Introduction
Osteoporosis presents a significant public health
challenge, which contributes a substantial cost economically and in
terms of morbidity and mortality. The disease is characterized by
low bone mineral density and degeneration of the bone
microarchitecture, which increases bone brittleness and fracture
risk. Four key mechanisms appear to be crucially involved in the
pathogenesis of this condition: i) Inhibition of osteoclast
recruitment; ii) inhibition of osteoclastic adhesion; iii)
shortening of the life span of osteoclasts due to earlier
apoptosis; and iv) the inhibition of osteoclast activity.
Bisphosphonates are the most widely available treatments for
osteoporosis in postmenopausal women. Among these, alendronate
(Aln), which has been used extensively and has the longest history
in clinical practice, is recognized as a first-line drug for the
treatment of OP (1–6). Previous studies have demonstrated that
Aln is able to effectively reduce the risk of vertebral,
non-vertebral, hip and wrist fractures (7–9). The
safety and tolerability of Aln has previously been investigated in
various randomized controlled trials (RCTs) and retrospective
studies (10–18), and the majority of these have
reported similar side effects, including gastrointestinal tract
adverse events (GIAEs), for the Aln-treated and placebo-treated
groups. It has been reported that oral administration of
bisphosphonates, particularly those containing a nitrogen atom, may
be accompanied by digestive tract disturbances (19). In addition, other bisphosphonates, as
well as Aln, have been associated with GIAEs, which may be linked
to reduced compliance (20–23). These findings suggest that Aln may
cause GI tract disorders. Furthermore, oesophageal- and
gastric-associated side effects are among the most common reasons
for terminating bisphosphonate therapy (24).
The present study conducted a meta-analysis of
randomized, placebo-controlled trials abstracted from databases, in
order to investigate the effects of Aln treatment on the risk of
GIAEs in postmenopausal women with OP.
Methods
Search strategy
The present study adhered to the Preferred Reporting
Items for Systematic Reviews and Meta-Analysis statement guidelines
for the meta-analysis of randomized controlled trials (25). A literature search for the purpose of
identifying RCTs was performed. In order to identify eligible
studies, the following databases were searched, with a date limit
of December 30th, 2014: Medical Literature Analysis and
Retrieval System Online, Medline (http://www.ncbi.nlm.nih.gov/pubmed), Embase
(http://www.embase.com), the Web of Science
(http://www.thomsonscientific.com.cn/productsservices/webofscience/)
and the Cochrane Central Register of Controlled Trials (http://www.cochrane.org). The search terms used were
as follows: ‘Osteoporosis’, ‘alendronate’ and ‘gastrointestinal’.
The search was limited to English-language publications and human
trials.
Study selection
Studies were included in the present meta-analysis
if they met the following criteria: i) They were randomized,
double-blind, placebo-controlled trials analyzed by
intention-to-treat (ITT); ii) the mean age of the trial
participants was at a >50 years old baseline; iii) the study
compared the safety or tolerability profile of Aln versus a placebo
for the treatment of low bone mineral density or postmenopausal
women with OP; and iv) the trial was >2 months. The exclusion
criteria were as follows: i) The study included men or lasted for
<2 months; ii) the study did not investigate upper GIAEs as an
outcome; iii) duplicate publications; and iv) only the abstract was
available.
Data abstraction
Data was tabulated by two independent investigators.
A double-check procedure was performed in order to ensure the
accuracy of the extracted data. The following information was
extracted from each study: First author, publishing year, study
design, patient number, treatment strategies, and outcomes.
Methodological quality of the studies was assessed using Jadad
scoring (26), in which studies were
scored from 0–5, where a score of 0 corresponded to the lowest
quality and a score of 5 represented the highest quality.
Statistical analysis
Analyses were conducted using the RevMan 5.3
software (The Nordic Cochrane Centre, The Cochrane Collaboration,
Copenhagen, Denmark). The safety profile of Aln was evaluated on
the basis of the total number of reported adverse events (AEs), AEs
resulting in discontinued Aln treatment, upper GIAEs, and GIAEs
resulting in discontinued Aln treatment.
The Mantel-Haenszel method was used to calculate
risk ratios (RRs), and their 95% confidence intervals (CI) were
determined using either the fixed or random effects model,
depending on the amount of observed heterogeneity. For
heterogeneous outcomes (I2>50%), a random-effects
model meta-analysis was conducted, whereas, for homogeneous
outcomes, a fixed-effects model meta-analysis was conducted. The
effects of heterogeneity were quantified as follows: I2
= 100% × (Q-df)/Q, where I2 corresponded to the degree
of inconsistency between the studies, and determined whether the
total percentage of variation across the studies was due to
heterogeneity or chance. I2 ranged between 0 and 100%,
where I2 values of 25, 50 and 75% indicated low,
moderate, and high estimates, respectively (27).
In order to detect publication bias, a sensitivity
analysis was conducted using the trim and fill method or subgroup
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Identification and selection of
studies
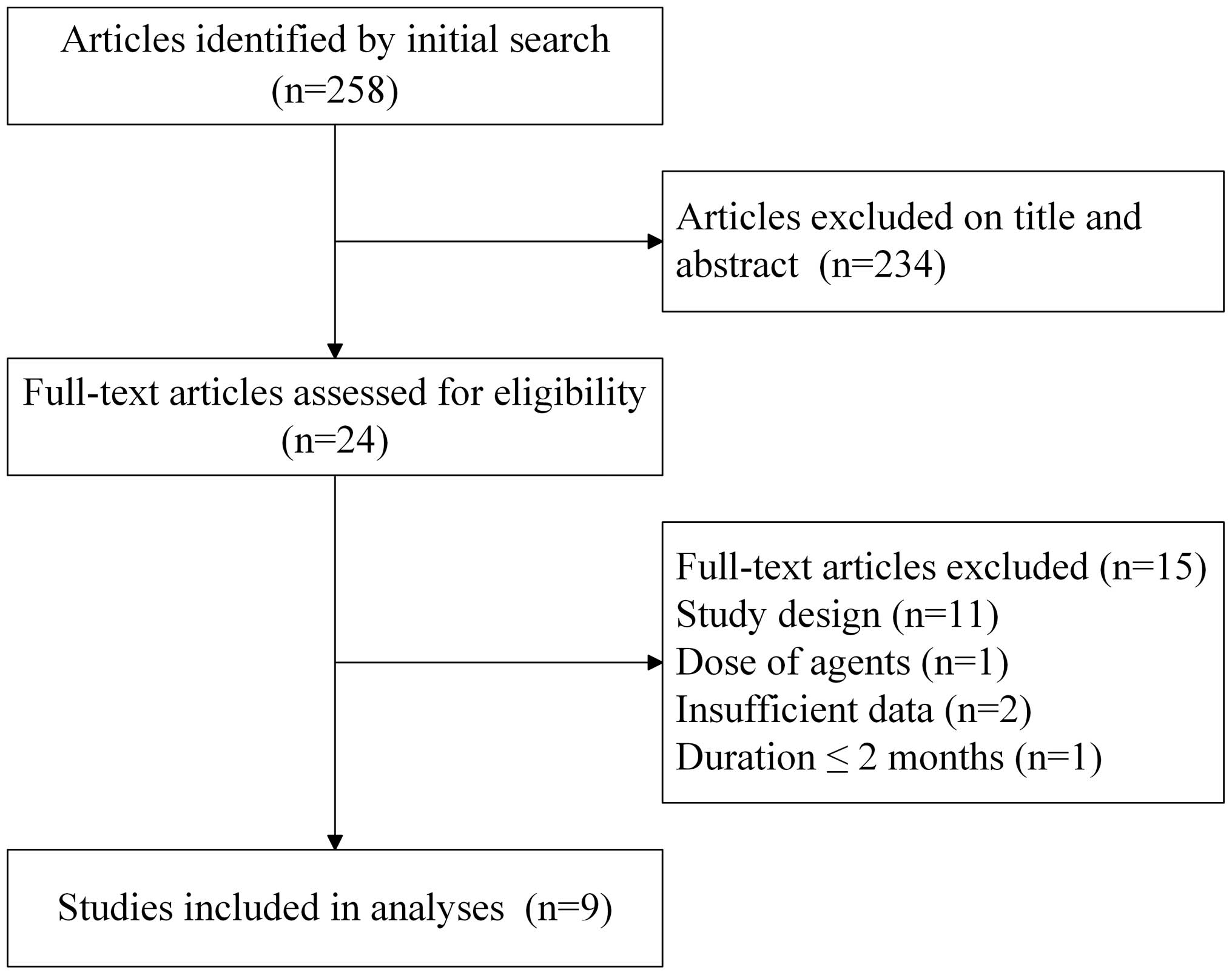
A total of 258 studies were retrieved in the initial
search of electronic databases, of which 234 were excluded on the
basis of their title and/or abstract. Of the remaining 24 studies,
a total of nine RCTs were selected for inclusion in the present
meta-analysis following full text reviews (28–36). A
flow chart of the study selection process is presented in Fig. 1.
Study characteristics
The characteristics of the included studies are
presented in Table I. A total of
15,192 participants (7,721 in the Aln group and 7,471 in the
placebo group) were included in the present meta-analysis; all of
which were postmenopausal women (mean age range, 62.8–69.6 years),
which had previously been diagnosed with a low bone density. All
the studies were randomized, double-blind, placebo-controlled
trials. Two of the studies (33,36)
initially administered 5 mg Aln daily; however this was later
increased to 10 mg daily, as the AEs associated with a 5 mg Aln
sodium dose have been shown to be markedly similar to those
associated with the 10 mg Aln sodium dose (37–39). As
the commercial dose of Aln is 10 mg, the analyses only used the
data from patients who had been treated with a 10 mg Aln dose.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
| First author
Year/(ref) | Study design | Sample size
(Aln/PBO) | Aln group age
(mean±SD) | PBO group age
(mean±SD) | Treatment | Duration
(months) | Use of aspirin or
NSAIDs [Aln(%)/PBO(%)] | Loss to follow up
[Aln(%)/PBO(%)] |
|---|
| Adachi
2009/(27) | Multicenter,
randomized, double-blind, placebo-controlled | 291/147 | 65.4±10.5 | 65.7±9.9 | 70 mg Aln/week | 3 | 27.8/27.9 | 18.6/11.6 |
| Yan 2009/(28) | Multicenter,
randomized, double-blind, placebo-controlled | 280/280 | 65.2±6.5 | 64.7±5.9 | 70 mg Aln/week | 12 | N/A | 18.9/15.0 |
| Cryer
2005/(29) | Randomized,
double-blind, placebo-controlled | 224/230 | 64.6±10.0 | 65.8±9.9 | 70 mg Aln/week | 6 | 43.8/44.8 | 13.8/13.5 |
| Hosking
2003/(30) | Multicenter,
randomized, double-blind, placebo-controlled | 219/108 | 69.2±6.6 | 69.6±7.0 | 70 mg Aln/week for
12 months | 12 | N/A | 21.5/17.6 |
| Miller
2000/(31) | Multicenter,
randomized, double-blind, placebo-controlled | 88/84 | 67.0±11.0 | 67.1±10.1 | 70 mg Aln/week | 2 | 33.0/29.8 | No |
| Bauer
2000/(32) | Randomized,
double-blind, placebo-controlled | 3236/3223 | 68.6±6.2 | 68.7±6.1 | 5 mg Aln/day; 2
years later 10 mg/day | 45 | 88.4/87.5 | No |
| Pols 1999/(33) | Randomized,
double-blind, placebo-controlled | 950/958 | 62.8±7.5 | 62.8±7.4 | 70 mg Aln/week | 12 | N/A | 8.9/13.2 |
| Felsenberg
1998/(34) | Randomized,
triple-blind, parallel group, placebo-controlled | 219/223 | 64.1±6.7 | 63.5±7.5 | 70 mg Aln/week | 12 | N/A | 15.1/14.8 |
| Cumming
1998/(35) | Randomized,
double-blind, placebo-controlled | 2214/2218 | 67.6±6.2 | 67.7±6.1 | 5 mg Aln/day; 2
years later 10 mg/day | 50 | N/A | No |
Four studies (28,30,32,33)
administered aspirin or a nonsteroidal anti-inflammatory drug
(NSAID), to the two groups. In addition, four studies conducted a 1
year follow-up (29,31,34,35),
whereas two studies had a follow-up at >1 year (33,36), and
the remaining three studies had a follow-up at <1 year. In
addition, four of the included studies (32–35)
lacked appropriately described randomization, and three studies
(32,33,36)
lacked a description of drop-outs. All the studies claimed to apply
ITT analysis. The level of evidence for each article was scored
from 3 to 5, according to the Jadad quality score (Table II).
 | Table II.Quality assessment of the included
studies (Jadad score). |
Table II.
Quality assessment of the included
studies (Jadad score).
| First author
(ref) | Random sequence
generation | Appropriate
randomization | Blinding of
participant of personnel | Blinding of outcome
assessors | Withdrawals and
dropouts | Sum (Jadad
score) | miTT |
|---|
| Adachi (27) | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
| Yan (28) | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
| Cryer (29) | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
| Miller (31) | 1 | 0 | 1 | 1 | 0 | 3 | Yes |
| Bauer (32) | 1 | 0 | 1 | 1 | 1 | 4 | Yes |
| Felsenberg
(34) | 1 | 0 | 1 | 1 | 1 | 4 | Yes |
| Cumming (35) | 1 | 1 | 1 | 1 | 0 | 4 | Yes |
| Pols (33) | 1 | 0 | 1 | 1 | 1 | 4 | Yes |
| Hosking (30) | 1 | 1 | 1 | 1 | 1 | 5 | Yes |
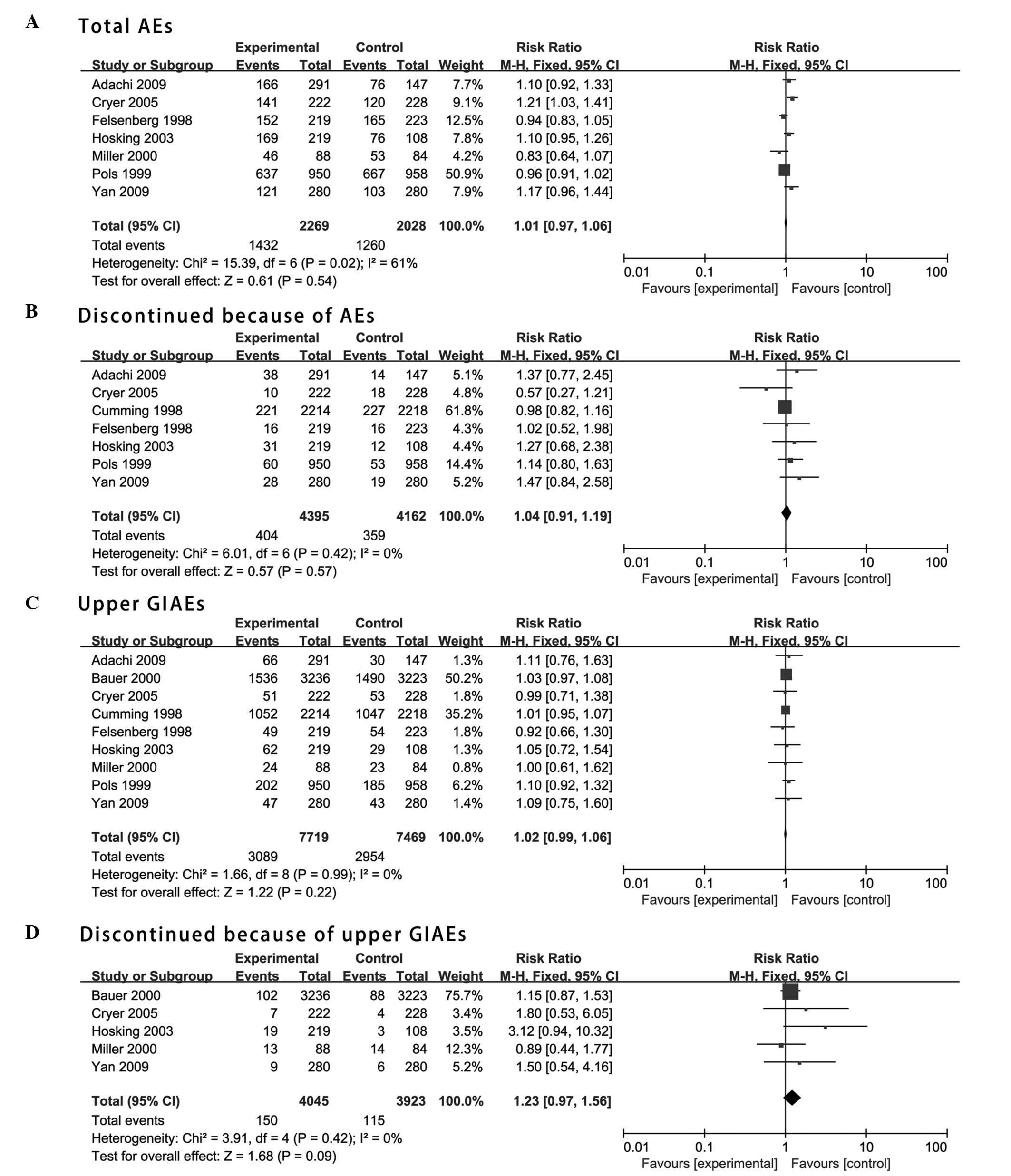
Total AEs
The risk ratio (95% CI) of AEs occurring in
postmenopausal women treated with Aln, as compared with the
placebo, was 1.01 (0.97–1.06), and this was not statistically
significant (P>0.05; Fig. 2A).
This outcome was attributed to heterogeneity among the included
studies. A sensitivity analysis was conducted by dividing the
studies into subgroups based on their duration; however, the
heterogeneity remained and the results were not statistically
significant. The risk ratio (95% CI) of discontinued Aln-treatment
due to the occurrence of AEs, as compared with the placebo, was
1.04 (0.91–1.19), and this was not statistically significant
(P>0.05; Fig. 2B). This outcome
could not be attributed to heterogeneity among the studies. The
sensitivity analysis demonstrated that this outcome could not be
altered by omitting any single trial.
Upper GI AEs
The risk ratio (95% CI) of upper GIAEs occurring in
postmenopausal women treated with Aln, as compared with the
placebo, was 1.02 (0.99–1.06), and this was not statistically
significant (P>0.05; Fig. 2C).
The heterogeneity among all studies was insignificant. The
sensitivity analysis demonstrated that the overall effect could not
be altered by omitting any single trial. The risk ratio (95% CI) of
the Aln-treatment of postmenopausal women being discontinued due to
the occurrence of upper GIAEs, as compared with the placebo, was
1.23 (0.97–1.56), and this was not statistically significant
(P>0.05; Fig. 2D). The
heterogeneity among all studies was insignificant. The sensitivity
analysis demonstrated that this outcome could not be altered by
omitting any single trial.
Individual upper GIAEs
Individual upper GIAEs, including abdominal pain,
nausea, dyspepsia, acid regurgitation, vomiting, gastroesophageal
reflux and esophagitis, were the most common upper GIAEs reported
among the studies. No significant differences in the incidence of
individual GIAEs between the Aln-treated and placebo-treated groups
were observed (Table III). The
heterogeneity across the studies was insignificant. The sensitivity
analysis demonstrated that this outcome could not be altered by
omitting any single trial.
 | Table III.Patients with upper GIAEs. |
Table III.
Patients with upper GIAEs.
|
|
| No. (%) of
patients |
|
|
|---|
|
|
|
|
|
|
|---|
| Upper GIAE | No. of studies | Aln | PBO | RR (95% CI) | P-value |
|---|
| Abdominal pain | 7 | 910 (12.6) | 877 (12.2) | 1.04
(0.95–1.13) | 0.41 |
| Nausea | 6 | 445 (8.9) | 458 (9.2) | 0.97
(0.86–1.10) | 0.62 |
| Dyspepsia | 5 | 639 (13.4) | 674 (14.1) | 0.94
(0.86–1.04) | 0.25 |
| Acid
regurgitation | 5 | 467 (6.9) | 445 (6.6) | 1.05
(0.92–1.19) | 0.47 |
| Vomiting | 5 | 136 (2.8) | 130 (2.7) | 1.04
(0.82–1.32) | 0.72 |
| Gastroesophageal
reflux | 4 | 81 (1.8) | 88 (2.0) | 0.91
(0.68–1.23) | 0.55 |
| Esophagitis | 4 | 49 (0.8) | 32 (0.5) | 1.53
(0.98–2.38) | 0.06 |
Discussion
The present study conducted a meta-analysis of
results from RCTs in the literature, in order to investigate the
effects of Aln treatment on the risk of upper GIAEs in
postmenopausal women with OP. A total of 258 studies were
identified in the initial database search, of which nine RCTs met
the inclusion criteria of the present study.
Previous studies have not identified a causative
link between upper GIAEs and Aln treatment; however, the GI safety
profile of bisphosphonates has been a concern in clinical practice
(20–23). The present study enrolled a broader
patient population and had a longer duration, as compared with
previous trials. In addition, all the included studies were of a
good quality. To the best of our knowledge, the present study is
the first to evaluate upper GIAEs as a primary outcome; thus
suggesting that our results would be more robust.
The duration of the studies included in the present
meta-analysis ranged from 2 months to 4.2 years. Landfeldt et
al (40) previously demonstrated
that the incidence of upper GIAEs was inversely associated with the
duration of treatment with Aln. However, some patients have
demonstrated superior GI tolerability, whereas others have
discontinued therapy after a shorter period of time. Therefore, it
would be unreliable to only measure the incidence of upper GIAEs in
patients who have persisted with therapy for a specific duration.
In addition, if the analysis was restricted to patients who had
remained on treatment for a specific duration, it would not be
possible to generalize the results to the population of
interest.
The treatment of patients with aspirin or NSAIDs was
not included in the exclusion criteria (28,30,32,33), yet
the presence of active GI tract disease, the need for
anti-secretory therapy, and the use of aspirin or NSAIDs may have
increased the risk of experiencing an upper GIAE in both the
Aln-treated and placebo-treated groups (41). However, the effects of these risk
factors were similar among the treatment groups.
The incidence of AEs was greater in the patients in
the Aln-treated group, as compared with the placebo-treated group;
however, the difference in the safety profile of Aln was not
statistically significant between these groups. It should be noted
that heterogeneity existed among the studies. A sensitivity
analysis was conducted by dividing the studies into subgroups based
on the study duration. In particular, the studies were divided into
three subgroups: i) Duration <1 year; ii) duration = 1 year; and
iii) duration >1 year. However, heterogeneity was still
detected. Notably, the overall outcome could not be altered by
omitting a single study.
The results of the present meta-analysis are
consistent with those from previous studies (28–36),
which also observed no significant difference in the frequency of
upper GIAEs between placebo-treated and Aln-treated groups, and
demonstrated that the GI safety and tolerability profile of Aln
resembles the placebo (10,11). However, the present results
contradict a previous study that suggested that Aln treatment may
be associated with an increased risk of upper GIAEs in patients, as
compared with no treatment (17).
Furthermore, in the present study, the incidences of the primary
individual upper GIAEs, including abdominal pain, nausea,
dyspepsia, acid regurgitation, vomiting, gastroesophageal reflux
and esophagitis, were not significantly different between the
placebo- and Aln-treated groups. This is inconsistent with a
previous study that suggested that patients treated with Aln
developed specific GIAEs, including dyspepsia and upper abdominal
pain (42).
Notably, oral bisphosphonates have fairly complex
administration instructions (taken alone with 240 ml of water after
fasting overnight, and remaining upright for at least 30 min), and
poor compliance to these has previously been associated with an
increased risk of GIAEs (11). This
may explain why some studies have reported an increased risk of
upper GIAEs in patients treated with Aln, and suggests that
patients should strictly adhere to the treatment instructions when
taking Aln.
The present study has some limitations: Firstly, the
demographic was restricted to postmenopausal OP, and thus the
results may not be extrapolated to patients with other conditions;
secondly, although the database searches were extensive, we cannot
be entirely sure that all relevant articles were included; and
thirdly, the analysis was only based on published data.
In conclusion, the results from the present
meta-analysis suggested that daily treatment with 10 mg Aln sodium
was not associated with an increased incidence of GIAEs, thus
suggesting that Aln may be considered safe for the treatment of
postmenopausal women with OP.
Acknowledgements
Dr Manru Zhou, Dr Yayuan Zheng and Professor Yuyu
Liu were involved in the study selection, quality assessment of the
selected studies, data extraction and analysis, and the writing of
the manuscript. Professor Yuyu Liu takes responsibility for the
integrity of the data analysis and critical revision of article.
The present study was funded by grants from the National Natural
Science Foundation of China (grant no. 81102450) and the Guangdong
Province Science and Technology Plan (grant no. 2012B031800225) and
the Characteristic Innovation Project (Natural Science) of the
Education Department of Guangdong Province (grant no.
2014KTSCX084).
References
|
1
|
Orimo H, Nakamura T, Hosoi T, Iki M,
Uenishi K, Endo N, Ohta H, Shiraki M, Sugimoto T, Suzuki T, et al:
Japanese 2011 guidelines for prevention and treatment of
osteoporosis - executive summary. Arch Osteoporos. 7:3–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brown JP and Josse RG: Scientific Advisory
Council of the Osteoporosis Society of Canada: 2002 clinical
practice guidelines for the diagnosis and management of
osteoporosis in Canada. CMAJ. 167(10 Suppl): S1–S34.
2002.PubMed/NCBI
|
|
3
|
Kanis JA, Burlet N, Cooper C, Delmas PD,
Reginster JY, Borgstrom F and Rizzoli R: European Society for
Clinical and Economic Aspects of Osteoporosis and Osteoarthritis
(ESCEO): European guidance for the diagnosis and management of
osteoporosis in postmenopausal women. Osteoporos Int. 19:399–428.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hodgson SF, Watts NB, Bilezikian JP,
Clarke BL, Gray TK, Harris DW, Johnston CC Jr, Kleerekoper M,
Lindsay R, Luckey MM, et al: AACE Osteoporosis Task Force: American
Association of Clinical Endocrinologists medical guidelines for
clinical practice for the prevention and treatment of
postmenopausal osteoporosis: 2001 edition, with selected updates
for 2003. Endocr Pract. 9:544–564. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Body JJ, Bergmann P, Boonen S, Boutsen Y,
Devogelaer JP, Goemaere S, Kaufman JM, Rozenberg S and Reginster
JY: Evidence-based guidelines for the pharmacological treatment of
postmenopausal osteoporosis: A consensus document by the Belgian
Bone Club. Osteoporos Int. 21:1657–1680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qaseem A, Snow V, Shekelle P, Hopkins R
Jr, Forciea MA and Owens DK: Clinical Efficacy Assessment
Subcommittee of the American College of Physicians: Pharmacologic
treatment of low bone density or osteoporosis to prevent fractures:
A clinical practice guideline from the American College of
Physicians. Ann Intern Med. 149:404–415. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murad MH, Drake MT, Mullan RJ, Mauck KF,
Stuart LM, Lane MA, Elnour Abu NO, Erwin PJ, Hazem A, Puhan MA, et
al: Clinical review. Comparative effectiveness of drug treatments
to prevent fragility fractures: A systematic review and network
meta-analysis. J Clin Endocrinol Metab. 97:1871–1880. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wells GA, Cranney A, Peterson J, Boucher
M, Shea B, Robinson V, Coyle D and Tugwell P: Alendronate for the
primary and secondary prevention of osteoporotic fractures in
postmenopausal women. Cochrane Database Syst Rev.
23:CD0011552008.
|
|
9
|
Mackey DC, Black DM, Bauer DC, McCloskey
EV, Eastell R, Mesenbrink P, Thompson JR and Cummings SR: Effects
of antiresorptive treatment on nonvertebral fracture outcomes. J
Bone Miner Res. 26:2411–2418. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bobba RS, Beattie K, Parkinson B, Kumbhare
D and Adachi JD: Tolerability of different dosing regimens of
bisphosphonates for the treatment of osteoporosis and malignant
bone disease. Drug Saf. 29:1133–1152. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Strampel W, Emkey R and Civitelli R:
Safety considerations with bisphosphonates for the treatment of
osteoporosis. Drug Saf. 30:755–763. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cadarette SM, Katz JN, Brookhart MA,
Stürmer T, Stedman MR, Levin R and Solomon DH: Comparative
gastrointestinal safety of weekly oral bisphosphonates. Osteoporos
Int. 20:1735–1747. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miller R, Bolognese M, Worley K, Sollis A
and Sheer R: Incidence of gastrointestinal events among
bisphosphonate patients in an observational setting. Am J Manag
Care. 10:S207–S215. 2004.
|
|
14
|
Kane S, Borisov N and Brixner D:
Pharmacoeconomic evaluation of gastrointestinal tract events during
treatment with risedronate or alendronate: A retrospective cohort
study. Am J Manag Care. 10:S216–S226. 2004.
|
|
15
|
Ste-Marie LG, Brown JP, Beary JF, Matzkin
E, Darbie LM, Burgio DE and Racewicz AJ: Comparison of the effects
of once-monthly versus once-daily risedronate in postmenopausal
osteoporosis: A phase II, 6-month, multicenter, randomized,
double-blind, active-controlled, dose-ranging study. Clin Ther.
31:272–285. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacLean C, Newberry S, Maglione M, McMahon
M, Ranganath V, Suttorp M, Mojica W, Timmer M, Alexander A,
McNamara M, et al: Systematic review: Comparative effectiveness of
treatments to prevent fractures in men and women with low bone
density or osteoporosis. Ann Intern Med. 148:197–213. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Landfeldt E, Lang A, Robbins S and Ström
O: Gastrointestinal tolerability and patterns of switching in
patients treated for primary osteoporosis: The Swedish Adherence
Register Analysis (SARA). Calcif Tissue Int. 89:234–245. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Reid IR: Osteoporosis treatment: Focus on
safety. Eur J Intern Med. 24:691–697. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fleisch H: Bisphosphonates in
osteoporosis. Eur Spine J. 12(Suppl 2): S142–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oh YH, Yoon C and Park SM: Bisphosphonate
use and gastrointestinal tract cancer risk: Meta-analysis of
observational studies. World J Gastroenterol. 18:5779–5788. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rossini M, Bianchi G, Di Munno O, Giannini
S, Minisola S, Sinigaglia L and Adami S: Treatment of Osteoporosis
in clinical Practice (TOP) Study Group: Determinants of adherence
to osteoporosis treatment in clinical practice. Osteoporos Int.
17:914–921. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shiraki M, Yamazaki Y, Kuroda T, Tanaka S
and Miyata K: Serum level of pepsinogen significantly associated
with gastric distress induced by amino-bisphosphonates. Osteoporos
Int. 22:1717–1723. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Papaioannou A, Kennedy CC, Dolovich L, Lau
E and Adachi JD: Patient adherence to osteoporosis medications.
Problems, consequences and management strategies. Drugs Aging.
24:37–55. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tosteson AN, Grove MR, Hammond CS, Moncur
MM, Ray GT, Hebert GM, Pressman AR and Ettinger B: Early
discontinuation of treatment for osteoporosis. Am J Med.
115:209–216. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liberati A, Altman DG, Tetzlaff J, Mulrow
C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J
and Moher D: The PRISMA statement for reporting systematic reviews
and meta-analyses of studies that evaluate health care
interventions: Explanation and elaboration. PLoS Med.
6:e10001002009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Higgins JPT and Green S: Cochrane Handbook
for Systematic Reviews of Interventions Version 5.0.1. The Cochrane
Collaboration. 2008.
|
|
28
|
Adachi JD, Faraawi RY, O'Mahony MF, Nayar
A, Massaad R, Evans JK and Yacik C: Upper gastrointestinal
tolerability of alendronate sodium monohydrate 10 mg once daily in
postmenopausal women: A 12-week, randomized, double-blind,
placebo-controlled, exploratory study. Clin Ther. 31:1747–1753.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yan Y, Wang W, Zhu H, Li M, Liu J, Luo B,
Xie H, Zhang G and Li F: The efficacy and tolerability of
once-weekly alendronate 70 mg on bone mineral density and bone
turnover markers in postmenopausal Chinese women with osteoporosis.
J Bone Miner Metab. 27:471–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cryer B, Binkley N, Simonelli C, Lewiecki
EM, Lanza F, Chen E, Petruschke RA, Mullen C and de Papp AE: A
randomized, placebo-controlled, 6-month study of once-weekly
alendronate oral solution for postmenopausal osteoporosis. Am J
Geriatr Pharmacother. 3:127–136. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hosking D, Adami S, Felsenberg D, Andia
JC, Välimäki M, Benhamou L, Reginster JY, Yacik C, Rybak-Feglin A,
Petruschke RA, et al: Comparison of change in bone resorption and
bone mineral density with once-weekly alendronate and daily
risedronate: A randomised, placebo-controlled study. Curr Med Res
Opin. 19:383–394. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miller PD, Woodson G, Licata AA, Ettinger
MP, Mako B, Smith ME, Wang L, Yates SJ, Melton ME and Palmisano JJ:
Rechallenge of patients who had discontinued alendronate therapy
because of upper gastrointestinal symptoms. Clin Ther.
22:1433–1442. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bauer DC, Black D, Ensrud K, Thompson D,
Hochberg M, Nevitt M, Musliner T and Freedholm D: Upper
gastrointestinal tract safety profile of alendronate: The fracture
intervention trial. Arch Intern Med. 160:517–525. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pols HA, Felsenberg D, Hanley DA, Stepán
J, Muñoz-Torres M, Wilkin TJ, Qin-sheng G, Galich AM, Vandormael K,
Yates AJ and Stych B: Multinational, placebo-controlled, randomized
trial of the effects of alendronate on bone density and fracture
risk in postmenopausal women with low bone mass: Results of the
FOSIT study. Fosamax International Trial Study Group. Osteoporos
Int. 9:461–468. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Felsenberg D, Alenfeld F, Bock O,
Hammermeister C and Gowan W: Placebo-controlled multicenter study
of oral alendronate in postmenopausal osteoporotic women.
Maturitas. 31:35–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cummings SR, Black DM, Thompson DE,
Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R,
Rubin SM, Scott JC, et al: Effect of alendronate on risk of
fracture in women with low bone density but without vertebral
fractures: Results from the Fracture Intervention Trial. JAMA.
280:2077–2082. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liberman UA, Weiss SR, Bröll J, Minne HW,
Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J and
Favus M: Effect of oral alendronate on bone mineral density and the
incidence of fractures in postmenopausal osteoporosis. The
Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J
Med. 333:1437–1443. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Tucci JR, Tonino RP, Emkey RD, Peverly CA,
Kher U and Santora AC II: Effect of three years of oral alendronate
treatment in postmenopausal women with osteoporosis. Am J Med.
101:488–501. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Devogelaer JP, Broll H, Correa-Rotter R,
Cumming DC, De Deuxchaisnes CN, Geusens P, Hosking D, Jaeger P,
Kaufman JM, Leite M, et al: Oral alendronate induces progressive
increases in bone mass of the spine, hip, and total body over 3
years in postmenopausal women with osteoporosis. Bone. 18:141–150.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Landfeldt E, Ström O, Robbins S and
Borgström F: Adherence to treatment of primary osteoporosis and its
association to fractures - the Swedish Adherence Register Analysis
(SARA). Osteoporos Int. 23:433–443. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Taggart H, Bolognese MA, Lindsay R,
Ettinger MP, Mulder H, Josse RG, Roberts A, Zippel H, Adami S,
Ernst TF and Stevens KP: Upper gastrointestinal tract safety of
risedronate: A pooled analysis of 9 clinical trials. Mayo Clin
Proc. 77:262–270. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hadji P, Gamerdinger D, Spieler W, Kann
PH, Loeffler H, Articus K, Möricke R and Ziller V: Rapid Onset and
Sustained Efficacy (ROSE) study: Results of a randomised,
multicentre trial comparing the effect of zoledronic acid or
alendronate on bone metabolism in postmenopausal women with low
bone mass. Osteoporos Int. 23:625–633. 2012. View Article : Google Scholar : PubMed/NCBI
|