Introduction
Epidermal growth factor receptor (EGFR) is a member
of the of the EGFR family proteins, which consists of at least four
receptor tyrosine kinases: EGFR, human epidermal growth factor
receptor (HER)2, HER3 and HER4.
The EGFR family regulates the tyrosine kinase
signaling pathway, which is responsible for cell cycle progression,
division and differentiation. EGFR has been found to be
overexpressed in certain types of solid tumors, such as colon,
pancreas, lung and breast tumors, and leads to the dysregulation of
cell growth (1).
Therapeutic agents with two main anti-EGFR
mechanisms are used in clinical practice: Low molecular-weight
tyrosine kinase inhibitors that compete with adenosine triphosphate
(ATP) for binding with the tyrosine kinase portion of a mutant EGFR
receptor, and monoclonal antibodies directed against the
ligand-binding extracellular domain (1).
Agents targeting EGFR comprise some of the most
extensively used drugs in the treatment of several common solid
tumors (2). Gefitinib is an orally
selective tyrosine kinase inhibitor of EGFR. Gefitinib and
erlotinib were the first EGFR tyrosine kinase inhibitors to be
approved by the US Food and Drug Administration for the treatment
of advanced non-small cell lung carcinoma (NSCLC) (3).
Although these agents do not exhibit many of the
classic side effects of cytotoxic chemotherapy, they are associated
with unpleasant cutaneous toxicities, which can affect treatment
compliance, as well as the quality of life of the patient (2,4).
Papulopustular acne-like eruption is the most common cutaneous
adverse event (5) with an incidence
rate of 55% (1). Xerosis, pruritus,
desquamation, eczematous eruptions, teleangectasia,
hyperpigmentation, trichomegalia, alopecia and nail alterations
have also been reported (6–8). The present case report describes an
atypical skin reaction in a patient treated with gefitinib.
Case report
The present study reports the case of a 73-year old
woman suffering from advanced NSCLC, who developed a squamous,
crusted eruption on her face after 4 weeks of treatment with
gefitinib. The patient had been initially diagnosed with advanced
lung adenocarcinoma in March 2013 and treated with gefinib at the
Unit of Medical Oncology of the Veneto Institute of Oncology
(Padua, Italy). The patient had never smoked and her initial
symptoms were a persistent dry cough and a dull low back pain,
which was unresponsive to corticosteroid therapy. A computed
tomography (CT) scan revealed the presence of a mass in the left
inferior lobe of the lung (average diameter, 5 cm), which was found
by histopathological examination to be a primary adenocarcinoma of
the lung. Further examinations with positron emission tomography/CT
revealed metastases in locoregional lymph nodes, bones and pleura.
Magnetic resonance imaging (MRI) of the lumbar spine demonstrated
secondary neoplastic lesions in multiple locations: D12, L2, L4,
S1, S2 and the sacral right wing. The brain CT scan was negative.
According to the TNM classification and staging system (9), lung adenocarcinoma stage IV was
diagnosed and the EGFR mutation status (in-frame deletion of
E746–750 in exon 19) was determined. The presence of an EGFR
mutation-positive neoplasm was thus identified, and oral treatment
with gefitinib was initiated at a dose of 250 mg once daily. After
4 weeks of treatment with gefitinib, the patient visited the
Dermatology Unit (Department of Medicine, University of Padua,
Padua, Italy) in May 2013, presenting with a squamous-crusted
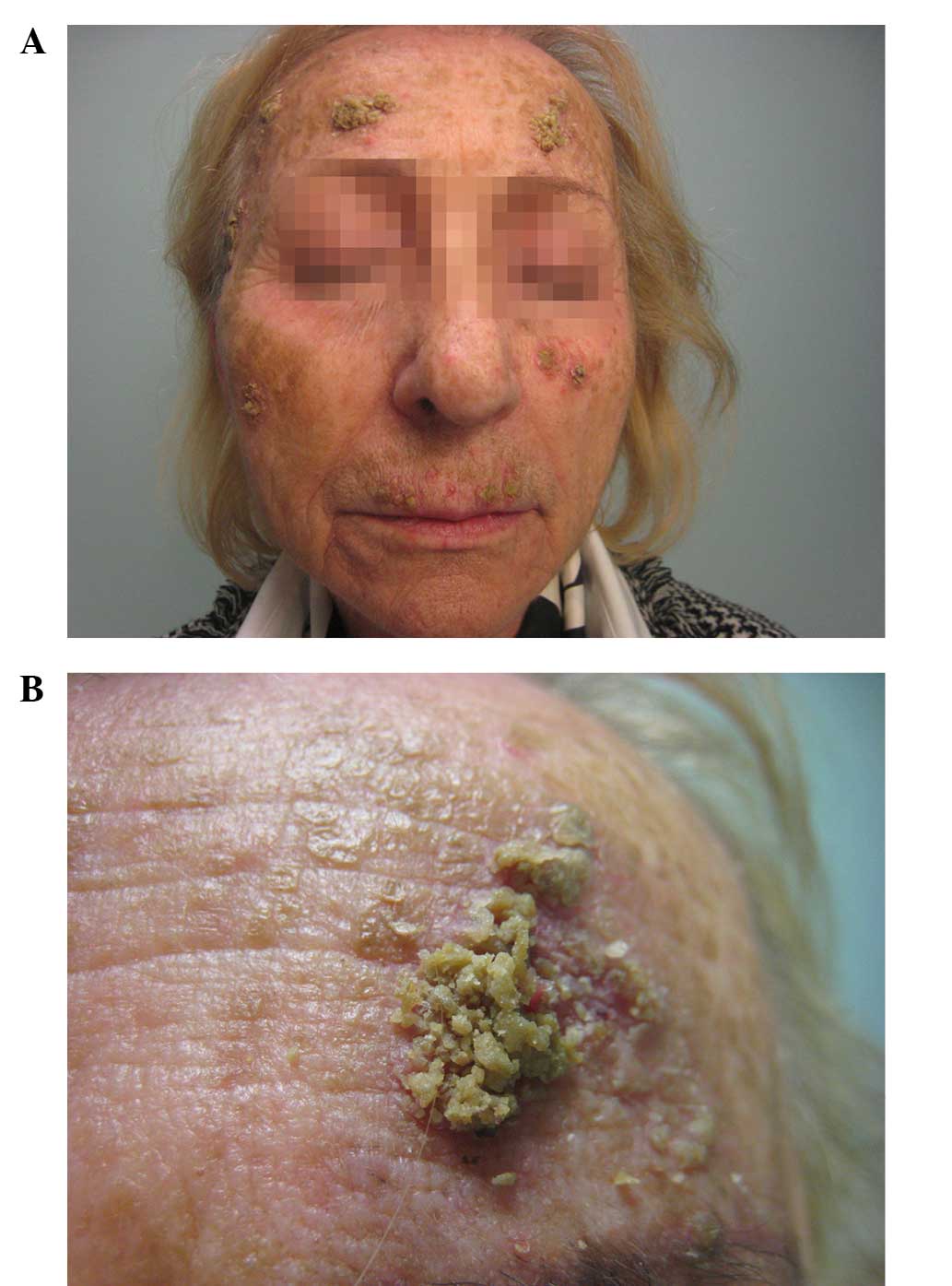
eruption on her face (Fig. 1). The
patient exhibited yellowish exophytic squamous-crusted lesions with
sharp edges and an erythematous base. She had not previously
applied topical products to the lesions, reported sun exposure or
contact with aeroallergens, taken any other medications, or
complained of fever, nausea, diarrhoea, abdominal pain or fatigue.
The patient reported a weight loss of 3 kg during the last month
prior to her visit to the hospital.
Dermatoscopic examination did not reveal
abnormalities in the lesions, thus excluding the diagnosis of
squamous cell carcinoma or seborrheic keratosis. The cutaneous swab
was positive for Staphylococcus aureus. Complete blood count
and liver and kidney function tests were normal, serum
carcinoembryonic antigen was elevated (1,300 ng/ml) and an
enzyme-linked immunosorbent assay for autoantibodies against
desmogleins 1 and 3 was negative.
Treatment with gefitinib was discontinued and a
systemic and topical antibiotic therapy was administered instead.
The patient was treated with 100 mg minocyclin (2 tablets/day)
orally, and with rifamycin topically (twice a day for 1 month).
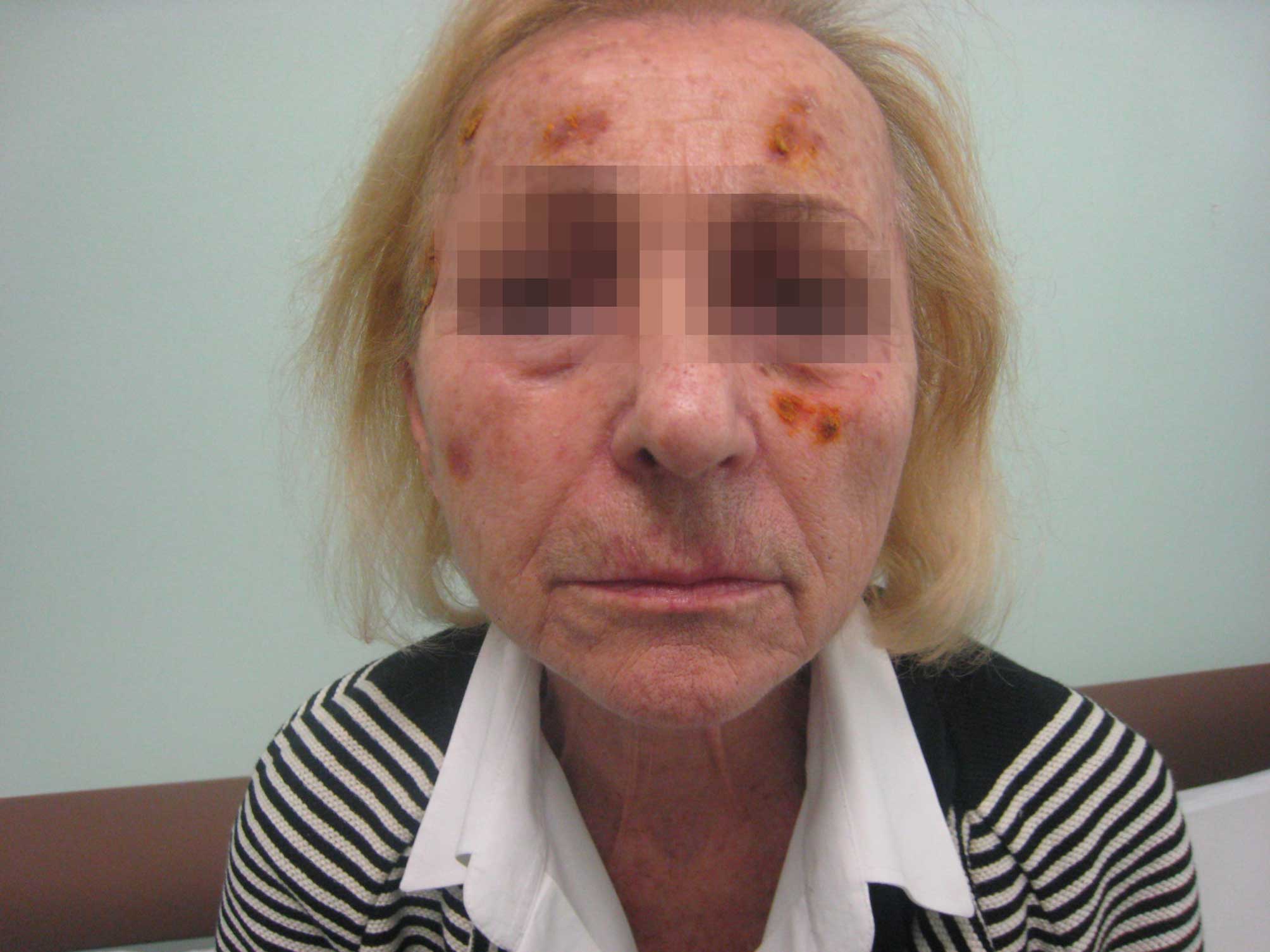
Two weeks later, the patient returned to the Unit
exhibiting an almost complete resolution of the lesions (Fig. 2), and therefore gefitinib treatment
(250 mg once daily) was resumed. Despite the gefitinib treatment,
the disease progressed, leading to the mortality of the patient 2
months after resuming the treatment. Written informed consent was
obtained from the patient for the publication of this case report
and any accompanying images.
Discussion
EGFR inhibitors are widely used in monotherapy, or
in combination with chemotherapy and/or radiotherapy, for the
treatment of advanced solid cancers, such as NSCLC, squamous cell
carcinoma of the head and neck, and colorectal and pancreatic
cancer (10).
Among EGFR inhibitors, gefitinib is generally
preferred because of its greater tolerability (2), despite the adverse events that are
commonly reported, such as diarrhea, fatigue, nausea, elevated
transaminase and skin rash (11).
The most common adverse effects associated with EGFR inhibitors
involve the skin. Several skin complications have been described,
with the most frequent being an inflammatory papulopustular rash
occurring within the first 2–4 weeks of treatment (12). The papulopustular eruption is usually
distributed in the seborrheic areas and the primary lesions are
follicular papules and pustules. Comedones are rarely observed
(13). Histopathologically, a T-cell
infiltrate around the follicular infundibulum is observed, which is
associated with a suppurative folliculitis (14). Dry skin is very commonly observed in
patients receiving treatment with EGFR inhibitors; vaginal dryness
and itching, perineal dryness and blepharitis have been reported
(15). The second most frequent
systemic adverse effect of EGFR inhibitors is diarrhea, whose
development can be associated with skin rash (16). Other cutaneous adverse reactions
include paronychia, mucositis and hair changes, such as scalp
alopecia, curling of the hair and facial hypertrichosis (17). Nail alterations are generally
observed between weeks 4 and 8 from the initiation of gefitinib
(13). The big toe is often affected
by paronychia, which can be very painful if pyogenic granuloma of
the nail fold develops (17). The
combination of the papulopustular eruption, xerosis and nail and
hair alterations along with pruritus is specific for this class of
agents. The term PRIDE syndrome (papulopustules and/or paronychia,
regulatory abnormalities of hair growth, itching, dryness caused by
epidermal growth factor inhibitors) has been proposed for EGFR
inhibitor-associated cutaneous complications (18,19).
Gefitinib-induced skin lesions usually occur on the face, scalp and
upper chest and back, but can be observed anywhere (16). The skin rash, particularly when it
occurs on the face, can affect the quality of life of the patient,
and it may even result in a modification or discontinuation of
their treatment. Furthermore, a high prevalence of cutaneous
bacterial infections has been reported among patients with
dermatological toxic effects following treatment with EGFR
inhibitors (20). Other reported
skin complications associated with gefitinib treatment are
small-vessel vasculitis (21),
psoriasis (22) and necrolytic
migratory erythema (23).
To the best of our knowledge, the present study
reports the first case of a squamous-crusted eruption on the face
of a patient undergoing treatment with gefitinib. The epidermal
alterations that this patient exhibited could have been the result
of the activation of both innate and acquired immunity (16), leading to the production of cytokines
acting on keratinocyte proliferation. A role of EGFR in the control
of skin inflammation has been proposed on the basis of the
observation that mice with an epidermis-restricted dominant
negative EGFR mutation display an abundant inflammatory infiltrate
in their skin, formed by macrophages, lymphocytes and granulocytes,
starting 4–6 days after birth and progressively aggravating with
the passage of time (24). Mascia
et al (25) demonstrated that
pharmacological blockade of EGFR boosted the expression of
monocytes, dendritic cells and the T-cell chemoattractants
chemokine (C-C motif) ligand (CCL)2 and CCL5, as well as the
T-cell-selective chemokine (C-X-C motif) ligand (CXCL)10, while it
markedly impaired the expression of granulocyte-macrophage
colony-stimulating factor and CXCL8. These molecular events are
dependent upon the concentration of the EGFR inhibitor, documenting
the existence of an EGFR-driven regulatory mechanism during the
keratinocyte response to inflammatory triggers (16). A strong upregulation of CCL2 and
CCL5, but also of CCL27 and CXCL14, was found in the lesional skin
of patients with cancer, who were undergoing treatment with EGFR
inhibitors (26,27). Finally, upregulated transcripts of
tumor necrosis factor-α and interleukin-1β, as well as CCL2, CCL5,
CCL11 and CCL22, were detected in the skin and circulation of mouse
models with EGFR ablation in the epidermis during their first week
of life, prior to infiltration by immune cell populations (27,28).
Due to the lack of clarity concerning the etiology
of the skin rash, there are no evidence-based recommendations for
treating it. Prophylactic treatments with local or systemic
antibiotics, such as minocycline, have been recommended (13,29), and
it is plausible that the effect of minocycline on gefitinib-induced
skin complications is mediated by its anti-inflammatory activities
(30).
Further studies are required in order to explain the
mechanisms through which EGFR-targeting agents, such as gefitinib,
induce cutaneous reactions. Additional studies may facilitate a
more natural approach to the treatment of these dermatologic
adverse effects and provide more information concerning the
physiological role of EGFR in the skin immune system. Informing the
patients about the nature of these unpleasant skin side effects may
restore their faith in the use of EGFR-targeted cancer treatments
and help to ensure compliance.
References
|
1
|
Wnorowski MA, de Souza A, Chachoua A and
Cohen DE: The management of EGFR inhibitor adverse events: A case
series and treatment paradigm. Int J Dermatol. 51:223–232. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ocvirk J, Heeger S, McCloud P and Hofheinz
RD: A review of the treatment options for skin rash induced by EGFR
targeted therapies: Evidence from randomized clinical trials and
meta-analysis. Radiol Oncol. 47:166–175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luo M and Fu LW: Redundunt kinase
activation and resistance of EGFR-tyrosine kinase inhibitors. Am J
Cancer Res. 4:608–628. 2014.PubMed/NCBI
|
|
4
|
Ricciardi S, Tomao S and de Marinis F:
Toxicity of targeted therapy in non-small cell lung cancer
management. Clin Lung Cancer. 10:28–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peréz-Soler R, Delord JP, Halpern A, Kelly
K, Krueger J, Sureda BM, von Pawel J, Temel J, Siena S, Soulières
D, et al: HER1/EGFR inhibitor-associated rash: Future directions
for management and investigation outcomes from the HER1/EGFR
inhibitor rash management forum. Oncologist. 10:345–356. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Balagula Y, Rosen ST and Lacouture ME: The
emergence of supportive oncodermatology: The study of dermatologic
adverse events to cancer therapies. J Am Acad Dermatol. 65:624–635.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eames T, Grabein B, Kroth J and
Wololenberg A: Microbiological analysis of epidermal growth factor
receptor inhibitor therapy-associated paronychia. J Eur Acad
Dermatol Venereol. 24:958–960. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rami-Porta R, Crowley JJ and Goldstraw P:
The revised TNM staging system for lung cancer. Ann Thorac
Cardiovasc Surg. 15:4–9. 2009.PubMed/NCBI
|
|
10
|
Ciardello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu HB, Wu Y, Lv TF, Yao YW, Xiao YY, Yuan
DM and Song Y: Skin rash could predict the response to EGFR
tyrosine kinase inhibitor and the prognosis for patients with
non-small cell lung cancer: A systematic review and meta-analysis.
PloS One. 8:e551282013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Potthoff K, Hofheinz R, Hassel JC,
Volkenandt M, Lordick F, Hartmann JT, Karthaus M, Riess H, Lipp HP,
Hauschild A, et al: Interdisciplinary management of
EGFR-inhibitor-induced skin reactions: A German expert opinion. Ann
Oncol. 22:524–535. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heidary N, Naik H and Burgin S:
Chemotherapeutic agents and the skin: An update. J Am Acad
Dermatol. 58:545–570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herbst RS, LoRusso PM, Purdom M and Ward
D: Dermatologic side effects associated with gefitinib therapy:
Clinical experience and management. Clin Lung Cancer. 4:366–369.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Herbst RS, Maddox AM, Rothenberg ML, Small
EJ, Rubin EH, Baselga J, Rojo F, Hong WK, Swaisland H, Averbuch SD,
et al: Selective oral epidermal growth factor receptor tyrosine
kinase inhibitor ZD1839 is generally well-tolerated and has
activity in non-small-cell lung cancer and other solid tumors:
Results of a phase I trial. J Clin Oncol. 20:3815–3825. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pastore S, Lulli D and Girolomoni G:
Epidermal growth factor receptor signalling in keratinocyte
biology: Implications for skin toxicity of tyrosine kinase
inhibitors. Arch Toxicol. 88:1189–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang GC, Yang TY, Chen KC, Yin MC, Wang
RC and Lin YC: Complications of therapy in cancer patients: Case 1.
Paronychia and skin hyperpigmentation induced by gefitinib in
advanced non-small-cell lung cancer. J Clin Oncol. 22:4646–4648.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lacouture ME and Lai SE: The PRIDE
(Papulopustules and/or paronychia, Regulatory abnormalities of hair
growth, Itching, and dryness due to epidermal growth factor
receptor inhibitors) syndrome. Br J Dermatol. 155:852–854. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Madke B, Gole P, Kumar P and Khopkar U:
Dermatological side effects of epidermal growth factor receptor
inhibitors: ‘PRIDE’ complex. Indian J Dermatol. 59:271–274. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eilers RE Jr, Gandhi M, Patel JD, Mulcahy
MF, Agulnik M, Hensing T and Lacouture ME: Dermatologic infections
in cancer patients treated with epidermal growth factor receptor
inhibitor therapy. J Natl Cancer Inst. 102:47–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kurokawa I, Endo K and Hirabayashi M:
Purpuric drug eruption possibly due to gefinitib (Iressa). Int J
Dermatol. 44:167–168. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zorzou MP, Stratigos A, Efstathiou E and
Bamias A: Exacerbation of psoriasis after treatment with an EGFR
tyrosine kinase inhibitor. Acta Derm Venereol. 84:308–309. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Trojan A, Jacky E, Follath F and Dummer R:
Necrolytic migratory erythema (glucagenoma)-like skin lesions
induced by EGF-receptor inhibition. Swiss Med Wkly. 133:22–23.
2003.PubMed/NCBI
|
|
24
|
Murillas R, Larcher F, Conti CJ, Santos M,
Ullrich A and Jorcano JL: Expression of a dominant negative mutant
of epidermal growth factor receptor in the epidermis of transgenic
mice elicits striking alterations in hair follicle development and
skin structure. EMBO J. 14:5216–5223. 1995.PubMed/NCBI
|
|
25
|
Mascia F, Cataisson C, Lee TC, Threadgill
D, Mariani V, Amerio P, Chandrasekhara C, Souto Adeva G, Girolomoni
G, Yuspa SH and Pastore S: EGFR regulates the expression of
granulocyte/macrophage colony-stimulating factor in vitro and in
vivo. J Invest Dermatol. 130:682–693. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamaki M, Sugiura K, Muro Y, Shimoyama Y
and Tomita Y: Epidermal growth factor receptor tyrosine kinase
inhibitors induce CCL2 and CCL5 via reduction in IL-1R2 in
keratinocytes. Exp Dermatol. 19:730–735. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lichtenberger BM, Gerber PA, Holcmann M,
Buhren BA, Amberg N, Smolle V, Schrumpf H, Boelke E, Ansari P,
Mackenzie C, et al: Epidermal EGFR controls cutaneous host defense
and prevents inflammation. Sci Transl Med. 5:199ra1112013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mascia F, Lam G, Keith C, Garber C,
Steinberg SM, Kohn E and Yuspa SH: Genetic ablation of epidermal
EGFR reveals the dynamic origin of adverse effects of anti-EGFR
therapy. Sci Transl Med. 5:199ra1102013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Scope A, Agero AL, Dusza SW, Myskowski PL,
Lieb JA, Saltz L, Kemeny NE and Halpern AC: Randomized double-blind
trial of prophylactic oral minocycline and topical tazarotene for
cetuximab-associated acne-like eruption. J Clin Oncol.
25:5390–5396. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Garrido-Mesa N, Zarzuelo A and Gálvez J:
Mynocicline: Far beyond an antibiotic. Br J Pharmacol. 169:337–352.
2013. View Article : Google Scholar : PubMed/NCBI
|