Introduction
Obesity is a metabolic syndrome caused by an
imbalance between energy intake and expenditure. It is associated
with a number of health problems, including hyperlipidemia,
hypertension, type 2 diabetes, coronary heart disease, cancer,
respiratory complications and osteoarthritis (1,2). The
degree of obesity is dependent on the amount of body fat and it has
been indicated that obesity is associated with various factors
(3,4). A high-fat diet (HFD) is one of the most
important environmental factors associated with obesity. Chronic
exposure to a HFD can affect the generation of and reaction to
meal-related signals that control food intake and metabolism
(5). When a diet with a high-fat
content is consumed on a regular basis, some of the fat remains in
the body and results in the accumulation of excessive body fat
(6). Visceral fat, which is formed
around the major organs, stores energy in the form of triglycerides
(TGs) and its accumulation has a pathophysiological role in the
development of metabolic syndromes including obesity,
hyperlipidemia and diabetes (7,8).
Anti-obesity drugs such as orlistat, sibutramine and
topiramate are currently used to treat obesity (9). However, these drugs have numerous
side-effects, including dry mouth, anorexia, insomnia and
gastrointestinal indisposition (10). Due to the adverse side-effects of
certain anti-obesity drugs, functional foods made from natural
plants are also being used to prevent and ameliorate obesity
(6). Deep sea water (DSW) generally
refers to sea water from a depth of >200 m (11). DSW has been the subject of many
studies due to its high content of inorganic nutrients such as
magnesium (Mg), calcium (Ca), potassium (K), zinc (Zn) and vanadium
(V), and has been indicated to be associated with prevention of
obesity (11–15). Sesame (Sesamum indicum L.),
which is an annual herbaceous plant, has long been an ingredient in
human foods (16). The leaves, seed
and oil from sesame plant are consumed locally as food products in
China, South Korea, Japan and many other countries (17). However, to the best of our knowledge
the biological functions of a combination of DSW and Sesamum
indicum leaf extract (SIE) in weight-reduction and its
regulatory effects on obesity-related metabolic diseases have not
been documented.
In the present study, the protective effects of a
combination of DSW and SIE were evaluated against HFD-induced
obesity and the underlying molecular mechanisms of the effect were
elucidated in the epididymal adipose tissues of ICR mice.
Materials and methods
Materials
DSW was obtained from Herb Valley Life Science
(Seoul, Korea); rabbit anti-mouse polyclonal phospho-adenosine
monophosphate-activated protein kinase (p-AMPKα; cat. no., 2531L;
dilution 1:2,000), rabbit anti-mouse polyclonal AMPKα (cat. no.,
2532S; dilution 1:2,000), rabbit anti-mouse polyclonal
phospho-acetyl-CoA carboxylase (p-ACC; cat. no., 3661L; dilution
1:2,000) and rabbit anti-mouse polyclonal ACC (cat. no., 3662;
dilution 1:2,000) antibodies were from Cell Signaling Technology
(Beverly, MA, USA); goat anti-mouse polyclonal immunoglobulin G
(IgG) actin antibody (cat. no. sc-1616; dilution, 1:1,000) was from
Santa Cruz Biotechnology (Dallas, TX, USA); and the HFD was
purchased from Research Diets Inc. (D12451; New Brunswick, NJ,
USA).
Preparation of SIE
Sesamum indicum leaves were purchased from
commercial sources and were dried in the shade for 1 week. An
extract was prepared by boiling the leaves in 70% ethanol for 5 h.
Following filtration and evaporation, the solution was evaporated
under vacuum to provide a powdered extract.
Animals
Four-week-old ICR seco were purchased from Orient
Bio Inc. (Seoul, Korea). The animals were housed for 1 week and fed
a standard diet under environmentally controlled conditions. The
mice were allowed to freely access drinking water and food under
constant room temperature (22±2°C) and humidity (50±10%) conditions
with an automatic 12-h light and 12-h dark cycle, and were cared
for and treated in accordance with the guidelines for the Care and
Use of Laboratory Animals (1996) (18). A tor mice were randomly
divided into three groups (6 mice per group): HFD control (HFC),
DSW and DSW + 125 mg/kg SIE (DSS). Mice in the HFC group had free
access to drinking water and the mice in the DSW and DSS groups had
free access to DSW instead. The mice in the DSS group were treated
with SIE by oral administration once per day for 8 weeks starting 3
days prior to the provision of the HFD. The mice in all groups were
allowed to freely access the HFD. The composition of the
experimental diet is shown in Table
I; it is a HFD in which 45% of the calories are provided by
fat. The body weight gain was measured once every week.
 | Table I.Composition of the experimental
diet. |
Table I.
Composition of the experimental
diet.
| A, Nutritional
profile |
|
|
|---|
|
|
|
|---|
| Component | g % | kcal % |
|---|
| Protein | 24 | 20 |
| Carbohydrate | 41 | 35 |
| Fat | 24 | 45 |
|
| B, Composition |
|
| Ingredient | g | kcal |
|
| Casein (from
milk) | 200 | 800 |
| Corn starch | 155.0 | 620 |
| Sucrose | 50 | 200 |
| Dextrose | 132 | 528 |
| Cellulose | 50 | 0 |
| Soybean oil | 25 | 225 |
| Corn oil | 175 | 1,575 |
| Mineral mixture | 35 | 0 |
| Vitamin mixture | 10 | 40 |
| TBHQ | 0.014 | 0 |
| L-Cystine | 3 | 12 |
| Choline
bitartrate | 2.5 | 0 |
| Total | 837.6 | 4,000 |
Determination of serum parameters
After 8 weeks of treatment, animals were fasted for
12 h and then blood samples were collected and centrifuged at 3,000
× g for 15 min at 4°C. The levels of serum glucose, insulin, TGs
and leptin in the serum were measured. Serum glucose concentrations
were determined using a commercial kit based on the glucose oxidase
method (Asan Pharmaceutical Co., Seoul, Korea). Serum insulin
concentrations were determined using a mouse insulin enzyme
immunoassay kit (Shibayagi, Gunma, Japan). Serum TG concentrations
were determined using a commercially available kit (Asan
Pharmaceutical Co.). Leptin levels were measured using a mouse
leptin enzyme immunoassay kit (Linco Research, Inc., St. Charles,
MO, USA). Homeostasis model assessment of insulin resistance
(HOMA-IR) values were calculated using the following formula:
Insulin (U/ml) × glucose (mM)/22.5.
Histological analysis
Epididymal white, retroperitoneal white and scapular
brown adipose tissues were removed and fixed in 10% neutral
buffered formalin. The tissues were subsequently embedded in
paraffin and sectioned with 5-µm thickness (Leica RM2245; Leica
Microsystems GmbH, Wetzlar, Germany), and then stained with
hematoxylin and eosin (H&E) for microscopic assessment (Olympus
BX51; Olympus Corporation, Tokyo, Japan). The mean adipocyte cell
number percentage of epididymal white and retroperitoneal white
adipose tissues were measured from the images obtained from H&E
staining using Image-Pro® Plus version 7.0 software (Media
Cybernetics, Inc., Bethesda, MD, USA).
Western blot analysis
At the end of the treatment, the mice were fasted
for 12 h, anaesthetized with diethyl ether and blood samples were
collected by cardiac puncture. Following blood collection, the mice
were sacrificed with diethyl ether. Immediately after sacrifice,
epididymal white adipose tissues were removed and instantly soaked
in liquid nitrogen for storage at −70°C. Protein extracts were
prepared using a protein extraction kit (Intron Biotechnology Inc.,
Seoul, Korea). Lysates (50 µg) were electroblotted onto a
nitrocellulose membrane following separation by 8% SDS
polyacrylamide gel electrophoresis. Blotted membranes were
incubated for 1 h with blocking solution (Tris-buffered
saline/Tween 20, TBST) containing 5% skimmed milk (w/v) at room
temperature, followed by incubation overnight at 4°C with a 1:2,000
dilution of primary antibody against AMPK, p-AMPK, ACC or p-ACC.
Membranes were washed four times with 0.1% TBST and incubated with
a 1:3,000 dilution of horseradish peroxidase-conjugated goat
anti-rabbit or donkey anti-rabbit IgG secondary antibody (cat. no.,
sc-2313) for 1 h at room temperature. Membranes were washed four
times in TBST and then developed by electrochemiluminescence
(Amersham, GE Healthcare, Uppsala, Sweden). Image J 1.49 software
(http://rsb.info.nih.gov/ij/download.html; National
Institute of Health) was used for the quantification of the results
of western blotting.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total mRNA was isolated from the epididymal adipose
tissue of mice from each group using an Easy-Blue kit (Intron
Biotechnology Inc.) according to the manufacturer's instructions.
From each sample, total RNA (10 µg) was reverse transcribed into
cDNA using the Moloney murine leukemia virus transcriptase and
Oligo(dT)15 primers (Promega Corporation, Madison, WI,
USA). The cDNA fragment was amplified by PCR using the following
specific primers: Cluster of differentiation 36 (CD36) sense,
5′-TCCTCTGACATTTGCAGGTCTATC-3′ and anti-sense,
5′-GTGAATCCAGTTATGGGTTCCAC-3′; proliferator-activated receptor-α
(PPAR-α), sense 5′-CCCTGAACATCGAGTGTCGA-3′ and anti-sense
5′-CTTGCCCAGAGATTTGAGGTCCT-3′; uncoupling protein 2 (UCP2), sense
5′-GCAAGCTCAATGTTGGTGTCTT-3′ and anti-sense
5′-ACTCTGCAGATAGACAGGCCTG-3′; carnitine palmitoyltransferase-1
(CPT1), sense 5′-CCTGGGCATGATTGCAAAG-3′ and anti-sense
5′-ACAGACTCCAGGTACCTGCTCA-3′; sterol regulatory element binding
protein-1 (SREBP1), sense 5′-GCGCTACCGGTCTTCTATCA-3′ and
anti-sense, 5′-TGCTGCCAAAAGACAAGGG-3′; and cyclophilin (CPN), sense
5′-ATGGTCAACCCCACCGTG-3′ and anti-sense
5′-TTAGAGTTGTCCACAGTCGGAGA-3′. For PPAR-α, UCP2 and CPT-1, PCR was
initiated with a thermal cycle of 95°C for 5 min, 95°C for 30 sec,
55°C for 30 sec, 72°C for 30 sec, with amplification for 30 cycles,
whereas for CD36, PCR was initiated with a thermal cycle of 95°C
for 5 min, 95°C for 30 sec, 51°C for 30 sec, 72°C for 30 sec, with
amplification for 30 cycles. For SREBP1 and CPN, PCR was initiated
with a thermal cycle of 95°C for 5 min, 95°C for 30 sec, 58°C for
30 sec, 72°C for 30 sec, with amplification for 30 cycles. The
RT-PCR products were electrophoresed on 1% agarose gels and
visualized by 0.5 µg/ml ethidium bromide staining. Scanning
densitometry was performed with the i-MAX Gel Image analysis system
(Core Bio System, Seoul, Korea). CPN was amplified as a control
gene.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean and differences between the groups were analyzed using
a Student's t-test. Mean values were considered significantly
different when P<0.05.
Results
Effects on body weight gain
Table II shows the
effects of DSW and SIE on body weight gain in the mice with
HFD-induced obesity following treatment for 8 weeks. The final body
weights were reduced by 3.95% in the DSW group and 8.42.%
(P<0.05) in the DSS group compared with those in the HFC group.
These results indicate that the DSS combination may have an
inhibitory effect against HFD-induced body weight gain. The HFC
group showed the highest food intake and lowest water intake among
the groups, whereas the DSS group showed a reduction in food intake
and increase in water intake (Table
II).
 | Table II.Effects of deep sea water and
Sesamum indicum leaf extract on mouse body weight, food
intake and water intake. |
Table II.
Effects of deep sea water and
Sesamum indicum leaf extract on mouse body weight, food
intake and water intake.
|
| Body weight
(g) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Group | Initial | Final | Weight gain
(g) | Food intake
(g/mouse) | Water intake
(ml/mouse) |
|---|
| HFC | 26.4±0.4 | 38.0±0.4 | 11.7±0.7 | 144.4 | 257.7 |
| DSW | 25.8±0.3 | 36.5±0.8 | 10.7±0.8 | 138.0 | 305.7 |
| DSS | 26.2±0.4 |
34.8±1.0a |
8.6±0.9a | 129.5 | 302.0 |
Effects on serum parameters
Table III shows the
effects of DSW and DSS on metabolic parameters in the mice with
HFD-induced obesity following treatment for 8 weeks. plasma glucose
levels were significantly decreased by 14.9% in the DSW group and
36.4% (P<0.01) in the DSS group compared with those in the HFC
group. However, no significant differences were observed in plasma
insulin levels between the HFC group and the DSW and DSS groups.
The insulin resistance index (HOMA-IR) values for the DSS-treated
group were markedly decreased by 38.2% when compared with those in
the HFC group (Table III). The
plasma TG and leptin levels of the DSW- and DSS-treated groups were
significantly lower than those in the HFC group (Table IV).
 | Table III.Effects of deep sea water and
Sesamum indicum leaf extract on fasting plasma glucose and
insulin levels, and homeostasis model assessment values for insulin
resistance. |
Table III.
Effects of deep sea water and
Sesamum indicum leaf extract on fasting plasma glucose and
insulin levels, and homeostasis model assessment values for insulin
resistance.
| Group | Blood glucose
(mM) | Insulin (U/ml) | HOMA-IR |
|---|
| HFC | 15.4±0.7 | 200.4±9.9 | 137.3±10.5 |
| DSW | 13.1±1.4 | 179.2±14.9 | 106.4±15.6 |
| DSS |
9.8±1.0a, b | 177.1±11.5 |
84.9±11.1c |
 | Table IV.Effects of deep water and Sesamum
indicum leaf extract on serum triglyceride and leptin
levels. |
Table IV.
Effects of deep water and Sesamum
indicum leaf extract on serum triglyceride and leptin
levels.
| Group | Triglyceride
(mg/dl) | Leptin (ng/ml) |
|---|
| HFC | 467.1±88.4 | 2.89±1.01 |
| DSW |
140.3±13.0a |
0.85±0.11b |
| DSS |
121.7±14.3a |
0.72±0.10b |
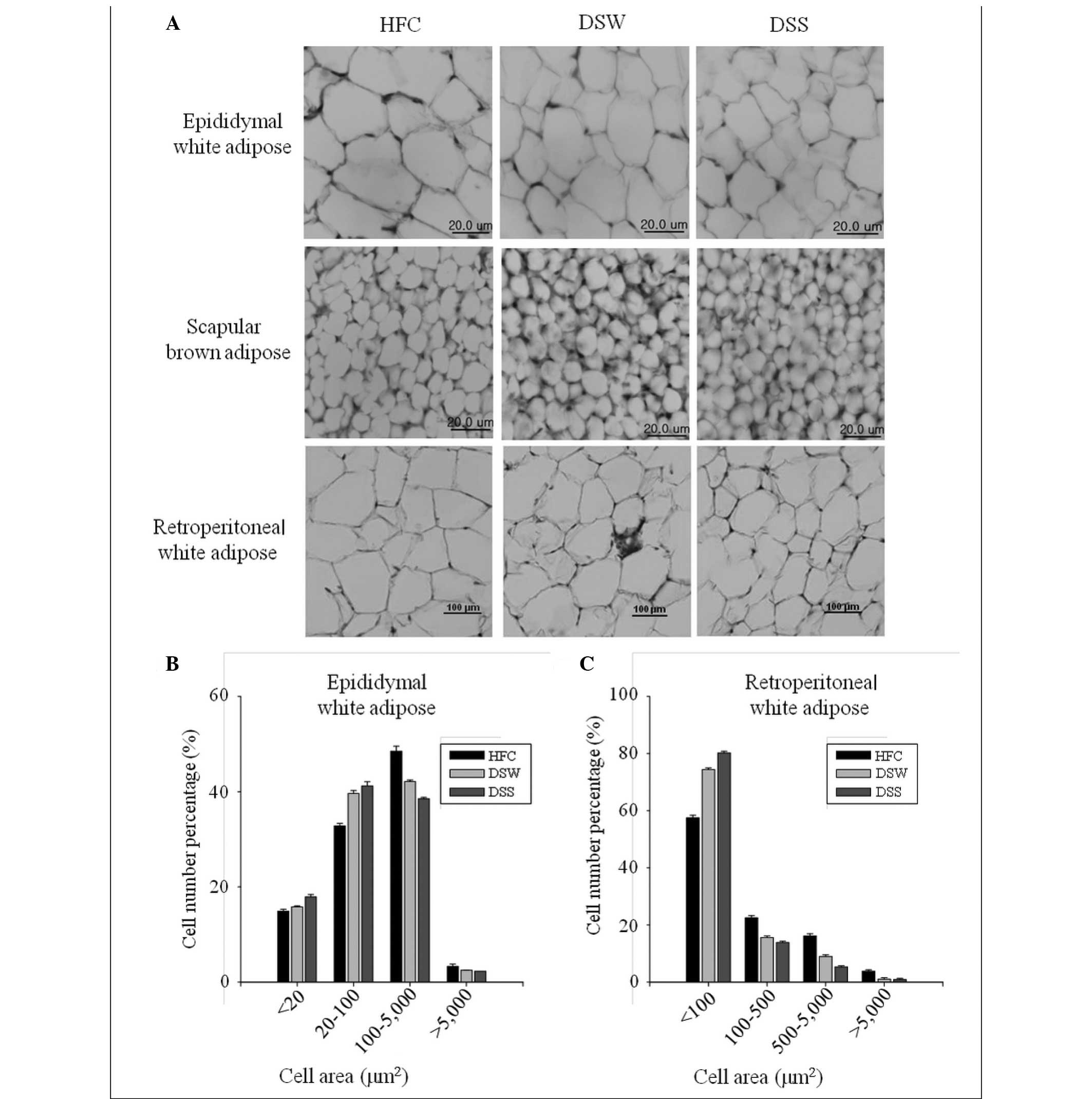
Histological observations
Histological analysis results showed that the sizes
of the epididymal fat and retroperitoneal fat cells in adipose
tissue from the DSW group were smaller than those from the HFC
group, and that fat cell sizes in the DSS-treated group were
smaller than those in the DSW-treated group. Similar results were
also observed in scapular brown adipose tissues (Fig. 1).
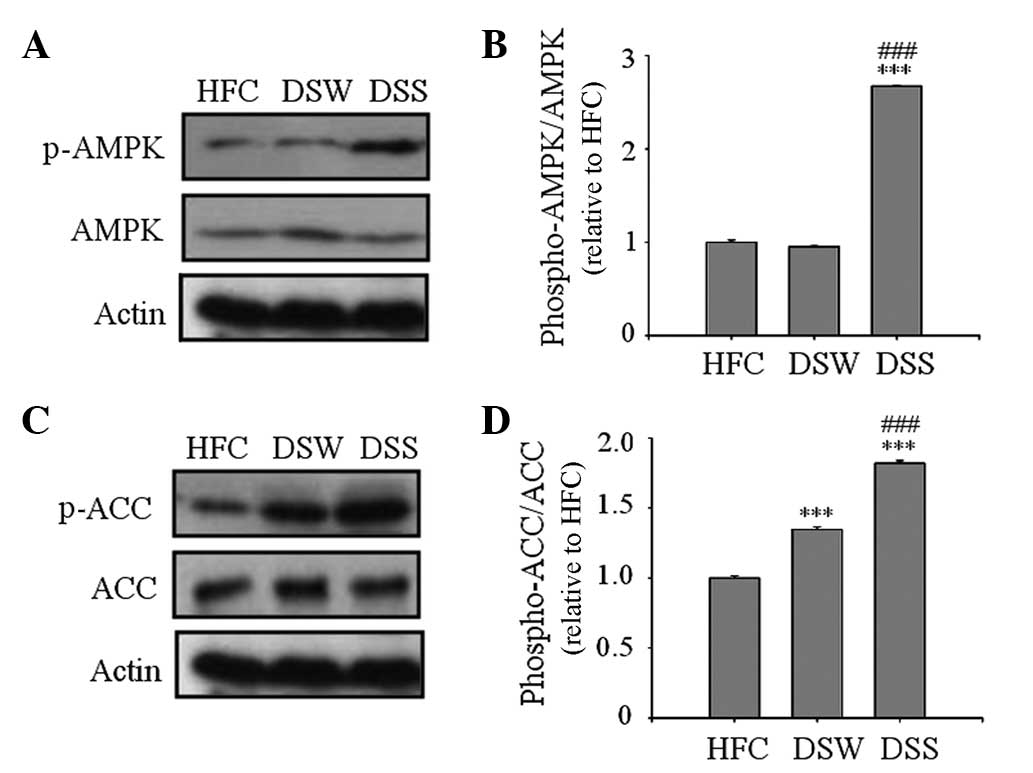
Western blot analysis of AMPK and ACC
activation
AMPK is a key regulator of intracellular fatty acid
metabolism and plays a central role in the regulation of whole body
energy homeostasis (19–21). In the present study, AMPK
phosphorylation was measured by western blot analysis of the
epididymal adipose tissue. As shown in Fig. 2, the levels of p-AMPK and p-ACC were
significantly increased in the DSS group compared with those in the
HFC and DSW groups, indicating the AMPL and ACC were activated
(Fig. 2).
Expression of mRNA in epididymal
adipose tissue
The results from the RT-PCR analysis of the total
RNA obtained from the epididymal adipose tissue of the mice showed
that DSS upregulated the expression of the lipolysis-associated
mRNA PPAR-α and CD36, and energy expenditure-associated# mRNA UCP2
and CPT1 (Fig. 3).
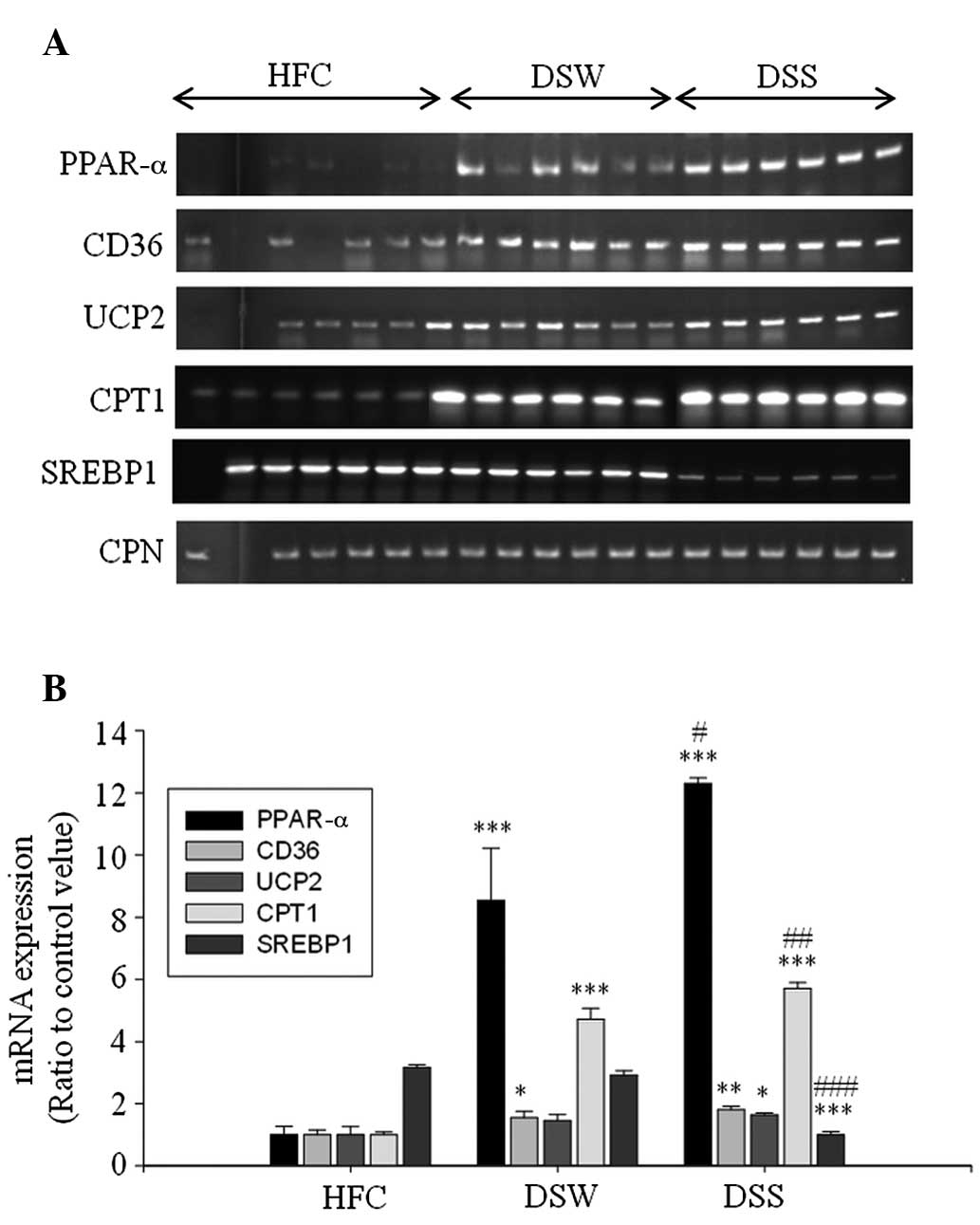 | Figure 3.Reverse transcription-polymerase chain
reaction analyses of PPAR-α, CD36, UCP2, CPT1, SREBP1 and CPN mRNA
expression in the epididymal adipose tissue of mice treated with
deep sea water and Sesamum indicum leaf extract. (A)
Electrophoretic results and (B) relative expression levels.
*P<0.05, **P<0.01 and ***P<0.001 vs. the HFC group;
#P<0.05, ##P<0.01 and
###P<0.001 vs. the DSW group. PPAR-α, peroxisome
proliferator-activated receptor-α; CD36, cluster of differentiation
36; UCP, uncoupling protein 2; CPT1, carnitine
palmitoyltransferase-1; SREBP1, sterol regulatory element-binding
protein-1; HFC, high-fat diet control; DSW, deep sea water; DSS,
DSW + 125 mg/kg Sesamum indicum leaf extract. CPN,
cyclophilin. |
Discussion
Obesity is a major health problem in developed and
developing countries (5,22). Obesity develops when energy intake
exceeds energy expenditure. HFD is a risk factor leading to
whole-body fat accumulation and distribution, particularly the
accumulation of visceral adipose tissue (23). Numerous studies have indicated that
high levels of body fat are associated with an increased risk of
the development of various diseases (1,2).
Anti-obesity foods and food ingredients may improve
the physical condition, and also possibly prevent
life-style-related diseases, if they are effective in reducing the
accumulation of visceral fat (24).
In the present study, whether DSS prevents HFD-induced obesity in
ICR mice was examined. It was found that DSS significantly
decreased body weight gain and adipose cell size in HFD-induced
obesity, indicating that DSS may have anti-obesity activity. The
reduction of body weight gain and adipose tissue size may be
associated with an effect on adipocyte metabolism. In addition,
plasma glucose levels, HOMA-IR values and plasma TG values were
significantly lowered in mice treated with DSW and DSS, compared
with those in the HFC group. This result suggests that DSW and DSS
may have a beneficial effect in improving the insulin resistance
induced by a HFD. Leptin is a major adipocyte-derived hormone that
is involved in the regulation of food intake and energy
expenditure. Therefore, the circulating leptin level correlates
with the extent of obesity (25).
According to the results of the present study, DSW and DSS
significantly decreased plasma leptin levels in mice fed with a
HFD. This result also indicates that DSS may have anti-obesity
activity. Although weight gains between the HFC and DSW groups were
not significantly different, fat mass and obesity-related
parameters in DSW group showed a marginal difference compared with
those in the HFC group. These results may be ascribed to the
reduced level of leptin in the DSW group.
AMPK is a serine/threonine kinase that is activated
when the intracellular AMP:ATP ratio increases. AMPK plays a key
role in regulating carbohydrate and fat metabolism, serving as a
metabolic master switch in response to alterations in cellular
energy charge (26–28). AMPK is also an intercellular signal
transmitting substance of leptin and adiponectin secretion from
adipocytes (29,30). ACC is a rate-limiting enzyme in the
synthesis of malonyl-CoA, which is a critical precursor in the
biosynthesis of fatty acids and a potent inhibitor of mitochondrial
fatty acid oxidation. Inhibition of ACC by AMPK through
phosphorylation leads to a fall in malonyl-CoA content and a
subsequent reduction in fatty acid synthesis and increase in
mitochondrial fatty acid oxidation via the allosteric regulation of
CPT-1, which catalyzes the entry of long-chain fatty acyl-CoA into
mitochondria (31). In the present
study, DSW and DSS resulted in the significant phosphorylation of
AMPK and ACC compared with that in the HFC group. These results may
elucidate the mechanism by which DSW and DSS promote fatty acid
oxidation, inhibit TG accumulation and enhance insulin sensitivity.
The expression levels of target genes responsible for lipogenesis,
lipolysis and energy expenditure were examined by RT-PCR. The
results showed that DSW and DSS upregulated the mRNA expression of
lipolysis-associated PPAR-α and CD36, and energy
expenditure-associated UCP2 and CPT1 (Fig. 3). By contrast, DSW and DSS
downregulated the mRNA expression of lipogenesis-related
SREBP1.
In conclusion, this study demonstrated that DSW and
DSS have preventive effects against HFD-induced obesity in ICR mice
through enhancing the expression of lipolysis-associated PPAR-α and
CD36 and energy expenditure-associated UCP2 and CPT1 at the mRNA
level, and increasing fatty acid oxidation via AMPK activation in
epididymal adipose tissue. Therefore, these results suggest that
this functional food may be beneficial for reducing body fat and/or
preventing obesity.
References
|
1
|
Kopelman PG: Obesity as a medical problem.
Nature. 404:635–643. 2000.PubMed/NCBI
|
|
2
|
Ahn J, Lee H, Kim S, Park J and Ha T: The
anti-obesity effect of quercetin is mediated by the AMPK and MAPK
signaling pathways. Biochem Biophys Res Commun. 373:545–549. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hill JO and Peters JC: Environmental
contributions to the obesity epidemic. Science. 280:1371–1374.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Beck B: Neuropeptides and obesity.
Nutrition. 16:916–923. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Woods SC, Seeley RJ, Rushing PA, D'Alessio
D and Tso P: A controlled high-fat diet induces an obese syndrome
in rats. J Nutr. 133:1081–1087. 2003.PubMed/NCBI
|
|
6
|
Woo MN, Bok SH, Lee MK, Kim HJ, Jeon SM,
Do GM, Shin SK, Ha TY and Choi MS: Anti-obesity and hypolipidemic
effects of a proprietary herb and fiber combination (S&S PWH)
in rats fed high-fat diets. J Med Food. 11:169–178. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Okamoto Y, Higashiyama H, Rong JX, McVey
MJ, Kinoshita M, Asano S and Hansen MK: Comparison of mitochondrial
and macrophage content between subcutaneous and visceral fat in
db/db mice. Exp Mol Pathol. 83:73–83. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shono M, Shimizu I, Aoyagi E, Taniguchi T,
Takenaka H, Ishikawa M, Urata M, Sannomiya K, Tamaki K, Harada N,
et al: Reducing effect of feeding powdered nacre of Pinctada
maxima on the visceral fat of rats. Biosci Biotechnol Biochem.
72:2761–2763. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hainer V, Toplak H and Mitrakou A:
Treatment modalities of obesity: What fits whom? Diabetes Care.
31:269–277. 2008. View Article : Google Scholar
|
|
10
|
Derosa G, Cicero AF, Murdolo G, Piccinni
MN, Fogari E, Bertone G, Ciccarelli L and Fogari R: Efficacy and
safety comparative evaluation of orlistat and sibutramine treatment
in hypertensive obese patients. Diabetes Obes Metab. 7:47–55. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hwang HS, Kim SH, Yoo YG, Chu YS, Shon YH,
Nam KS and Yun JW: Inhibitory effect of deep-sea water on
differentiation of 3T3-L1 adipocytes. Mar Biotechnol. 11:161–168.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hataguchi Y, Tai H, Nakajima H and Kimata
H: Drinking deep-sea water restores mineral imbalance in atopic
eczema/dermatitis syndrome. Eur J Clin Nutr. 59:1093–1096. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Katsuda S, Yasukawa T, Nakagawa K, Miyake
M, Yamasaki M, Katahira K, Mohri M, Shimizu T and Hazama A:
Deep-sea water improves cardiovascular hemodynamics in Kurosawa and
Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol Pharm Bull.
31:38–44. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang HS, Kim HA, Lee SH and Yun JW:
Anti-obesity and antidiabetic effects of deep sea water on ob/ob
mice. Mar Biotechnol (NY). 11:531–539. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ha BG, Park JE, Shin EJ and Shon YH:
Effects of balanced deep sea water on adipocyte hypertrophy and
liver steatosis in high-fat diet induced obese mice. Obesity
(Silver Spring). 22:1669–1678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hahm TS, Park SJ and Martin Lo Y: Effects
of germination on chemical composition and functional properties of
sesame (Sesamum indicum L.) seeds. Bioresour Technol.
100:1643–1647. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee J, Kausar T and Kwon JH:
Characteristic hydrocarbons and 2-alkylcyclobutanones for detecting
gamma-irradiated sesame seeds after steaming, roasting and oil
extraction. J Agric Food Chem. 56:10391–10395. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Institute of Laboratory Animal Research.
Commission on Life Sciences and National Research Council: Guide
For The Care And Use Of Laboratory Animals (Washington, DC).
National Academy Press. 1–136. 1996.
|
|
19
|
Yin HQ, Kim M, Kim JH, Kong G, Kang KS,
Kim HL, Yoon BI, Lee MO and Lee BH: Differential gene expression
and lipid metabolism in fatty liver induced by acute ethanol
treatment in mice. Toxicol Appl Pharmacol. 223:225–233. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Horton JD, Goldstein JL and Brown MS:
SREBPs: Activators of the complete program of cholesterol and fatty
acid synthesis in the liver. J Clin Invest. 109:1125–1131. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hardie DG: The AMP-activated protein
kinase pathway - new players upstream and downstream. J Cell Sci.
117:5479–5487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jeon JR and Kim JY: Effects of pine needle
extract on differentiation of 3T3-L1 preadipocyte. Biol Pharm Bull.
29:2111–2115. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jun HS, Hwang K, Kim Y and Park T:
High-fat diet alters PP2A, TC10 and CIP4 expression in visceral
adipose tissue of rats. Obesity (Silver Spring). 16:1226–1231.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saito M, Ueno M, Ogino S, Kubo K, Nagata J
and Takeuchi M: High dose of Garcinia cambogia is effective in
suppressing fat accumulation in developing male Zucker obese rats,
but highly toxic to the testis. Food Chem Toxicol. 43:411–419.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ogawa Y, Masuzaki H, Ebihara K, Shintani
M, Aizawa-Abe M, Miyanaga F and Nakao K: Pathophysiogical role of
leptin in lifestyle-related diseases: Studies with transgenic
skinny mice overexpressing leptin. J Diabetes Complications.
16:119–122. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hardie DG and Carling D: The
AMPK-activated protein kinase-fuel gauge of mammalian cells. Eur J
Biochem. 246:259–273. 1998. View Article : Google Scholar
|
|
27
|
Hardie DG, Carling D and Carlson M: The
AMP-activated/SNF1 protein kinase subfamily: Metabolic sensors of
the eukaryotic cell? Ann Rev Biochem. 67:821–855. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hardie DG, Scott JW, Pan DA and Hudson ER:
Management of cellular energy by energy by the AMP-activated
protein kinase system. FEBS Lett. 546:113–120. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yano W, Kubota N, Itoh S, Kubota T,
Awazawa M, Moroi M, Sugi K, Takamoto I, Ogata H, Tokuyama K, et al:
Molecular mechanism of moderate insulin resistance in
adiponectin-knockout mice. Endocr J. 55:515–522. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Janovská A, Hatzinikolas G, Staikopoulos
V, McInerney J, Mano M and Wittert GA: AMPK and ACC
phosphorylation: Effect of leptin, muscle fibre type and obesity.
Mol Cell Endocrinol. 284:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Velasco G, Geelen MJ and Guzmán M: Control
of hepatic fatty acid oxidation by 5′-AMP-activated protein kinase
involves a malonyl-CoA-dependent and a malonyl-CoA-independent
mechanism. Arch Biochem Biophys. 337:169–175. 1997. View Article : Google Scholar : PubMed/NCBI
|