|
1
|
Orimo H, Nakamura T, Hosoi T, Iki M,
Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, et al:
Japanese 2011 guidelines for prevention and treatment of
osteoporosis - executive summary. Arch Osteoporos. 7:3–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown JP and Josse RG: Scientific Advisory
Council of the Osteoporosis Society of Canada: 2002 clinical
practice guidelines for the diagnosis and management of
osteoporosis in Canada. CMAJ. 167(10 Suppl): S1–S34.
2002.PubMed/NCBI
|
|
3
|
Kanis JA, Burlet N, Cooper C, Delmas PD,
Reginster JY, Borgstrom F and Rizzoli R: European Society for
Clinical and Economic Aspects of Osteoporosis and Osteoarthritis
(ESCEO): European guidance for the diagnosis and management of
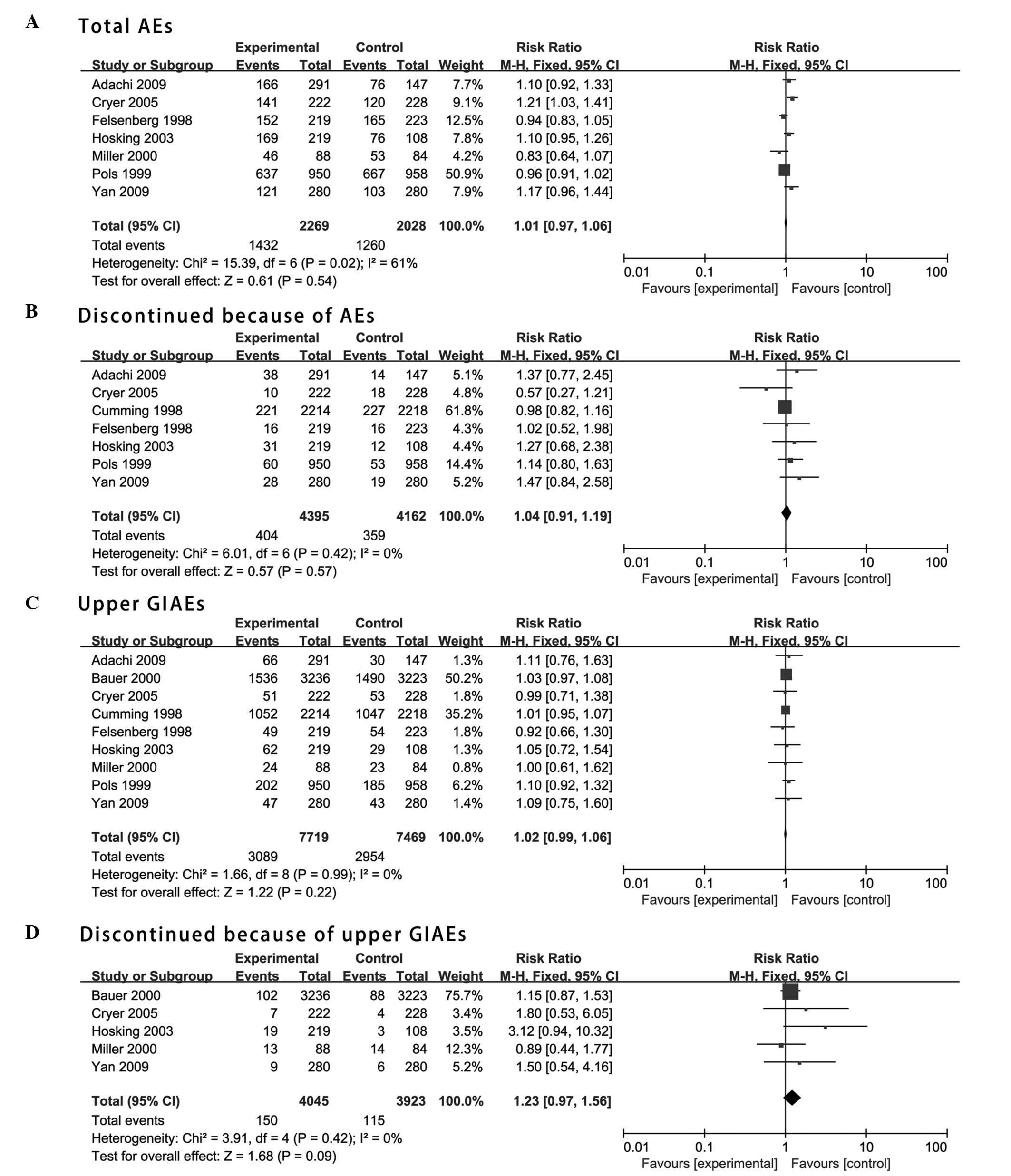
osteoporosis in postmenopausal women. Osteoporos Int. 19:399–428.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hodgson SF, Watts NB, Bilezikian JP,
Clarke BL, Gray TK, Harris DW, Johnston CC Jr, Kleerekoper M,
Lindsay R, Luckey MM, et al: AACE Osteoporosis Task Force: American
Association of Clinical Endocrinologists medical guidelines for
clinical practice for the prevention and treatment of
postmenopausal osteoporosis: 2001 edition, with selected updates
for 2003. Endocr Pract. 9:544–564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Body JJ, Bergmann P, Boonen S, Boutsen Y,
Devogelaer JP, Goemaere S, Kaufman JM, Rozenberg S and Reginster
JY: Evidence-based guidelines for the pharmacological treatment of
postmenopausal osteoporosis: A consensus document by the Belgian
Bone Club. Osteoporos Int. 21:1657–1680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qaseem A, Snow V, Shekelle P, Hopkins R
Jr, Forciea MA and Owens DK: Clinical Efficacy Assessment
Subcommittee of the American College of Physicians: Pharmacologic
treatment of low bone density or osteoporosis to prevent fractures:
A clinical practice guideline from the American College of
Physicians. Ann Intern Med. 149:404–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murad MH, Drake MT, Mullan RJ, Mauck KF,
Stuart LM, Lane MA, Elnour Abu NO, Erwin PJ, Hazem A, Puhan MA, et
al: Clinical review. Comparative effectiveness of drug treatments
to prevent fragility fractures: A systematic review and network
meta-analysis. J Clin Endocrinol Metab. 97:1871–1880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wells GA, Cranney A, Peterson J, Boucher
M, Shea B, Robinson V, Coyle D and Tugwell P: Alendronate for the
primary and secondary prevention of osteoporotic fractures in
postmenopausal women. Cochrane Database Syst Rev.
23:CD0011552008.
|
|
9
|
Mackey DC, Black DM, Bauer DC, McCloskey
EV, Eastell R, Mesenbrink P, Thompson JR and Cummings SR: Effects
of antiresorptive treatment on nonvertebral fracture outcomes. J
Bone Miner Res. 26:2411–2418. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bobba RS, Beattie K, Parkinson B, Kumbhare
D and Adachi JD: Tolerability of different dosing regimens of
bisphosphonates for the treatment of osteoporosis and malignant
bone disease. Drug Saf. 29:1133–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strampel W, Emkey R and Civitelli R:
Safety considerations with bisphosphonates for the treatment of
osteoporosis. Drug Saf. 30:755–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cadarette SM, Katz JN, Brookhart MA,
Stürmer T, Stedman MR, Levin R and Solomon DH: Comparative
gastrointestinal safety of weekly oral bisphosphonates. Osteoporos
Int. 20:1735–1747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller R, Bolognese M, Worley K, Sollis A
and Sheer R: Incidence of gastrointestinal events among
bisphosphonate patients in an observational setting. Am J Manag
Care. 10:S207–S215. 2004.
|
|
14
|
Kane S, Borisov N and Brixner D:
Pharmacoeconomic evaluation of gastrointestinal tract events during
treatment with risedronate or alendronate: A retrospective cohort
study. Am J Manag Care. 10:S216–S226. 2004.
|
|
15
|
Ste-Marie LG, Brown JP, Beary JF, Matzkin
E, Darbie LM, Burgio DE and Racewicz AJ: Comparison of the effects
of once-monthly versus once-daily risedronate in postmenopausal
osteoporosis: A phase II, 6-month, multicenter, randomized,
double-blind, active-controlled, dose-ranging study. Clin Ther.
31:272–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacLean C, Newberry S, Maglione M, McMahon
M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A,
McNamara M, et al: Systematic review: Comparative effectiveness of
treatments to prevent fractures in men and women with low bone
density or osteoporosis. Ann Intern Med. 148:197–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landfeldt E, Lang A, Robbins S and Ström
O: Gastrointestinal tolerability and patterns of switching in
patients treated for primary osteoporosis: The Swedish Adherence
Register Analysis (SARA). Calcif Tissue Int. 89:234–245. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reid IR: Osteoporosis treatment: Focus on
safety. Eur J Intern Med. 24:691–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fleisch H: Bisphosphonates in
osteoporosis. Eur Spine J. 12(Suppl 2): S142–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh YH, Yoon C and Park SM: Bisphosphonate
use and gastrointestinal tract cancer risk: Meta-analysis of
observational studies. World J Gastroenterol. 18:5779–5788. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rossini M, Bianchi G, Di Munno O, Giannini
S, Minisola S, Sinigaglia L and Adami S: Treatment of Osteoporosis
in clinical Practice (TOP) Study Group: Determinants of adherence
to osteoporosis treatment in clinical practice. Osteoporos Int.
17:914–921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiraki M, Yamazaki Y, Kuroda T, Tanaka S
and Miyata K: Serum level of pepsinogen significantly associated
with gastric distress induced by amino-bisphosphonates. Osteoporos
Int. 22:1717–1723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papaioannou A, Kennedy CC, Dolovich L, Lau
E and Adachi JD: Patient adherence to osteoporosis medications.
Problems, consequences and management strategies. Drugs Aging.
24:37–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tosteson AN, Grove MR, Hammond CS, Moncur
MM, Ray GT, Hebert GM, Pressman AR and Ettinger B: Early
discontinuation of treatment for osteoporosis. Am J Med.
115:209–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. PLoS Med.
6:e10001002009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.0.1. The Cochrane
Collaboration. 2008.
|
|
28
|
Adachi JD, Faraawi RY, O'Mahony MF, Nayar
A, Massaad R, Evans JK and Yacik C: Upper gastrointestinal
tolerability of alendronate sodium monohydrate 10 mg once daily in
postmenopausal women: A 12-week, randomized, double-blind,
placebo-controlled, exploratory study. Clin Ther. 31:1747–1753.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan Y, Wang W, Zhu H, Li M, Liu J, Luo B,
Xie H, Zhang G and Li F: The efficacy and tolerability of
once-weekly alendronate 70 mg on bone mineral density and bone
turnover markers in postmenopausal Chinese women with osteoporosis.
J Bone Miner Metab. 27:471–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cryer B, Binkley N, Simonelli C, Lewiecki
EM, Lanza F, Chen E, Petruschke RA, Mullen C and de Papp AE: A
randomized, placebo-controlled, 6-month study of once-weekly
alendronate oral solution for postmenopausal osteoporosis. Am J
Geriatr Pharmacother. 3:127–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hosking D, Adami S, Felsenberg D, Andia
JC, Välimäki M, Benhamou L, Reginster JY, Yacik C, Rybak-Feglin A,
Petruschke RA, et al: Comparison of change in bone resorption and
bone mineral density with once-weekly alendronate and daily
risedronate: A randomised, placebo-controlled study. Curr Med Res
Opin. 19:383–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miller PD, Woodson G, Licata AA, Ettinger
MP, Mako B, Smith ME, Wang L, Yates SJ, Melton ME and Palmisano JJ:
Rechallenge of patients who had discontinued alendronate therapy
because of upper gastrointestinal symptoms. Clin Ther.
22:1433–1442. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bauer DC, Black D, Ensrud K, Thompson D,
Hochberg M, Nevitt M, Musliner T and Freedholm D: Upper
gastrointestinal tract safety profile of alendronate: The fracture
intervention trial. Arch Intern Med. 160:517–525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pols HA, Felsenberg D, Hanley DA, Stepán
J, Muñoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K,
Yates AJ and Stych B: Multinational, placebo-controlled, randomized
trial of the effects of alendronate on bone density and fracture
risk in postmenopausal women with low bone mass: Results of the
FOSIT study. Fosamax International Trial Study Group. Osteoporos
Int. 9:461–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Felsenberg D, Alenfeld F, Bock O,
Hammermeister C and Gowan W: Placebo-controlled multicenter study
of oral alendronate in postmenopausal osteoporotic women.
Maturitas. 31:35–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cummings SR, Black DM, Thompson DE,
Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R,
Rubin SM, Scott JC, et al: Effect of alendronate on risk of
fracture in women with low bone density but without vertebral
fractures: Results from the Fracture Intervention Trial. JAMA.
280:2077–2082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liberman UA, Weiss SR, Bröll J, Minne HW,
Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J and
Favus M: Effect of oral alendronate on bone mineral density and the
incidence of fractures in postmenopausal osteoporosis. The
Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J
Med. 333:1437–1443. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tucci JR, Tonino RP, Emkey RD, Peverly CA,
Kher U and Santora AC II: Effect of three years of oral alendronate
treatment in postmenopausal women with osteoporosis. Am J Med.
101:488–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Devogelaer JP, Broll H, Correa-Rotter R,
Cumming DC, De Deuxchaisnes CN, Geusens P, Hosking D, Jaeger P,
Kaufman JM, Leite M, et al: Oral alendronate induces progressive
increases in bone mass of the spine, hip, and total body over 3
years in postmenopausal women with osteoporosis. Bone. 18:141–150.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landfeldt E, Ström O, Robbins S and
Borgström F: Adherence to treatment of primary osteoporosis and its
association to fractures - the Swedish Adherence Register Analysis
(SARA). Osteoporos Int. 23:433–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Taggart H, Bolognese MA, Lindsay R,
Ettinger MP, Mulder H, Josse RG, Roberts A, Zippel H, Adami S,
Ernst TF and Stevens KP: Upper gastrointestinal tract safety of
risedronate: A pooled analysis of 9 clinical trials. Mayo Clin
Proc. 77:262–270. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hadji P, Gamerdinger D, Spieler W, Kann
PH, Loeffler H, Articus K, Möricke R and Ziller V: Rapid Onset and
Sustained Efficacy (ROSE) study: Results of a randomised,
multicentre trial comparing the effect of zoledronic acid or
alendronate on bone metabolism in postmenopausal women with low
bone mass. Osteoporos Int. 23:625–633. 2012. View Article : Google Scholar : PubMed/NCBI
|