Appl
Physiol Nutr Metab. 32:197–205. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Meeus M, Nijs J, Hermans L, Goubert D and
Calders P: The role of mitochondrial dysfunctions due to oxidative
and nitrosative stress in the chronic pain or chronic fatigue
syndromes and fibromyalgia patients: peripheral and central
mechanisms as therapeutic targets? Expert Opin Ther Targets.
17:1081–1089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Phaneuf S and Leeuwenburgh C: Apoptosis
and exercise. Med Sci Sports Exerc. 33:393–396. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McClung JM, Deruisseau KC, Whidden MA, Van
Remmen H, Richardson A, Song W, Vrabas IS and Powers SK:
Overexpression of antioxidant enzymes in diaphragm muscle does not
alter contraction-induced fatigue or recovery. Exp Physiol.
95:222–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rodríguez-Alonso M, Fernández-García B,
Pérez-Landaluce J and Terrados N: Blood lactate and heart rate
during national and international women's basketball. J Sports Med
Phys Fitness. 43:432–436. 2003.PubMed/NCBI
|
|
14
|
Finaud J, Lac G and Filaire E: Oxidative
stress: relationship with exercise and training. Sports Med.
36:327–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schippinger G, Fankhauser F, Abuja PM,
Winklhofer-Roob BM, Nadlinger K, Halwachs-Baumann G and Wonisch W:
Competitive and seasonal oxidative stress in elite alpine ski
racers. Scand J Med Sci Sports. 19:206–212. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Spanidis Y, Goutzourelas N, Stagos D,
Kolyva AS, Gogos CA, Bar-Or D and Kouretas D: Assessment of
oxidative stress in septic and obese patients using markers of
oxidation-reduction potential. Vivo. 29:595–600. 2015.
|
|
17
|
Stagos D, Goutzourelas N, Ntontou AM,
Kafantaris I, Deli CK, Poulios A, Jamurtas AZ, Bar-Or D and
Kouretas D: Assessment of eccentric exercise-induced oxidative
stress using oxidation-reduction potential markers. Oxid Med Cell
Longev. 2015:2046152015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stagos D, Goutzourelas N, Bar-Or D,
Ntontou AM, Bella E, Becker AT, Statiri A, Kafantaris I and
Kouretas D: Application of a new oxidation-reduction potential
assessment method in strenuous exercise-induced oxidative stress.
Redox Rep. 20:154–162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Keles MS, Taysi S, Sen N, Aksoy H and
Akçay F: Effect of corticosteroid therapy on serum and CSF
malondialdehyde and antioxidant proteins in multiple sclerosis. Can
J Neurol Sci. 28:141–143. 2001.PubMed/NCBI
|
|
20
|
Lykkesfeldt J: Malondialdehyde as
biomarker of oxidative damage to lipids caused by smoking. Clin
Chim Acta. 380:50–58. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reddy YN, Murthy SV, Krishna DR and
Prabhakar MC: Role of free radicals and antioxidants in
tuberculosis patients. Indian J Tuberc. 51:213–218. 2004.
|
|
22
|
Patsoukis N, Zervoudakis G, Panagopoulos
NT, Georgiou CD, Angelatou F and Matsokis NA: Thiol redox state
(TRS) and oxidative stress in the mouse hippocampus after
pentylenetetrazol-induced epileptic seizure. Neurosci Lett.
357:83–86. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Janaszewska A and Bartosz G: Assay of
total antioxidant capacity: comparison of four methods as applied
to human blood plasma. Scand J Clin Lab Invest. 62:231–236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aebi H: Catalase in vitro. Methods
Enzymol. 105:121–126. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Margaritelis NV, Veskoukis AS, Paschalis
V, Vrabas IS, Dipla K, Zafeiridis A, Kyparos A and Nikolaidis MG:
Blood reflects tissue oxidative stress: a systematic review.
Biomarkers. 20:97–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Margaritelis NV, Kyparos A, Paschalis V,
Theodorou AA, Panayiotou G, Zafeiridis A, Dipla K, Nikolaidis MG
and Vrabas IS: Reductive stress after exercise: The issue of redox
individuality. Redox Biol. 2:520–528. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rankinen T and Bouchard C: Gene-physical
activity interactions: Overview of human studies. Obesity (Silver
Spring). 16(Suppl 3): S47–S50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zembron-Lacny A, Slowinska-Lisowska M and
Ziemba A: Integration of the thiol redox status with cytokine
response to physical training in professional basketball players.
Physiol Res. 59:239–245. 2010.PubMed/NCBI
|
|
29
|
Melikoglu MA, Kaldirimci M, Katkat D, Sen
I, Kaplan I and Senel K: The effect of regular long term training
on antioxidant enzymatic activities. J Sports Med Phys Fitness.
48:388–390. 2008.PubMed/NCBI
|
|
30
|
Zembron-Lacny A, Naczk M, Gajewski M,
Ostapiuk-Karolczuk J, Dziewiecka H, Kasperska A and Szyszka K:
Changes of muscle-derived cytokines in relation to thiol redox
status and reactive oxygen and nitrogen species. Physiol Res.
59:945–951. 2010.PubMed/NCBI
|
|
31
|
Ji LL, Gomez-Cabrera MC and Vina J:
Exercise and hormesis: activation of cellular antioxidant signaling
pathway. Ann N Y Acad Sci. 1067:425–435. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kerksick C and Willoughby D: The
antioxidant role of glutathione and N-acetyl-cysteine supplements
and exercise-induced oxidative stress. J Int Soc Sports Nutr.
2:38–44. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Steensberg A, van Hall G, Osada T,
Sacchetti M, Saltin B and Klarlund Pedersen B: Production of
interleukin-6 in contracting human skeletal muscles can account for
the exercise-induced increase in plasma interleukin-6. J Physiol.
529:237–242. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chatzinikolaou A, Draganidis D, Avloniti
A, Karipidis A, Jamurtas AZ, Skevaki CL, Tsoukas D, Sovatzidis A,
Theodorou A, Kambas A, et al: The microcycle of inflammation and
performance changes after a basketball match. J Sports Sci.
32:870–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wiggs MP: Can endurance exercise
preconditioning prevention disuse muscle atrophy? Front Physiol.
6:632015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oh-ishi S, Kizaki T, Nagasawa J, Izawa T,
Komabayashi T, Nagata N, Suzuki K, Taniguchi N and Ohno H: Effects
of endurance training on superoxide dismutase activity, content and
mRNA expression in rat muscle. Clin Exp Pharmacol Physiol.
24:326–332. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smuder AJ, Kavazis AN, Min K and Powers
SK: Exercise protects against doxorubicin-induced oxidative stress
and proteolysis in skeletal muscle. J Appl Physiol 1985.
110:935–942. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mangner N, Linke A, Oberbach A, Kullnick
Y, Gielen S, Sandri M, Hoellriegel R, Matsumoto Y, Schuler G and
Adams V: Exercise training prevents TNF-α induced loss of force in
the diaphragm of mice. PLoS One. 8:e522742013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fatouros IG, Jamurtas AZ, Villiotou V,
Pouliopoulou S, Fotinakis P, Taxildaris K and Deliconstantinos G:
Oxidative stress responses in older men during endurance training
and detraining. Med Sci Sports Exerc. 36:2065–2072. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wiecek M, Maciejczyk M, Szymura J and
Szygula Z: Changes in oxidative stress and acid-base balance in men
and women following maximal-intensity physical exercise. Physiol
Res. 64:93–102. 2015.PubMed/NCBI
|
|
41
|
Bloomer RJ and Fisher-Wellman KH: Blood
oxidative stress biomarkers: influence of sex, exercise training
status, and dietary intake. Gend Med. 5:218–228. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gul M, Demircan B, Taysi S, Oztasan N,
Gumustekin K, Siktar E, Polat MF, Akar S, Akcay F and Dane S:
Effects of endurance training and acute exhaustive exercise on
antioxidant defense mechanisms in rat heart. Comp Biochem Physiol A
Mol Integr Physiol. 143:239–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Powers SK and Jackson MJ: Exercise-induced
oxidative stress: Cellular mechanisms and impact on muscle force
production. Physiol Rev. 88:1243–1276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Rahal A, Kumar A, Singh V, Yadav B, Tiwari
R, Chakraborty S and Dhama K: Oxidative stress, prooxidants, and
antioxidants: the interplay. BioMed Res Int. 2014:7612642014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nikolaidis MG, Jamurtas AZ, Paschalis V,
Fatouros IG, Koutedakis Y and Kouretas D: The effect of
muscle-damaging exercise on blood and skeletal muscle oxidative
stress: magnitude and time-course considerations. Sports Med.
38:579–606. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pliauga V, Kamandulis S, Dargevičiūtė G,
Jaszczanin J, Klizienė I, Stanislovaitienė J and Stanislovaitis A:
The effect of a simulated basketball game on players' sprint and
jump performance, temperature and muscle damage. J Hum Kinet.
46:167–175. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pyne DB: Exercise-induced muscle damage
and inflammation: a review. Aust J Sci Med Sport. 26:49–58.
1994.PubMed/NCBI
|